Abstract
Peri-implantitis is one of the major clinical conditions associated with dental implant failure. Adhesion of bacterial biofilm is considered as the primary etiological factor for this condition. A commonly used therapeutic method for surgical removal of adhered biofilm is mechanical debridement, which may cause detrimental effects on the implant surface. Post-treatment, implants are expected to re-osseointegrate with bone tissue, providing mechanical stability. However, it is important to understand that both bacterial adhesion and detoxification procedures can affect the titanium surface, which is vital for growth of bone-forming cells, osteoblasts. The goal of this study was to evaluate the synergistic effect of bacterial adhesion and detoxification treatment method on subsequent bone cell growth on implant surface. Polished titanium specimens underwent bacterial contamination and debridement/detoxification treatment with acidic and neutral chemicals to model a treatment for a peri-implantitis-infected dental implant. Subsequently, bone cell activity and surface morphology were evaluated using standard cell viability/differentiation assays, scanning electron and optical microscopies, respectively. The synergistic activity of bacterial contamination and detoxification with acidic chemicals generally lowered cell viability and proliferation rates. This suggested higher toxicity of titanium surfaces imparted by detoxification methods on osteoblasts. Electrochemical testing corroborated visual signs of corrosion attack and revealed that immersion-treated specimens had higher corrosion resistance than their corresponding rubbing-treated counterparts, excluding saline. Overall, surface damage induced by detoxification methods must be considered when selecting the most appropriate therapy to increase the probability of re-osseointegration of titanium substrates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Dental implants have altered the face of dentistry over the last 25 years. More than 500,000 implants are placed every year [1] with a reported success rate of approximately 90–95% [2]. Success of a dental implant is primarily associated with implant surface interaction and integration with surrounding hard and soft tissues to achieve mechanical support and stability, which depends on surface properties. Materials currently used to design and develop dental implants include metals, carbons, polymers, ceramics and a combination of them [3]. Of these, commercially pure titanium (cpTi) and its alloys have conquered majority use in the dental implant industry due to their biocompatibility, durability [3] and mechanical properties [4, 5]. In addition, titanium has a naturally forming oxide layer [6], which provides corrosion resistance and also facilitates osseointegration. Other chemical and physical properties of the implant surface including surface roughness and hydrophilicity regulate the quality and speed of osseointegration [3].
Despite high success rates, 5–10% of implants fail, which results in economical and health burden to many patients [2]. Implant failures are mainly classified into two stages: early- and late-stage failures; early-stage failure occurs before attachment of prosthetic components primarily due to failure to establish osseointegration [7, 8]. Late-stage complications occur after osseointegration is established between the implant and surrounding bone. Factors that contribute to late implant failures include excessive loading, peri-implantitis and inadequate prosthetic construction [9]. However, bacterial biofilm has been found to be the primary reason for both early- and late-stage complications [10].
Recently, a rising number of implant failures have been reported due to peri-implantitis, which is a clinical condition characterized by inflammation and continued loss of integrated bone around an implant caused by the formation of a bacterial biofilm [3, 5]. A study reported that 28–56% of patients who receive an implant suffer from this severe clinical condition [6]. There are mainly two types of peri-implant disease: peri-implant mucositis and peri-implantitis. Peri-implant mucositis is the reversible inflammatory disease that affects only the soft tissue around an implant [11] and is identified by the presence of bleeding on probing with no evidence of radiographic loss of bone around an implant [12]. On the other hand, peri-implantitis is a site-specific inflammation of soft tissues with bleeding on probing and suppuration accompanied by progressive destruction of supporting bone around an implant [13]. Common pathogenic microorganisms associated with peri-implantitis are gram-negative anaerobes such as Prevotella intermedia, Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, Tannerella forsythia, Treponema denticola, Prevotella nigrescens, Peptostreptococcus micros and Fusobacterium nucleatum [14,15,16]. In addition to these, Streptococcus sanguinis [17,18,19,20] and Staphylococcus aureus [11] are known as pioneering colonizers in oral biofilms and identified to bind to hard substrates such as implant surfaces [13, 21].
When peri-implantitis does occur, clinicians have the option to either remove the infected implant or perform debridement and detoxification to remove bacteria and its metabolites present on the surface to re-establish osseointegration [22]. There are many different methods to treat implants affected by peri-implantitis including chemical, mechanical and laser treatments [22]. Chemical treatment is employed for debridement of surfaces with biofilm; commonly used chemicals include citric acid, tetracycline, saline, chlorhexidine, hydrogen peroxide and doxycycline [21, 23,24,25,26]. Laser treatments such as neodymium-doped yttrium aluminum garnet (Nd:YAG) and erbium-doped yttrium aluminum garnet (Er:YAG) lasers along with mechanical abrasion by curettes and air powder abrasives are also used [12]. However, this study focuses on evaluating mechanical debridement in combination with chemicals as a treatment method for peri-implantitis.
The solutions used to facilitate debridement are effective at removing bacteria from the surface [27]. However, many of these solutions are low in pH and high in fluoride concentration, which are known to cause damage to the surface of titanium [28]. Though both prescribed oral mouthwash and detoxification treatments are effective for biofilm removal, they might hinder the recovery of the de-osseointegrated interface between supporting bone and implant surface. Studies have shown that these treatment options damage implant surfaces by introducing defects, delamination of top layers and cracks, which can result in weakening of the metal structure, thereby hindering re-osseointegration [21, 28, 29].
Understanding the effects of bacterial adhesion and peri-implantitis detoxification treatment methods on implant surfaces is crucial to drive innovations in implant design and to better inform clinicians performing such procedures in their practices. There is a lack of controlled in vitro studies that investigate the synergistic impact of bacterial adhesion and detoxification treatments on cellular growth. In this study, a new testing methodology was developed to evaluate titanium surface changes when exposed to oral pathogenic bacteria associated with peri-implantitis and to determine the effects of commonly used chemicals for detoxification on the implant surface to simulate oral conditions and treatment. The aim of this study was to investigate the impact of (1) bacterial biofilm on surface of titanium, (2) combination of chemicals and mechanical debridement on titanium surface, (3) and synergistic activity of bacterial biofilm and treatment process on growth of pre-osteoblasts. The hypothesis was that the combined effect of mechanical abrasion and detoxification procedures would hinder growth of pre-osteoblasts, thus enhancing corrosion susceptibility. This experimental model has the versatility to accommodate different dental implant materials as well as different implant surface detoxification methods.
