Abstract
Resurgence is observed when a previously extinguished behavior reemerges while a more recently reinforced behavior is extinguished. Resurgence is further defined as responding that is greater than an inactive control response that has never produced reinforcement. Recent studies of resurgence using neurotypical adults as participants in human-laboratory investigations have produced discrepant patterns of responding compared to nonhuman animal laboratory studies when comparing control response performance. Namely, human-laboratory investigations have produced no differences between target and control responding, and persistence of all response types across the resurgence-test phase. In the present study, we conducted two human-laboratory experiments to determine if these effects were a product of the history of reinforcement associated with the target response as well as the types of technology used in human-laboratory studies. For all participants, we found no differences in levels of resurgence and occurrence for the target and control response, respectively. Moreover, we observed persistence of all response types across the resurgence-test phase in a manner consistent with prior research. This finding was apparent even when the length of baseline (i.e., reinforcement for the target) was increased, when the length of extinction was increased, and when low-technology stimuli were used. We highlight the implications of this outcome in the context of recent human-laboratory studies that have used arbitrary responses to study resurgence, and discuss the possible role of verbal mediation in these investigations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
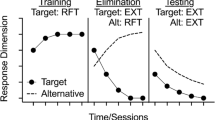
Resurgence is defined as the recurrence of a target behavior that has previously been eliminated while a more recently reinforced alternative behavior is placed on extinction (Doughty & Oken, 2008; Lattal & St. Peter Pipkin, 2009; Podlesnik & Shahan, 2010; Pritchard et al., 2014). Resurgence is typically evaluated in a three-phase procedure (Leitenberg et al., 1970). In the first phase, a target response is reinforced (e.g., pressing a lever). In the second phase, the target response is placed on extinction and an alternative response (e.g., pulling a chain) is reinforced (i.e., extinction plus differential reinforcement of alternative behavior [DRA]). In the final phase (i.e., the resurgence-test phase), reinforcement for the alternative response is discontinued while the target response continues to undergo extinction. Recurrence of the target behavior during this final phase is termed resurgence (Epstein, 1983). Stimuli is typically associated with a response that is never reinforced (e.g., an inactive lever) are presented throughout all phases such that resurgence of the target response during the test phase can be demarcated from extinction-induced response variability (e.g., Craig & Shahan, 2016; Podlesnik & Kelley, 2015; Sweeney & Shahan, 2015). Thus, resurgence is further defined by an increase in responding above an inactive control response with no history of reinforcement (Epstein, 1983).
Along with nonhuman-animal laboratory demonstrations of resurgence (e.g., Podlesnik & Shahan, 2009), this phenomenon has been observed in clinical situations with vulnerable populations such as children with intellectual disabilities who engage in severe destructive behavior (e.g., self-injury; Nevin et al., 2016; Sullivan et al., 2020; Volkert et al., 2009). The collective findings of studies from the nonhuman animal laboratory as well as applied investigations with vulnerable populations may have important implications for understanding relapse of human behavior (Podlesnik & Kelley, 2015; Pritchard et al., 2014). For example, these investigations may identify key variables that can be leveraged in treatment to mitigate resurgence in clinical populations.
Given the significance that studies of resurgence may have for understanding relapse under naturalistic conditions, there has been a recent increase in translational research on relapse broadly (Podlesnik & Kelley, 2015), and resurgence in particular (Lattal et al., 2017). In particular, there has been a growing trend for translational research studies of resurgence to use human-laboratory preparations that rely on methodologies that parallel nonhuman experiments (e.g., arbitrarily selected responses that are easy to measure, computerized tasks, nonsocially significant behavior; Cox et al., 2019; Bolívar et al., 2017; Kuroda et al., 2016; Marsteller & St. Peter, 2012; Sweeney & Shahan, 2016).
Although human-laboratory preparations have a long-standing history of translational research in the experimental analysis of human behavior (Saini & Roane, 2018), studies of resurgence that have recruited neurotypical adults to engage in computer tasks have led to results inconsistent with studies of resurgence using nonhuman animals as subjects. In particular, human-laboratory experimenters that included inactive controls have observed resurgence of target responding and occurrence of inactive control responding at usually equal rates during tests of resurgence (e.g., Bolívar et al., 2017; Cox et al., 2019; Sweeney & Shahan, 2016). In these studies, response persistence of target and control responding has been observed across the entire resurgence test phase. This finding represents a departure from nonhuman animal studies that have included inactive control responses, which typically produce differences in target and control responses, as well as extinction of the target response across sessions (e.g., Podlesnik et al., 2006).
Sweeney and Shahan (2016) conducted a brief, trial-based human-laboratory procedure with undergraduate students responding on a computerized task (i.e., clicking on different shapes on a screen), but they did not observe resurgence (i.e., differences in target and control responding during the test phase). Instead, they observed persistence of all response forms (target, alternative, control) across the entire test phase. However, these results may have been a product of the relatively brief baseline procedure used, which may have not been sufficiently long to establish a history of reinforcement for the target response. Some parametric analyses have suggested that the length of baseline reinforcement for the target response influences levels of resurgence, where longer histories of reinforcement for target behaviors produce greater levels of resurgence (Doughty et al., 2010; Fisher et al., 2019; Winterbauer et al., 2013).
Similar response-persistence patterns were obtained by Bolívar et al. (2017) in their human-laboratory investigation of resurgence. These effects were observed even when the number of response options available in each phase and how stimuli associated with each response, varied across conditions. Cox et al. (2019) found that having additional inactive control responses did not reduce overall responding to control options, and aggregated responding to control options was similar to target responding during tests of resurgence.
Each of the prior three human-laboratory studies of resurgence that have included a control response (i.e., Bolívar et al., 2017; Cox et al., 2019; Sweeney & Shahan, 2016), all conducted studies using computers wherein participants would click a computer mouse or press a touchscreen to earn points, which were to serve as positive reinforcement. It is interesting that there is some evidence to suggest that differences in target and control responding can be achieved when the experimental task does not involve high-technology stimuli such as interacting with a computer to complete a task (e.g., Bruzek et al., 2009; Ho et al., 2018). For example, Ho et al. (2018) obtained lower levels of a control response (emotional responding, requesting) relative to the target response in their resurgence study that required children with and without autism spectrum disorder to complete a task that involved manipulating a Montessori object permanence box. This finding suggests that indifference across the two response types (i.e., target and control), and persistence observed during resurgence-test phases, may be a product of the type of stimuli used during human-laboratory studies (i.e., high tech versus low tech).
The purpose of this study was to further evaluate response-persistence effects and indifferences in target and control responding during human-laboratory investigations of resurgence. We conducted two experiments in order to determine if the patterns of responding observed in human-laboratory preparations of resurgence were a product of (1) the history of reinforcement associated with the target response and (2) the type of technology used in human-laboratory studies of resurgence.
Experiment 1
In Experiment 1 we conducted a parametric analysis to determine whether a longer history of reinforcement (i.e., longer baseline phase) resulted in resurgence of target responding greater than the occurrence of inactive control responding. In addition, we conducted a parametric analysis of the resurgence test phase to determine if responding (target, alternative, control) would eventually extinguish when the length of the test phase varied.
Method
Participants and Apparatus
Eight neurotypical adults between 21 to 35 years old participated (two were male and six were female). Experiments were completed in one 60- to 180-min visit to the experimental environment, which consisted of a quiet room that was free of distractions. The environment consisted of a table, chair, and the response apparatus.
We developed a behavioral task that was presented on an Apple iPad®. Display screens were 32.7 cm in diagonal diameter. The task used capacitive touchscreen technology to record responses by monitoring the electrical field of the screen when response keys were selected. The interface was locked in an upright position and rotating the iPad did not affect the stimulus display. In the center of the screen was a scoreboard that was 5.0 cm by 7.6 cm large and was surrounded (in a triangular formation) by three different colored circle keys of 12.7 cm diameter. Earning points on the scoreboard was functionally intended to serve as reinforcement for key presses. Swift and Xcode executed and recorded experimental events, which were stored on the iPad® for later retrieval by the experimenter. Arranged experimental events were not visible to subjects during interaction with the behavioral task.
Procedure
We used a three-phase resurgence paradigm to evaluate recurrent behavior. Target, alternative, and control responses were randomly assigned to one of the colored circle keys and colors were counterbalanced across participants. In Phase 1, reinforcement was delivered for the target response and the alternative and control responses produced no consequences. In Phase 2, the target response was placed on extinction and the alternative response was reinforced. The control response continued to go unreinforced. In Phase 3, both the target and alternative responses were placed on extinction and the control response continued to produce no consequences.