2 Materials and Methods
2.1 Preparation of Samples
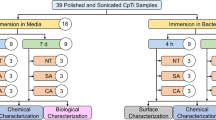
Cold-worked, grade 2 commercially pure titanium (cpTi) stocks were cut and mounted in an acrylic mold and subsequently polished using increasingly finer grit sizes (240, 360, 600, 800 and 1200) of silicon carbide (SiC) paper followed by 1-µm polycrystalline diamond and 0.05-µm alumina suspensions. Specimens were then removed from the acrylic mold and cleaned with ultra-sonication while immersed in acetone, deionized (DI) water and ethanol for 15 min each before being placed in an oven at 60 °C to dry overnight.
2.2 Contamination of Titanium Surface
Early-colonizing Streptococcus mutans (UA 159), Streptococcus sanguinis (ATCC 10556), Streptococcus salivarius (ATCC 13419) and late-colonizing Aggregatibacter actinomycetemcomitans (VT 1169) were cultured in brain heart infusion (BHI) agar plates (BD, Franklin Lakes) and incubated at 37 °C in 5% CO2 microaerophilic condition (BD GasPak) for 48 h until colonies were formed. Next, individual colonies from each strain of bacteria were inoculated into 2 ml of BHI broth medium in separate wells of a 24-well plate. Each of the 33 cpTi samples was immersed in individual wells containing the bacterial polyculture. The plate was then incubated for 5 days at 37 °C in 5% CO2 microaerophilic condition (BD GasPak) to develop a biofilm. Turbidity of the BHI broth medium was checked every 48 h. After 5 days, the cpTi specimens were removed from each well, individually wrapped in aluminum foil and autoclaved.
2.3 Detoxification of Titanium Surface
2.3.1 Preparation of Chemicals
Citric acid (30%) was prepared by dissolving 30 g of citric acid monohydrate (Fisher Scientific, Hampton, NH) in 100 ml of DI water. Chlorhexidine gluconate oral rinse solution (0.12%) was used as provided by the manufacturer (Chlorheximed GSK, Middlesex, UK). Saline solution (0.9%) was used as prepared, and doxycycline (50%) was prepared in solution by mixing doxycycline powder (Actavis, Dublin, Ireland) in DI water. The pH of each chemical was measured before and after treatments using a pH meter (FiveEasy, Mettler Toledo).
2.3.2 Rubbing and Immersion Treatment Methods
To stimulate clinical procedure, this study included two methods for detoxification: rubbing and immersion methods. The rubbing method involved soaking a cotton swab in the chemical solution and manually rubbing the sample surface in a circular motion. The immersion method involved immersing a specimen in a given detoxification chemical. Each technique was carried out for 8 min. CpTi samples (n = 4) underwent immersion treatment for each of the 4 detoxification chemicals, while another group (n = 4) of cpTi samples received the rubbing treatment for each detoxification chemical. For each treatment group, three specimens were subsequently used for cell compatibility studies, while one was used for surface analysis. One cpTi specimen was left untreated as control.
2.4 Evaluation of Cell Compatibility on Detoxified Surfaces
Cytocompatibility of pre-osteoblasts to detoxification-treated specimens was assessed using the ISO 10993-5:2009 standard method. This standard provides a testing method to assess in vitro cytotoxicity of medical device surfaces and/or extracts of a device to cells in direct or indirect contact with these surfaces. This method considers 70% or lower cell viability as cytotoxic.
2.4.1 Pre-osteoblast (MC3T3-E1) Cell Growth
Cultured pre-osteoblasts (MC3T3-E1) (American Type Culture Collection) were grown in Minimum Essential Medium (MEM) Alpha Modification (1X) media (American Type Culture Collection) supplemented with 10% fetal bovine serum (FBS) (GE Healthcare Life Sciences) in T-75 flasks. Post-detoxification, each treated sample (n = 3) was placed in individual wells of a 24-well plate. Pre-osteoblast cells (MC3T3-E1) were placed on each cpTi surface at a seeding density of 0.05 × 106 cells/well along with 1 ml of MEM. Pre-osteoblasts were then incubated at 37 °C for 7 days; media in each well was changed every 2 days.
2.4.2 Cellular Viability Assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium-bromide (MTT) assay was performed to evaluate cell viability for each chemical and detoxification method performed. First, media from each well in the 24-well plate was aspirated. Pre-osteoblast cells were washed with 1X phosphate-buffered saline (PBS) and then trypsinized to detach cells from the bottom of the wells and cpTi surface. Detached cells from each well were transferred into separate 50-ml centrifuge tubes with 1 ml media and centrifuged at 1200 rpm for 5 min after which the supernatant was removed. Cells were re-suspended in 250 µl of media; 100 µl of this cell solution from each centrifuge tube was added to separate wells in 96-well plate, and 100 µl of MEM was added into 3 separate wells as blank control. For each well, 10 µl of MTT reagent was added, and the 96-well plate was incubated at 37 °C for 4 h in a dark environment. After 4 h, 100 µl of detergent reagent was added, and the plate was placed in a dark environment overnight. Optical density was measured using an automatic plate reader (Synergy Mx, Biotek) after 12 h at 570 nm. Intensity of the blue formazan produced by viable cells resulted in distinct optical density values. These values were used to calculate percentage cell viability after the blank optical density was subtracted.
2.4.3 Cellular Differentiation Assay
Alkaline phosphatase (ALP) assay was performed to quantify the degree of differentiation of pre-osteoblasts into osteoblasts. From the 250 µl cell suspension obtained after centrifuging, 50 µl was added to a separate 96-well plate for ALP Assay (Abcam), in addition to 50 µl of MEM in 3 other wells to serve as a blank. Next, 30 µl of assay buffer and 50 µl of 5 mM pNPP solution were added to each well. At this point, 20 µl of stop solution was transferred to the blank wells only. Then, the plate was incubated for 1 h in dark environment at room temperature. Afterward, 20 µl of stop solution was added to the remaining wells and optical density was read at 405 nm. ALP activity was then calculated by comparing against a calibration curve.
2.4.4 ALP Cell Staining
ALP staining procedure was carried out on treated samples (n = 2) to observe pre-osteoblast growth on cpTi surfaces. After 7 days of cell growth, media was aspirated, and each well was rinsed with PBS and then aspirated; 2 ml of neutral buffered formalin (10%) was added into each well to cover the monolayer of cells. After 60 s, the formalin was aspirated and cells were washed with washing buffer (0.05% Tween 20 to Dulbecco’s PBS, w/o Ca2+/Mg2+) and then aspirated again; 2 ml of 5-bromo-4-chloro-3-indolyl-phosphate/nitro blue tetrazolium (My BioSource) was added to immerse the monolayer of cells, and the plate was then incubated in the dark for 20 min; the plate was checked every 5 min for the purple color stain. Once the color was seen, the solution was aspirated, washed with washing buffer, and aspirated again. Finally, 2 ml of PBS was added into each well.
2.5 Surface Analysis
Surface analysis of cpTi surface was conducted prior to contamination, post-bacterial contamination, after detoxification and after ALP staining. One cpTi specimen from each treatment group including a non-treated control (n = 1) specimen underwent surface analysis with an optical microscope (Keyence VHX-2000) and scanning electron microscope (SEM, JEOL JSM 6010). Optical microscopy images were taken using high dynamic range (HDR) setting at both low (5–50X) and high magnifications (100X–1000X). SEM images were taken using a beam accelerating voltage ranging from 5 to 20 kV.
2.5.1 Electrochemical Testing
Electrochemical testing of cpTi specimens (n = 3) was adapted from ASTM F2129 standard protocol and performed to assess corrosion behavior of the material after treatment by detoxification chemicals. The electrochemical setup consisted of a potentiostat (Interface 1000, Gamry Instruments) connected to a standard three-electrode electrochemical cell. A saturated calomel electrode was used as the reference electrode, while a graphite rod was used as the counter electrode. The electrolyte was 1X PBS maintained at 37 °C throughout testing. First, the open-circuit potential (OCP) was monitored, and the 1-h value was recorded as the corrosion potential (E corr). Next, linear polarization resistance measurements were made within ± 10 mV versus E corr at a scan rate of 0.1667 mVs to yield polarization resistance (R p). Finally, anodic Tafel polarization from E corr to 250 mV versus E corr at a scan rate of 1 mV/s was used to extrapolate a corrosion current density (I corr) which in turn was used to calculate a corrosion rate (CR).
2.6 Statistical Analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by post hoc Tukey’s test with Origin Pro 8 Software. Statistical significance was observed when the p value was 0.05 or less (95% confidence level).
3 Results
3.1 Evaluation of Cell Compatibility
3.1.1 Cell Viability of Pre-osteoblasts After Detoxification Treatment
Host cell response to treatment is observed in Fig. 1. Cell viability of pre-osteoblasts on sample surfaces treated with mechanical abrasion or immersion was compared to the cell viability on non-treated samples (control). Overall, average cell viability of pre-osteoblasts on samples that experienced abrasion (rubbing) was lower than that on samples that did not experience abrasion (immersion), with the exception of citric acid. There was a significant difference in cell viability between rubbing and immersion methods when treated with doxycycline (p < 0.05) or citric acid (p < 0.05). In addition, cell viability of pre-osteoblasts on rubbing-treated samples was lower on average relative than that on the non-treated sample. On the other hand, average cell viability for saline- and doxycycline-immersed specimens exceeded that of the control, while those immersed in citric acid and chlorhexidine resulted in lower viability than the control. Although saline immersion resulted in much higher cell viability compared to saline rubbing, there was no statistical difference between these treatments (p > 0.05). Similarly, no significant difference between rubbing and immersion was observed for chlorhexidine-treated samples (p > 0.05), although rubbing with chlorhexidine induced lower cell viability than immersion with chlorhexidine.
3.1.2 Cell Differentiation of Pre-osteoblasts After Detoxification Treatment
Figure 2 demonstrates ALP activity of pre-osteoblasts differentiating into osteoblasts on cpTi specimens post-treatment. On average, higher ALP activity was seen on cpTi specimens that experienced mechanical abrasion (rubbing) than with samples that were immersed, excluding doxycycline. No statistical difference (p > 0.05) was found for ALP activity between rubbing and immersion methods, nor were there any statistical differences (p > 0.05) between chemicals used for the two treatment methods. Also, in general, ALP activity of samples immersed in all 4 chemicals was lower than the ALP activity on non-treated specimens (control). For samples rubbed with doxycycline and chlorhexidine, the ALP activity was lower than the control, whereas samples rubbed with citric acid and saline exhibited ALP activity greater than that on non-treated samples.
Osteoblast adhesion on control samples and treated specimens was visualized using optical microscopy. All test specimen surfaces were stained to detect ALP enzyme, and the differentiated cells were identified by the purple color of the stain. Figure 3a shows the well with only media and cells, whereas Fig. 3f shows the non-treated sample surface with a monolayer of cells present. Samples treated via rubbing method seemed to have a lot more differentiated cells attached to the surface as compared to immersion-treated samples. For citric acid, it was observed that both rubbing (Fig. 3e) and immersion (Fig. 3j) methods resulted in high coverage of differentiated cells. The same observation was obtained for specimens treated with saline (Fig. 3d, i). There was a considerable number of differentiated cells adherent to the surface of specimens treated by rubbing method using chlorhexidine (Fig. 3c).
3.2 Surface Analysis of cpTi Surface
3.2.1 Surface Evaluation of Contaminated Samples
CpTi samples were immersed in a polyculture bacterial broth for 5 days consisting of S. mutans, S. salivarius, S. sanguinis and A. actinomycetemcomitans grown in BHI medium. Before bacterial growth, BHI medium was observed to have a clear yellow coloration. After 5 days post-inoculation, a thin white bacterial film was visible on sample surfaces in addition to increased turbidity of the BHI immersion media.
Figure 4 shows representative SEM images of cpTi samples. In Fig. 4a, the control sample exhibited pristine surface condition with superficial scratches characteristic of polishing visible on the surface. Figure 4b shows the SEM image of structures resembling bacterial clusters adhering to the specimen surface, which was seen like a film covering the surface. Figure 4c shows the SEM image of the same sample with biofilm removed by sonication, and a feature resembling pitting damage was observed (yellow arrow). Correspondingly, Fig. 4d depicts representative optical microscope (OM) image of the same sample that underwent SEM imaging. Severe discoloration (yellow and blue) was observed around the feature resembling bacterial biofilm on the surface (Fig. 4e), which became more evident after the biofilm was removed (yellow arrows in Fig. 4f).
Chemicals included in this study had a pH range from very acidic to neutral. Table 1 lists the pH of each chemical measured before treatment. Citric acid was the most acidic followed by doxycycline, while chlorhexidine and saline were slightly basic.