Participants were randomly assigned to either the short-baseline, long-baseline, and extended-exposure groups. Participants in the short-baseline group were exposed to 10 sessions of Phase 1 (baseline), whereas participants in the long-baseline group were exposed to 20 sessions of Phase 1. Participants in both groups were exposed to 10 sessions of Phase 2 (DRA plus extinction) and 10 sessions of Phase 3 (extinction). That is, the only difference between short- and long-baseline groups was the length of Phase 1 (i.e., reinforcement for the target). Participants in the extended-exposure group were exposed to 30 sessions of each phase in order to determine if responding would extinguish if the duration of exposure to extinction was increased for all responses.
We provided vocal instructions regarding the behavioral task and how to interact with the iPad:
Welcome to our study of reward learning. Your task today will be completed on an iPad. You will use your finger to respond by pressing on the iPad. The iPad will present you with a game with three circles: red, blue, and green. Pressing the different colored circles will sometimes earn you points. How you respond is completely up to you and you may stop responding at any time. No credit is assigned to how well you play, however, the participant who scores the most total points will receive a $25 gift card so try to earn as many points as you can. When the screen displays “game over,” you will return the iPad to me. Please hand the iPad back only when the screen says “game over,” in the event of an emergency, or if you wish to withdraw from the study. Watches and cellular phones are not allowed in the experimental room. They must be safely stored away during session time. Do you have any questions?
Prior to each session, the iPad displayed the instructions “Score as many points as you can” and button text, “Press here to begin.” Pressing the button text began the session proper. All sessions were 2 min in duration separated by a 15-s intersession interval. The scoreboard read “0” at the beginning of each session and subsequent point accumulation was always visible to participants.
Phase 1 (Baseline)
All reinforcers were arranged according to variable-interval (VI) schedules, sampled without replacement from 13 intervals (Flesher & Hoffman, 1962). Engaging in the target response was reinforced on a VI 10-s schedule, wherein the scoreboard would increase by 15 points. Engaging in the alternative or inactive control response produced no consequences.
After each response (independent of whether the response was target, alternative, or inactive), a 1-s change-over delay (COD) was imposed in which all keys disappeared and only the scoreboard was visible. After the COD, the colored circles reappeared in a different position within the triangular formation. The COD was in place to orient subjects back to the center of the screen and mitigate bias towards a particular position.
Phase 2 (DRA)
Phase 2 was identical to Phase 1 except that the target response was placed on extinction and engaging in the alternative response was reinforced on a VI 10-s schedule. The inactive control response continued to produce no consequences.
Phase 3 (Extinction)
Phase 3 was identical to Phase 1 except that all responses were placed on extinction. The scoreboard read “0” throughout and never blinked.
Results
Figure 1 displays results of participant performance in the short-baseline (top panel; P1, P2, P3), long-baseline (middle panel; P4, P5, P6), and extended-exposure (bottom panel; P7, P8) groups. Participants in every group acquired the target response within the first session of Phase 1 (i.e., higher levels of target responding relative to alternative and inactive control responses) and differentiation among response types continued throughout the phase.
Individual Response Patterns in Experiment 1 in the Short-Baseline (top panel), Long-Baseline (middle panel), and Extended-Exposure (bottom panel) Groups
During Phase 2, we observed little response persistence of the target behavior and fairly rapid acquisition of the alternative response for all participants in all groups. All participants acquired the alternative response within the first experimental session, and responding towards the target stimulus reduced to near-zero levels. The alternative response persisted across the duration of Phase 2 for all participants.
When the extinction phase was introduced (Phase 3), we observed undifferentiated response allocation across all three response options (i.e., target, alternative, inactive control) for all participants in all groups. If resurgence was distinguishable from extinction-induced variability, we would have expected a higher frequency for the target during the first five sessions of Phase 3 and no change in frequency for the control response (i.e., no trend, or level of data points remains near zero; Epstein, 1983). However, for all groups, an increase in frequency was observed for both target and inactive control responses, suggesting indifference in the final phase. Increasing the length of Phase 1, as was done for participants in the long-baseline group (Fig. 1, middle panel), did not result in differentiation between target and inactive control responding. Likewise, extending the length of all phases, as was done in the extended-exposure group (Fig. 1, bottom panel), did not result in any response extinguishing. Taken together, we observed no differences in responding between all response forms in the resurgence-test phase for all participants, and response persistence of all response forms occurred throughout this phase in all groups.