3.2.2 Surface Evaluation of Contaminated Samples Treated by Rubbing Method
Figure 5 illustrates the surface of 4 samples that endured rubbing with a detoxification chemical. SEM and OM were performed for each sample to visualize and compare changes inflicted on the surface. Citric acid inflicted significant damage to the surface of cpTi compared to the other chemicals investigated. Severe discoloration and pitting attack (yellow arrows) were observed with this treatment as can be seen in the SEM and OM images illustrated in Fig. 5b and g. Samples rubbed with doxycycline showed mostly minor pitting (yellow arrow) local to particular areas on the surface and no discoloration (Fig. 5i). Residual doxycycline after treatment was seen on the surface as dark agglomerations in the SEM image (Fig. 5d). Rubbing with chlorhexidine generated discoloration on the specimens as shown in the SEM image (Fig. 5e) and in the OM image (Fig. 5j). Samples treated by saline with rubbing (Fig. 5c, h) showed no discoloration or pitting and were observed to exhibit similar surface features as control specimens (Fig. 5a, f).
3.2.3 Surface Evaluation of Contaminated Samples Treated by Immersion Method
Figure 6 shows images of 4 samples that were subjected to immersion with a chemical. SEM and OM were performed for each sample to visualize and compare changes to the surface. Immersion in citric acid resulted in discoloration (indicated by yellow arrows) within superficial scratches made during polishing present on the cpTi surface (Fig. 6g). As observed for samples subjected to rubbing, immersion in saline did not deteriorate the surface of cpTi specimens (Fig. 6c, h) as compared to the control specimen (Fig. 6a, f). Similar to the rubbing method, immersion in doxycycline resulted in a significant amount of residue left on the sample surface as can be observed by SEM (Fig. 6d) and OM (Fig. 6i) images. However, no morphological changes distinct from the untreated control were observed for this specimen. Lastly, immersion in chlorhexidine created minor discoloration (blue and purple as shown by yellow arrows) illustrated in Fig. 6j and e.
3.3 Corrosion Behavior
CpTi specimens treated with saline and citric acid had higher E corr values as compared to their rubbing-treated counterparts (Fig. 7a). In contrast, samples treated by immersion in chlorhexidine or doxycycline had lower E corr values relative to those treated by the rubbing method. Also, all immersion- and rubbing-treated specimens had E corr values greater than or similar to control, excluding those debrided with citric acid. However, no significant differences in E corr were found between the treated specimens and control (p > 0.05), excluding the saline-immersed and citric acid-rubbed groups. Based on linear polarization curves, no significant differences were found for R p values between control and treated specimens (Fig. 7b). However, R p values in general were higher for immersed specimens relative to rubbed specimens for each detoxification chemical, excluding saline. Furthermore, citric acid-immersed cpTi specimens had the highest R p among all groups, while citric acid-rubbed ones had the lowest. Finally, Tafel plots revealed all treatment methods had a higher corrosion rate (CR) when compared to non-treated control (Fig. 7c). Although no significant differences (p > 0.05) were found between immersion and rubbing in citric acid, the citric acid-rubbed group had a much higher corrosion rate (about 3X) than its corresponding immersion group. Chlorhexidine- and doxycycline-rubbed specimens also had higher CR compared to their respective immersion groups but to a lesser extent (about 2X), while the reverse trend was observed for those treated with saline.
4 Discussion
The main goal of this study was to develop an in vitro testing model to investigate the effects of bacterial adhesion and mechanical debridement on the morphology of cpTi surface in addition to the effect of mechanical/chemical debridement on corrosion behavior of cpTi. Subsequently, growth of osteoblast cells on titanium surface was assessed after the synergistic activity of bacterial adhesion and detoxification. The study was designed to simulate the human oral environment and detoxification treatment methods typically used by clinicians. There are numerous clinical studies that have examined the impact acidic chemicals have on titanium surfaces [22, 25, 28], but only a few have looked at the synergistic activity of bacterial adhesion and mechanical debridement on bone cell growth.
Based on preliminary studies (data not shown) of immersion of titanium samples in the selected polyculture bacterial strains used in this study, growth of a biofilm on sample surfaces was expected to occur along with a reduction in pH to about a value of 5. Subsequently, it was hypothesized that this bacterial adhesion on cpTi would create an acidic environment due to production of lactic acid and a crevice-like environment, which would result in surface corrosion. The incorporation of early- and late-colonizing bacteria (S. mutans, S. sanguinis, S. salivarius and A. actinomycetemcomitans) was chosen to ensure clinical relevance to the oral cavity of a peri-implantitis-infected patient. Streptococcus mutans is a gram-positive bacterium, which is one of the primary colonizers of biofilm on tooth surfaces and is the most abundant bacteria found in peri-implant tissue compared to other species comprising the periodontal microflora [30]. Streptococcus mutans along with the other bacteria are known to create acidic environments in the mouth as a result of metabolizing carbohydrates from food intake due to the release of organic acids [31]. Metabolites such as lactic acids are produced by bacteria, which can contribute to pH reduction. Even though titanium has a naturally forming oxide layer, if this layer is disturbed or covered by bacterial adhesion, continuous metal dissolution and corrosion may occur, which can be identified by surface features such as discoloration, pitting attack and delamination [24].
It has been shown that the presence of bacteria on implant surfaces can reduce the pH and may contribute to oxidation of the implant surface [32,33,34]. In previous studies conducted by Sridhar et al. [27] and Rodrigues et al. [29], two possible mechanisms of corrosion involving bacteria have been proposed: (1) after adhesion and during glycolysis, early-colonizing planktonic bacteria release lactic acid which decreases the pH of the oral environment. When titanium experiences low pH, the oxidation state of its surface changes leading to metal ion dissolution; (2) once a biofilm is formed on a surface, a crevice environment is created with restricted aeration and fluid exchange. This creates localized oxygen-depleted zones where pH is further decreased and subsequently resulting in accelerated metal dissolution.
Results from the first part of this study revealed that bacterial adhesion does indeed change the titanium surface morphology. Optical microscopy and scanning electron microscopy showed severe discoloration and pitting attack along the bacterial adhesion agglomerates found on the sample surfaces as indicated in Fig. 4b and e. Once the biofilm was removed by sonication, discoloration was more prominently observed throughout specimen surfaces (Fig. 4c, f). This can be corroborated with previously mentioned mechanisms of corrosion triggered by bacteria where a non-uniform biofilm layer possibly created oxygen-depleted zones resulting in crevice corrosion, which was observed by the yellow and blue discoloration of the surface found around bacterial agglomerates. This discoloration can be attributed to the acidic environment created by the lactic acid-producing bacteria (Streptococci sp.).
In the second portion of the study, detoxification of contaminated samples was carried out. Currently, there is an ongoing debate in the literature regarding the effectiveness of this procedure in the treatment of peri-implantitis-infected implants. This is because there is a lack of agreement to which chemical agent and technique are the most efficient for treatment. Furthermore, this is aggravated by the fact that current studies greatly differ in implant type, concentration of chemical and technique used to detoxify implants [21, 25, 28]. However, the majority of these clinical and in vitro studies point to some evidence of change in surface morphology after detoxification with acidic chemicals.
In the present study, discoloration and pitting were mainly observed when surfaces were treated with citric acid and doxycycline, which were the two most acidic chemicals (Figs. 5, 6:b, d, g and i). The morphological changes were more prominently observed for samples that were rubbed with these two chemicals than immersed in them. Chlorhexidine and saline, two of the neutral chemicals evaluated, inflicted little to no effect on specimen morphology, with both rubbing and immersion methods (Figs. 5, 6:c, e, h and j).
Corroborating the observations made on cpTi specimens post-detoxification, the results of electrochemical testing confirmed that citric acid rubbing increased corrosion susceptibility the greatest on average as compared to control cpTi (Fig. 7). That is, citric acid-rubbed specimens were the only group to have E corr values lower than control (Fig. 7a). In general, lower E corr values suggest lower thermodynamic stability of the passive oxide layer. In contrast, all other treated specimens had E corr values similar to or greater than that of control, suggesting that oxide film was stable after treatment. Among the treated specimens, immersed specimens were found to have greater R p values and therefore higher corrosion resistance as compared to their corresponding rubbed specimens, excluding saline-treated groups (Fig. 7b). This result can be explained by mechanical debridement physically contacting and damaging the oxide layer. Without rubbing, immersion of cpTi in corrosive media can actually be expected to increase corrosion resistance by promoting growth of a thicker oxide layer, which was observed for cpTi immersed in citric acid. Furthermore, the trends observed for R p values were found to be reversed for corrosion rate measurements (Fig. 7c). This result can be expected since R p and CR are inversely proportional to each other. Moreover, the fact that both parameters were measured independently further confirmed the accuracy of the trends observed. Despite citric acid-rubbed cpTi specimens having a higher CR in comparison with control and all other treated groups, the increase was less than tenfold and therefore may not pose a significant clinical risk. Among the remaining treated groups, the order in terms of increasing corrosion rate observed was doxycycline-immersed, citric acid-immersed, doxycycline-rubbed, saline-rubbed, chlorhexidine-immersed, chlorhexidine-rubbed and saline-immersed.
Similar to this study, the impact of mechanical motion on titanium surfaces has been evaluated to play a significant role in the degradation of titanium surfaces, especially when occurring synergistically with corrosion processes [27]. A previous study conducted by Wheelis et al. [28] evaluated the effects of 3 chemicals, peroxyacetic acid (35% in acetic acid, pH ~ 0), citric acid (40% in DI water pH ~ 1) and 0.12% sodium fluoride (in DI water pH ~ 8), on cpTi and Ti-6Al-4 V alloy samples. In that study, the same treatment methods were employed (rubbing and immersion). Optical microscopy and atomic force microscopy (AFM) revealed that acidic chemicals (pH < 3) inflicted mild corrosion on surfaces immersed in the chemical, whereas specimens that underwent mechanical debridement (rubbing) exhibited exaggerated corrosive effect. Similarly, a study done by Ericsson et al. using pure titanium implants showed acidic chemicals having a pH of less than 3 damaged the titanium oxide layer causing discoloration, corrosion, pitting and etching on the surface [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50]. From these studies, a correlation between pH and corrosion of titanium surface can be established [29, 51, 52]. Specifically, titanium has a higher vulnerability for changes in surface morphology when in contact with acidic substances because the acidic chemicals have a higher concentration of dissolved H+ ions, which can easily dissolute the titanium oxide layer [53].
Discoloration of titanium surface is the result of electrochemical attack; this happens when the surface becomes oxidized. Titanium, when oxidized, produces Ti3+ and Ti4+ ions. The Ti3+ oxidation state produces a characteristic purple color, while Ti4+ produces a distinctive yellow discoloration on the surface as demonstrated in previous studies [29, 54]. The presence of yellow and purple discoloration on treated titanium surfaces in this study corroborates the conclusion of corrosion induced by the performed procedures.
Growth and proliferation of osteoblasts on implant surfaces ensure the natural process of osseointegration, which determines the stability of a dental implant. The concern with the detoxification method is that once debridement is completed and the exposed area of the implant is cleaned, adherent bone-forming cells on the surface can be scrubbed off. Numerous studies have tried to evaluate re-growth of bone-forming cells on implant surfaces post-detoxification, but the results are mostly inconclusive due to differences in experimental design [26, 55,56,57,58]. Results from cell compatibility revealed chemicals used along with mechanical force had a considerable consequence on the proliferation and differentiation of pre-osteoblasts as shown in Figs. 1, 2 and 3. In general, cell viability was found to be on average lower on samples subjected to rubbing method in relation to samples subjected to immersion (Fig. 1). In addition, when comparing to non-treated specimens (control), treated titanium surfaces exhibited lower cell viability and differentiation (Figs. 1, 2). Although it was hypothesized that acidic chemicals would hinder growth of bone-forming cells, citric acid, being the most acidic chemical included in this study, surprisingly had the second highest cell viability and ALP activity for rubbing method, when compared to the other treatments investigated (Figs. 1, 2). The use of citric acid for detoxification has been well studied, and the positive effect of this chemical revealed that it may increase the chances of new attachment of cells on root surfaces [59,60,61,62]. A study done by Alhag et al. in 2008 [63] and Kolonidis et al. in 2003 [64] in dogs assessed three different treatment techniques: surface treatment with (1) supersaturated citric acid, (2) brushing with toothbrush and (3) swabbing with hydrogen peroxide for 1 min. All three techniques were followed by rinsing with saline. In both studies, it was concluded that new bone-to-implant contact was established and that this was associated with an increase in surface roughness induced by the treatment.
The study conducted by Wheelis et al. revealed that almost all treatment methods (rubbing and immersion) using the chemicals investigated in this study caused an increase in surface roughness of titanium. Specifically, citric acid was seen to have the most distinct increase in roughness from immersion (approximately 5 nm) to rubbing (approximately 25 nm) [28]. This is an interesting observation because in vivo studies have shown that increase in dental implant surface roughness offers a more suitable anchorage surface for bone cells to adhere to the surface [65, 66].
When analyzing both cell viability and ALP activity, a trend observed was that the combination of mechanical abrasion with acidic chemicals initially induced low proliferation of bone-forming cells but ultimately resulted in higher degree of cell differentiation. On the other hand, samples that did not experience mechanical abrasion initially had high proliferation but led to low differentiation rates. This trend was observed with the other chemicals as well, including chlorhexidine, doxycycline and saline. This observation disproved the initial hypothesis which stated that both proliferation and differentiation of pre-osteoblasts would be hindered.