Experiment 2
In Experiment 2, we replicated the resurgence procedure used in Experiment 1 with low-technology stimuli (i.e., those that did not rely on electronics). Bolívar et al. (2017) suggested that persistence during the resurgence test phase, and indiscriminate responding across target and control responses, might be due to participants’ extraexperimental histories of reinforcement when interacting with technology. In Experiment 1, we relied on technologically sophisticated tasks that required participants to interact with computers. Given the pervasive nature of interacting with technology outside of experimental situations, it is possible that the reinforcement history of neurotypical adults with computers could come to bare on a human-laboratory experiment that uses technology to study extinction and resurgence. Thus, the purpose of Experiment 2 was to determine if obtained results in Experiment 1 were affected by the computerized task.
Method
Participants and Apparatus
Six neurotypical adults between 22 and 25 years old participated (one was male and five were female). Data collection was completed in one 60–240-min visit to the experimental environment, which consisted of a quiet room with a table, four chairs, and the response apparatus. One or two experimenters and two data collectors were in the room. One experimenter was seated directly in front of the participant, implemented the COD, and delivered points for reinforcement. The second experimenter was situated behind the participant, out of their view, and signaled to the first experimenter when reinforcement became available. When the second experimenter was not present, the availability of reinforcement was singled by a nearby computer, which was outside of the participant’s view and programmed to provide a signal to the first experimenter according to VI schedules. The data collectors were positioned to the right side of the participants and remained as unobtrusive as possible.
The task in Experiment 2 was a low-technology replication of the task in Experiment 1. Three differently colored (e.g., purple, green, and orange) BIGmack manual buttons were presented on a table in a triangular formation, approximately 30 cm apart from one another. Above the buttons, closest to the first experimenter, a 10 x 10 cm laminated piece of paper was used to signal reinforcement. That is, earning points resulted in the first experimenter holding up a laminated piece of paper displaying the number of points and then placing it down on the table.
Procedure
We used a similar three-phase resurgence paradigm and general procedures described in Experiment 1 to evaluate resurgence in Experiment 2. Two participants in Experiment 2 were exposed to the contingencies for the short-baseline group described in Experiment 1, two participants were exposed to the contingencies of the long-baseline group, and two participants were exposed to the contingencies of the extended-exposure group. Instructions similar to those provided in Experiment 1 were also provided to participants in Experiment 2, with the exception of experiment-specific information (i.e., the use of BIGmack buttons instead of an iPad). All sessions were 2 min in duration separated by a 15–30-s intersession interval to allow data collectors to arrange the subsequent experimental session. The scoreboard read “0” at the beginning of each session and subsequent point accumulation was always visible to subjects.
Data were collected manually by experimenters on iPads using the Countee application or on laptop computers using the BDataPro application (Bullock et al., 2017). Interobserver agreement was collected by having two observers independently and simultaneously collect data. At the end of each session, we divided the smaller frequency by the larger frequency for each response type, as well as deliveries of reinforcement. Each quotient was then converted to a percentage. Interobserver agreement was collected during 100% of sessions for P9, P10, P11, and P12, and 33.3% of sessions for P13 and P14. Mean agreement across participants for all responses and reinforcer deliveries was 98% for P9, 99% for P10, 98% for P11, 97% for P12, 98% for P13, and 98% for P14.
Phase 1 (Baseline)
All reinforcers were arranged according to VI schedules, sampled without replacement from 13 intervals (Flesher & Hoffman, 1962). During Phase 1, engaging in the target response was reinforced on a VI 10-s schedule. Following each reinforcement, the scoreboard would be held in the participants line of sight for 1 s and increase by 15 points. Engaging in the alternative or control response produced no consequences.
In order to accurately replicate Experiment 1, a 1-s COD was imposed in which the experimenter would place a black, 22 cm x 28 cm paper barrier over the response buttons and only the scoreboard was visible.
Following the COD, all buttons reappeared in the same triangular formation, differing from Experiment 1. That is, the physical position of the buttons remained constant. However, between each session the target, alternative, and control response buttons were randomly repositioned in a manner consistent with Experiment 1.
Phase 2 (DRA)
Phase 2 was conducted in the same manner as in Phase 2 of Experiment 1 using the low-technology modifications necessary for Experiment 2.
Phase 3 (Extinction)
Phase 3 was conducted in the same manner as in Phase 3 of Experiment 1 using the low-technology modifications necessary for Experiment 2.