From this observation, it was deduced that cells strongly adherent to titanium surface but in low populations were able to differentiate, while non- or weakly adherent cells were not able to differentiate. Furthermore, cellular differentiation to a greater extent was observed on samples that were subjected to mechanical abrasion as compared to those that were only exposed to chemical immersion, which ultimately led to better growth of bone-forming cells. This improvement in cellular behavior was hypothesized to occur due to increased surface roughness imparted by mechanical abrasion. So, it can be concluded from this study and supporting literature that an increase in surface roughness can be attributed to the higher cell viability observed with the citric acid-rubbing treatment.
In summary, this study demonstrated that bacteria can create suitable conditions for oxide layer damage. In addition to this effect, detoxification of contaminated titanium surfaces using acidic chemicals and mechanical forces also induced changes in surface morphology and oxidation state of titanium resulting in discoloration and pitting attack as shown in Figs. 5 and 6. The combination of these two actions led to a significant change in morphology of the surface as hypothesized. This change in morphology did impact cell behavior on treated surfaces but not as hypothesized. Although cell proliferation was low on samples treated with a combination of mechanical abrasion and acidic chemicals, there was a higher differentiation on these samples compared to samples that did not endure mechanical abrasion. This leads to the presumption that pre-osteoblasts could differentiate into osteoblasts on surfaces that were roughened with the detoxification method. It can be inferred from this study that morphology of titanium surface plays a key role in cellular attachment and differentiation.
Some of the limitations of this study included sterilization of specimen surface post-contamination but prior to detoxification which does not mimic detoxification in the clinical setting. However, bacterial biofilm and products were still present post-sterilization and removed during detoxification treatment. In addition, verification of the homogeneity and quality of polishing the titanium specimen surface were lacking in this study. Future studies will address this concern by measuring surface roughness both before and after bacterial adhesion and detoxification to look at how surface roughness changes at each stage of methodology employed in this study. Additionally, it is necessary to further investigate why bone-forming cells are more compatible on titanium surfaces subjected to mechanical abrasion.
5 Conclusion
In conclusion, it was observed that bacterial adhesion on titanium surface inflicted severe discoloration and pitting. In addition, manual rubbing combined with acidic chemicals exacerbated this effect by producing more pronounced discoloration, which indicates drastic changes in the oxidation state of titanium. The treatment that was found to cause the greatest damage to cpTi surface in this study was rubbing with citric acid, which corroborated with the electrochemical testing results, while cpTi treated by immersion or rubbing with chlorhexidine, doxycycline or saline demonstrated corrosion susceptibility intermediate between that of non-treated control and citric acid-rubbed cpTi. The combination of manual application of force (rubbing method) and chemicals resulted in low proliferation rates which indicated cytotoxicity of treated titanium surfaces to pre-osteoblasts. Immersion in saline and doxycycline produced the highest percentage of cell viability, while immersion with citric acid produced the lowest cell viability. In general, ALP activity of pre-osteoblasts was higher on samples treated by rubbing method than on samples treated by immersion method. Although pre-osteoblast proliferation was lower for samples subjected to rubbing method compared to immersion, ALP activity was higher for rubbing-treated samples than for immersion-treated ones overall. It can be concluded that the combination of mechanical debridement with chemicals did hinder cell proliferation but ultimately led to a higher differentiation of bone-forming cells. Overall, careful consideration must be given when applying detoxification chemical and methods that can affect the surface morphology of titanium surfaces and subsequent cellular behavior, which in turn may influence outcome of re-osseointegration.
References
Dental Implants Facts and Figures. American Academy of Implant Dentistry. http://www.aaid.com/about/Press_Room/Dental_Implants_FAQ.html. Published 2017
Mellado-Valero A, Buitrago-Vera P, Solá-Ruiz MF, Ferrer-García JC (2013) Decontamination of dental implant surface in peri-implantitis treatment: a literature review. Med Oral Patol Oral Cir Bucal. doi:10.4317/medoral.19420
Pier-Francesco A, Adams RJ, Waters MGJ, Williams DW (2006) Titanium surface modification and its effect on the adherence of Porphyromonas gingivalis: an in vitro study. Clin Oral Implants Res 17(6):633–637. doi:10.1111/j.1600-0501.2006.01274.x
Elias CN (2011) Factors affecting the success of dental implants. INTECH. doi:10.5772/18746
Ozkurt Z (2011) Zirconia dental implants: a literature review. Oral Implantol 3(37):367–376. http://www.joionline.org/doi/abs/10.1563/AAID-JOI-D-09-00079?code=aaid-premdev
Luo L, Jiang ZY, Bin Wei D, He XF (2014) Surface modification of titanium and its alloys for biomedical application. Adv Mater Res 887–888:1115–1120. doi:10.4028/www.scientific.net/AMR.887-888.1115
Esposito M, Thomsen P, Ericson LE, Sennerby L, Lekholm U (2000) Histopathologic observations on late oral implant failures. Clin Implant Dent Relat Res 2(1):18–32. doi:10.1111/j.1708-8208.2000.tb00103.x
Santos MCLG (2002) MIGCSRP. Early dental implant failure: a review of the literature. Braz J Oral Sci 1(3):103–111
Sakka S, Baroudi K, Nassani MZ (2012) Factors associated with early and late failure of dental implants. J Investig Clin Dent 3(4):258–261. doi:10.1111/j.2041-1626.2012.00162.x
Rodrigues DC, Sridhar S, Gindri IM et al (2016) Spectroscopic and microscopic investigation of the effects of bacteria on dental implant surfaces. RSC Adv 6(54):48283–48293. doi:10.1039/C6RA07760A
Jovanovic SA (1993) The management of peri-implant breakdown around functioning osseointegrated dental implants. J Periodontol 64((11 Suppl)):1176–1183. doi:10.1902/jop.1993.64.11s.1176
Pontoriero R, Carnevale DG (2001) Surgical crown lengthening: a 12-month clinical wound healing study. Periodontology 72(7):841–848. http://www.joponline.org/doi/abs/10.1902/jop.2001.72.7.841
Mombelli A (1993) Microbiology of the dental implant. Adv Dent Res 7(2):202–206. doi:10.1177/08959374930070021201
Mombelli A, Lang NP (1998) The diagnosis and treatment of peri-implantitis evidence for a microbial cause of peri-implant infections. Periodontology 17:63–76. doi:10.1016/j.jaci.2010.06.022
Busscher HJ, Van Der Mei RBHC (1995) Initial microbial adhesion is a determinant for the strength of biofilm adhesion. FEMS Microbiol Lett 128(3):229–234. doi:10.1016/0378-1097(95)00103-C
Manor Y, Oubaid S, Mardinger O, Chaushu G, Nissan J (2009) Characteristics of early versus late implant failure: a retrospective study. J Oral Maxillofac Surg 67(12):2649–2652. doi:10.1016/j.joms.2009.07.050
Scarano A, Piattelli M, Caputi S, Favero GA, Piattelli A (2004) Bacterial adhesion on commercially pure titanium and zirconium oxide disks: an in vivo human study. J Periodontol 75(2):292–296. doi:10.1902/jop.2004.75.2.292
Elter C, Heuer W, Demling A et al (2008) Supra- and subgingival biofilm formation on implant abutments with different surface characteristics. Int J Oral Maxillofac Implants 23(2):327–334
Burgers R, Gerlach T, Hahnel S, Schwarz F, Handel G, Gosau M (2010) In vivo and in vitro biofilm formation on two different titanium implant surfaces. Clin Oral Implant Res 21(2):156–164. doi:10.1111/j.1600-0501.2009.01815.x
Wadhwani C, Rapoport D, La Rosa S, Hess T, Kretschmar S (2012) Radiographic detection and characteristic patterns of residual excess cement associated with cement-retained implant restorations: a clinical report. J Prosthet Dent 107(3):151–157. doi:10.1016/S0022-3913(12)60046-8
Valderrama P, Wilson TG (2013) Detoxification of implant surfaces affected by peri-implant disease: an overview of surgical methods. Int J Dent. doi:10.1155/2013/740680
Mouhyi J, Dohan Ehrenfest DM, Albrektsson T (2012) The peri-implantitis: implant surfaces, microstructure, and physicochemical aspects. Clin Implant Dent Relat Res 14(2):170–183. doi:10.1111/j.1708-8208.2009.00244.x
Subramani K, Wismeijer D (2012) Decontamination of titanium implant surface and re-osseointegration to treat peri-implantitis: a literature review. Int J Oral Maxillofac Implants 27(5):1043–1054
Finnegan M, Linley E, Denyer SP, McDonnell G, Simons C, Maillard JY (2010) Mode of action of hydrogen peroxide and other oxidizing agents: differences between liquid and gas forms. J Antimicrob Chemother 65(10):2108–2115. doi:10.1093/jac/dkq308
Gosau M, Hahnel S, Schwarz F, Gerlach T, Reichert TE, Bürgers R (2010) Effect of six different peri-implantitis disinfection methods on in vivo human oral biofilm. Clin Oral Implants Res 21(8):866–872. doi:10.1111/j.1600-0501.2009.01908.x
de Waal YCM, Raghoebar GM, Meijer HJA, Winkel EG, van Winkelhoff AJ (2015) Implant decontamination with 2% chlorhexidine during surgical peri-implantitis treatment: a randomized, double-blind, controlled trial. Clin Oral Implants Res 26(9):1015–1023. doi:10.1111/clr.12419
Sridhar S, Wilson TG, Palmer KL et al (2015) In vitro investigation of the effect of oral bacteria in the surface oxidation of dental implants. Clin Implant Dent Relat Res 17:e562–e575. doi:10.1111/cid.12285
Wheelis SE, Gindri IM, Valderrama P, Wilson TG, Huang J, Rodrigues DC (2016) Effects of decontamination solutions on the surface of titanium: investigation of surface morphology, composition, and roughness. Clin Oral Implants Res 27(3):329–340. doi:10.1111/clr.12545
Rodrigues DC, Valderrama P, Wilson TG et al (2013) Titanium corrosion mechanisms in the oral environment: a retrieval study. Materials (Basel) 6(11):5258–5274. doi:10.3390/ma6115258
Cosyn J, van Aelst L, Collaert B, Persson GR, de Bruyn H (2011) The peri-implant sulcus compared with internal implant and suprastructure components: a microbiological analysis. Clin Implant Dent Relat Res 13(4):286–295. doi:10.1111/j.1708-8208.2009.00220.x
Chin MYH, Sandham A, de Vries J, van der Mei HC, Busscher HJ (2007) Biofilm formation on surface characterized micro-implants for skeletal anchorage in orthodontics. Biomaterials 28(11):2032–2040. doi:10.1016/j.biomaterials.2006.12.014
Mathew MT, Abbey S, Hallab NJ, Hall DJ, Sukotjo C, Wimmer MA (2012) Influence of pH on the tribocorrosion behavior of CpTi in the oral environment: synergistic interactions of wear and corrosion. J Biomed Mater Res Part B Appl Biomater 100 B(6):1662–1671. doi:10.1002/jbm.b.32735
Barão Va, Mathew MT, Assunção WG, Yuan JC, Wimmer Ma, Sukotjo C (2011) The role of lipopolysaccharide on the electrochemical behavior of titanium. J Dent Res 90(5):613–618. doi:10.1177/0022034510396880
Souza JCM, Henriques M, Oliveira R, Teughels W, Celis J-P, Rocha La (2010) Do oral biofilms influence the wear and corrosion behavior of titanium? Biofouling 26(4):471–478. doi:10.1080/08927011003767985
Thierry B, Tabrizian M, Savadogo O, Yahia L (2000) Effects of sterilization processes on NiTi alloy: surface characterization. J Biomed Mater Res 49(1):88–98. doi:10.1002/(SICI)1097-4636(200001)49:1<88:AID-JBM11>3.0.CO;2-I
Schwarz F, Sculean A, Romanos G et al (2005) Influence of different treatment approaches on the removal of early plaque biofilms and the viability of SAOS2 osteoblasts grown on titanium implants. Clin Oral Investig 9(2):111–117. doi:10.1007/s00784-005-0305-8
Chaturvedi TP (2009) An overview of the corrosion aspect of dental implants (titanium and its alloys). Indian J Dent Res 20(1):91–98. doi:10.4103/0970-9290.49068