Results
Figure 2 displays results of participant performance in the short-baseline (top panel; P9, P10), long-baseline (middle panel; P11, P12), and extended-exposure (bottom panel; P13, P14) groups when Experiment 1 was replicated using low-technology stimuli.
Individual Response Patterns in Experiment 2 in the Short-Baseline (top panel), Long-Baseline (middle panel), and Extended-Exposure (bottom panel) Groups
In the short-baseline group we observed that all three response types persisted across the majority of Phase 1 for both participants. However target responding occurred more frequently than alternative or control responding in at least the final five sessions of the phase for both participants, indicating target-response acquisition (and no systematic differences between the alternative and control responses). Response persistence of the target and control responses continued in Phase 2. However, response allocation was greatest toward the alternative response, suggesting response acquisition. Highest rates of the alternative response persisted across the duration of Phase 2 for both participants. Target and inactive control responding was undifferentiated across the length of this phase. Similar to Experiment 1, for both participants when the extinction phase was introduced (Phase 3), we observed undifferentiated response allocation toward all three response options (i.e., target, alternative, inactive control). Undifferentiation observed in this phase was near identical to that observed in Experiment 1 (i.e., indiscriminate responding across task stimuli). However, unlike Experiment 1, we observed greater levels of variability toward the end of Phase 3 with both participants. That is, although in Experiment 1 undifferentiated responding persisted across the entire phase, in Experiment 2 we observed response allocation toward one of the response types and high levels of that response in one session in each of the final three to four sessions.
In the long-baseline group (Fig. 2, middle panel), we did not observe any differentiation in any of the response types across all phases for P11. That is, this participant’s responding did not appear to be sensitive to the reinforcement contingencies arranged in Phases 1 and 2 (despite the fact that the participant frequently contacted reinforcement in these phases). It is interesting, however, responding during Phase 3 was consistent with responding observed in participants in other groups (including Experiment 1, long-baseline). That is, all response forms persisted across the duration of Phase 3, and no response extinguished. For P12, responding across phases was consistent with response patterns observed for participants in the long-baseline group in Experiment 1. That is, we observed high and persistent levels of responding toward the target in Phase 1, high and persistent levels of responding in Phase 2, and undifferentiation and persistence of all responses during Phase 3. However, unlike Experiment 1, there was some responding toward each response type in Phases 1 and 2, even when these responses did not contact reinforcement. Although this appears discrepant with participants in Experiment 1, this finding is consistent with other participants in Experiment 2.
In the extended-exposure group (Fig. 2, bottom panel), responding for both participants was consistent with response patterns observed in Experiment 1 (i.e., high rates and persistence of the response form that contacted reinforcement across Phases 1 and 2, followed by indifference across response types and persistence of all responses in Phase 3). However, similar to other participants in Experiment 2, and unlike the participants in Experiment 1, there was some level of variability in responding across response forms that did not contact reinforcement in Phases 1 and 2.
Taken together, the results of Experiment 2 appear to replicate the findings of Experiment 1, with the exception of Phase 1 and 2 responding for P11. These results suggest that the substitution of low-technology stimuli during human-laboratory investigations of resurgence do not significantly alter response patterns observed across target, alternative, and control response options. That is, undifferentiation and persistence of response forms during tests of resurgence do not appear to be affected by the type of technology used during resurgence tasks.
Discussion
In the present study we used adult, neurotypical human participants to examine the role of baseline-phase length, extinction-phase length, and types of stimuli used (high technology vs. low technology) on resurgence and response persistence. First, using the logic described in previous studies (e.g., Winterbauer et al., 2013), we attempted to facilitate resurgence by providing a longer history of baseline reinforcement (short- vs. long-baseline groups) to assess whether that history would be influential in producing a difference between target and inactive responding during Phase 3. However, similar to the results obtained by Sweeney and Shahan (2016), we did not observe differences in resurgence of the target response and occurrence of the control response. Second, we attempted to extinguish all responses by extending the length of all phases including the extinction phase (extended-exposure group). However, similar to results obtained by Bolívar et al. (2017), none of the three response types extinguished during Phase 3. Finally, we replicated Experiment 1 using low-technology stimuli in order to assess whether responding in Experiment 1 was a product of participants interacting with the computerized task, which might have been influenced by participants’ extra-experimental history with high-technology operandum. However, when low-technology stimuli were incorporated, we continued to see no difference between target and control responding for all participants, and response persistence of all response types continued during the extinction phase for all participants.