Mabilleau G, Bourdon S, Joly-Guillou ML, Filmon R, Baslé MF, Chappard D (2006) Influence of fluoride, hydrogen peroxide and lactic acid on the corrosion resistance of commercially pure titanium. Acta Biomater 2(1):121–129. doi:10.1016/j.actbio.2005.09.004
Anil S, Anand PS, Alghamdi H, Jansen Ja (2011) Dental implant surface enhancement and osseointegration. Implant Dent A Rapidly Evol Pract. doi:10.5772/16475
Albrektsson T, Hansson HA, Ivarsson B (1985) Interface analysis of titanium and zirconium bone implants. Biomaterials 6(2):97–101. doi:10.1016/0142-9612(85)90070-5
Pontoriero R, Tonelli MP, Carnevale G, Mombelli A, Nyman SR, Lang NP (1994) Experimentally induced peri-implant mucositis. A clinical study in humans. Clin Oral Implants Res 5(4):254–259. doi:10.1034/j.1600-0501.1994.050409.x
Takasaki AA, Aoki A, Mizutani K, Kikuchi S, Oda S, Ishikawa I (2007) Er:YAG laser therapy for peri-implant infection: a histological study. Lasers Med Sci 22(3):143–157. doi:10.1007/s10103-006-0430-x
Heydenrijk K, Meijer HJA, van der Reijden WA, Raghoebar GM, Vissink A, Stegenga B (2002) Microbiota around root-form endosseous implants: a review of the literature. Int J Oral Maxillofac Implants 17(6):829–838
Leonhardt A, Adolfsson B, Lekholm U, Wikstrom M, Dahlen G (1993) A longitudinal microbiological study on osseointegrated titanium implants in partially edentulous patients. Clin Oral Implant Res 4(3):113–120. doi:10.1034/j.1600-0501.1993.040301.x
Shibli JA, Martins MC, Lotufo RF, Marcantonio E Jr (2003) Microbiologic and radiographic analysis of ligature-induced peri-implantitis with different dental implant surfaces. Int J Oral Maxillofac Implant 18(3):383–390
Hensten-Pettersen A, Helgeland K (1981) Sensitivity of different human cell line in the biologic evaluation of dental resin-based restorative materials. Scand J Dent Res 89(1):102–107
Costa CADS, Hebling J, Hanks CT (2000) Current status of pulp capping with dentin adhesive systems: a review. Dent Mater 16(3):188–197. doi:10.1016/S0109-5641(00)00008-7
Ribeiro DA, Marques MESD (2006) Genotoxicity and cytotoxicity of glass ionomer cements on Chinese hamster ovary (CHO) cells. J Mater Sci Mater Med 17:495–500
Grivet M, Morrier JJ, Souchier C, Barsotti O (1999) Automatic enumeration of adherent streptococci or actinomyces on dental alloy by fluorescence image analysis. J Microbiol Methods 38(1–2):33–42. doi:10.1016/S0167-7012(99)00074-3
Fürst MM, Salvi GE, Lang NP, Persson GR (2007) Bacterial colonization immediately after installation on oral titanium implants. Clin Oral Implants Res 18(4):501–508. doi:10.1111/j.1600-0501.2007.01381.x
Rodrigues DC, Urban RM, Jacobs JJ, Gilbert JL (2009) In vivo severe corrosion and hydrogen embrittlement of retrieved modular body titanium alloy hip-implants. J Biomed Mater Res Part B Appl Biomater 88(1):206–219. doi:10.1002/jbm.b.31171
Golvano I, Garcia I, Conde A, Tato W, Aginagalde A (2015) Influence of fluoride content and pH on corrosion and tribocorrosion behaviour of Ti13Nb13Zr alloy in oral environment. J Mech Behav Biomed Mater 49:186–196. doi:10.1016/j.jmbbm.2015.05.008
Sato N (1989) Toward a more fundamental understanding of corrosion processes. Corrosion 45(5):354–368. doi:10.5006/1.3582030
Gilbert JL, Buckley CA, Jacobs JJ (1993) In vivo corrosion of modular hip prosthesis components in mixed and similar metal combinations. The effect of crevice, stress, motion, and alloy coupling. J Biomed Mater Res 27(12):1533–1544. doi:10.1002/jbm.820271210
Triplett RG, Frohberg U, Sykaras N, Woody RD (2003) Implant materials, design, and surface topographies: their influence on osseointegration of dental implants. J Long Term Eff Med Implants 13(6):485–501. doi:10.1615/JLongTermEffMedImplants.v13.i6.50
Albrektsson T, Zarb G, Worthington P, Eriksson AR (1986) The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants 1(1):11–25
Lee T-H, Hu C-C, Lee S-S, Chou M-Y, Chang Y-C (2010) Cytotoxicity of chlorhexidine on human osteoblastic cells is related to intracellular glutathione levels. Int Endod J 43(5):430–435. doi:10.1111/j.1365-2591.2010.01700.x
Schwarz F, John G, Mainusch S, Sahm N, Becker J (2012) Combined surgical therapy of peri-implantitis evaluating two methods of surface debridement and decontamination. A two-year clinical follow up report. J Clin Periodontol 39(8):789–797. doi:10.1111/j.1600-051X.2012.01867.x
Nalbandian JCN (1982) Direct histological comparison of periodontal wound healing in the beagle dog with and without citric acid conditioning. Periodontology 13:538–549
Crigger M, Bogle GNR (1978) The effect of topical citric acid application on the healing of experimental furcation defects in dogs. Periodontology 13:538–549
Klinge B, Nilveus R, Egelberg J (1985) Effect of periodic tooth displacement on healing of experimental furcation defects in dogs. J Clin Periodontol 12(3):239–246
Wikesjo UME, Selvig KA, Zimmerman G, Nilveus R (1991) Periodontal repair in dogs—healing in experimentally created chronic periodontal defects. J Periodontol 62(4):258–263. doi:10.1902/jop.1991.62.4.258
Alhag M, Renvert S, Polyzois I, Claffey N (2008) Re-osseointegration on rough implant surfaces previously coated with bacterial biofilm: an experimental study in the dog. Clin Oral Implants Res 19(2):182–187. doi:10.1111/j.1600-0501.2007.01429.x
Kolonidis SG, Renvert S, Hämmerle CHF, Lang NP, Harris D, Claffey N (2003) Osseointegration on implant surfaces previously contaminated with plaque. An experimental study in the dog. Clin Oral Implants Res 14:373–380. doi:10.1034/j.1600-0501.2003.01871.x
Noguti J, De Oliveira F, Peres RC, Renno ACM, Ribeiro DA (2012) The role of fluoride on the process of titanium corrosion in oral cavity. Biometals 25(5):859–862. doi:10.1007/s10534-012-9570-6
Garg H, Bedi G, Garg A (2012) Implant surface modifications: a review. J Clin Diagn Res 6(2):319–324
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Ramesh, D., Sridhar, S., Siddiqui, D.A. et al. Detoxification of Titanium Implant Surfaces: Evaluation of Surface Morphology and Bone-Forming Cell Compatibility. J Bio Tribo Corros 3, 50 (2017). https://doi.org/10.1007/s40735-017-0111-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-017-0111-2