There were no notable differences in Phase 3 responding among the three response types between groups in Experiment 1 or when using low-technology stimuli in Experiment 2. This suggests that the increased length of baseline in the long-baseline groups did not promote increased rates of the target behavior greater than responding towards the inactive control stimulus. Further research is warranted to determine the conditions under which baseline length impacts later response resurgence, which ultimately may be a function of the rate of reinforcement in combination with baseline length (e.g., Fisher et al., 2019). However, in the present study we used a dense VI 10-s schedule of reinforcement which, when combined with a longer baseline phase, did not significantly affect levels of resurgence beyond inactive control occurrence.
In Experiment 2, we observed greater variability in responding across Phases 1 and 2 (i.e., responding occurred to some extent on all response options). However, in Experiment 1, participants allocated responding almost exclusively to the response that produced reinforcement in Phases 1 and 2, respectively. It is possible that this difference is due to the slight delay that occurred between response and reinforcer delivery in Experiment 2, given that reinforcers were delivered by the experimenter. Said another way, there was greater contiguity between the response and reinforcer in Experiment 1 compared to Experiment 2. However, this delay was so short that the difference in reinforcer delivery between Experiment 1 and 2 is likely negligible. As an alternative, it is possible that variability in Experiment 2 was related to the positioning of the stimuli, which remained constant across phases (i.e., the buttons remained in the same physical position). In Experiment 1, the position of each response key changed following each response, which required participants to visually track the response option that produced reinforcement. Future research is warranted to determine reasons for response variability that occurs during human-laboratory preparations that involve high- versus low-technology stimuli.
In Phase 3 of Experiment 2, we observed greater variability towards response options during the final three to four sessions for participants in the short-baseline group (Figure 2, P9 and P10). Because target and control responding did not completely extinguish during Phase 2, it is possible that participants inadvertently learned a contingency related to a unique pattern of responding rather than a single response. Thus, over extended exposures to extinction in Phase 3, these unintended response patterns may have extinguished, resulting in invariant responding (i.e., responding toward only one response option at a time). It is interesting that this pattern in response allocation to only one response option was not observed for participants in the long-baseline and extended-exposure groups (nor was it observed for any participants in Experiment 1). Therefore, it is unclear as to if this invariant responding is influenced by the duration of the baseline phase, the duration of the extinction phase, a function of the type of task stimuli used, or the results of other participant characteristics that may have differed between groups and across experiments.
In Experiment 2 we used low-technology stimuli to replicate Experiment 1 in order to determine if indifferences in response types and response persistence during Phase 3 could be a product of extra-experimental histories with high-technology stimuli (e.g., neurotypical adult humans have an extensive reinforcement history with touchscreen phones, touchscreen tablets, and computers). Lattal and Oliver (2020) noted that the participant’s extra-experimental history with technology could influence the results of resurgence studies that rely on these technologies. They suggested there is a high probability that participants contact dense schedules of reinforcement when manipulating these stimuli, which may promote response persistence. They further reported that 6 of 6 reviewed resurgence studies that have used human participants have shown some responding toward the control stimulus in at least one participant whereas 13 of 14 reviewed resurgence studies using nonhuman animals as subjects have shown no or minimal responding toward the control stimulus. That is, undifferentiated responding among target and control responses was common in studies including human participants but almost never occurred in nonhuman studies. Furthermore, in the present investigation, although we did not directly compare high- versus low-technology stimuli within subjects, the response pattern observed in Phase 3 for participants in Experiment 2 was similar to the response patterns observed for participants in Experiment 1 (with the exception of Phases 1 and 2 for P11). Taken together, it is likely that those variables (e.g., ontogeny) that contribute to indifference between target and control responses, as well as response persistence of all response types, operate on human-laboratory investigations independent of the type of stimuli used. Discrepant results between human- and animal-laboratory evaluations of resurgence may be the result of a characteristic difference between neurotypical adult participants and nonhuman animal subjects.
In addition to observing increases in the target and inactive control responses during Phase 3, relative to Phase 2, we also observed persistence of all responses throughout Phase 3 in both experiments. Unlike nonhuman animal studies where responding during extinction decreases with time, we observed approximately equal levels of responding across all response types for all participants, independent of the group or arrangement (high technology vs. low technology) they experienced. It is possible that during Phase 3, participant behavior was operating under self-directed rules due to the presence of the experimental setting and instructions given to participants prior to the experiment. That is, it is possible that subjects had an extraexperimental history of rule following in highly controlled settings such that they continued to respond during the behavioral task instead of behaving more typically under free-operant extinction arrangements (i.e., a gradual decrease in responding). The presence of the experimenter and experimental setting may have induced compliance among participants (i.e., participants continued to respond to avoid responding “incorrectly,” or stimulus conditions evoked compliance).
Some have criticized the approach of using human-laboratory preparations for studying relapse because it is possible that results from studies of this kind may be a product of verbal mediation or rules, given that human-laboratory studies typically recruit neurotypical adults as participants (Baron et al., 1969; Craig et al., 2019; Madden et al., 1998). Although we did not manipulate self-directed rules in the present investigation, it is possible that participants in human-laboratory studies of resurgence are not sensitive to extinction contingencies because of these rules. In addition to pointing to participant’s history as contributing factor to indifference between target resurgence and control-response occurrence, Lattal and Oliver (2020) suggested that control responding during extinction (and possible response persistence) during human-laboratory studies may be a product of verbal behavior (i.e., rule-governed behavior).
In addition to self-directed rules that may have influenced Phase 3 response persistence in the present study, there may be procedural differences across studies that lead to response persistence in some cases (e.g., present study) and lack of persistence under other arrangements (e.g., Diaz-Salvat et al., 2020). For example, in the present study we used single-schedules of reinforcement, whereas studies that have observed minimal Phase 3 persistence have used multiple schedules of reinforcement (e.g., Diaz-Salvat et al., 2020; Kuroda et al., 2016). In the present study we used a VI 10-s schedule whereas Diaz-Salvat et al. (2020) used a dense fixed-interval 2-s schedule, which may have increased the discriminability between Phases 2 and 3. Some studies have provided participants with multiple-control response options (e.g., Cox et al., 2019; Diaz-Salvat et al., 2020) whereas in the present study there was only one control response. Last, Bolívar et al. (2017) required greater effort (i.e., six clicks) before reinforcement was obtained compared to the present study (i.e., one button press). This difference in response effort may have promoted response persistence in the present study. Future researchers might consider investigating how procedural differences across human-laboratory studies affect the outcome of resurgence studies (including the degree of response persistence in Phase 3).
It is important to note that stimuli associated with the alternative response are not usually presented in Phase 1, and engaging in the alternative response is not possible until Phase 2 of animal studies (e.g., Nevin et al., 2016; Podlesnik & Shahan, 2009). In the present study, the stimulus associated with the alternative response was available, and therefore engaging in the alternative response was possible during Phase 1 (reinforcement of the target). Although this procedural variation differed from previous animal research (e.g., Podlesnik & Shahan, 2009), the presence of the alternative stimulus and engaging in the alternative response during Phase 1 may have promoted latent inhibition. Trask et al. (2015) attribute resurgence to contextual differences demarcated by the change from high to low density reinforcement brought on by different schedules of reinforcement. It is possible that the alternative response contacting extinction in Phase 3 resembled the stimulus conditions (i.e., context) of Phase 1, wherein the alternative response did not produce reinforcement. Future research might consider how to account for species differences in human and nonhuman animal studies of resurgence. That is, it may be important to acknowledge or examine to what extent self-directed rules account for differences in resurgence (or persistence) between brief, human-laboratory simulations, the animal research it is compared to (typically conducted across days with several exposures to conditions and steady-state criteria for phase changes), and the clinical spaces it is designed to model (e.g., relapse of clinically relevant maladaptive behavior). This discovery may also help elucidate the extent to which rules and instructions influence responding in some human-laboratory studies (e.g., the present data; Bolívar et al., 2017; Cox et al., 2019; Sweeney & Shahan, 2016) but not in others (e.g., Bolívar & Dallery, 2020; Lambert et al., 2015).
A third possibility is that increases in control responding observed in human studies could be a result of response induction. Response induction (or generalization) might be considered a form of response recurrence where responding that is topographically similar to the topography reinforced begins to occur (Mackintosh, 1955). Da Silva et al. (2008) suggested that what resurges during Phase 3 is not a specific target response but instead a pattern or topography of responses that were previously reinforced or similar to that reinforced (see also Cançado & Lattal, 2011; Reed & Morgan, 2006; Schwartz, 1980). That is, the behavior that resurges closely resembles the original response topography. In the present study, the target, alternative, and inactive control responses were topographically similar, which could have resulted in generalized responding during Phase 3. The control response form was also topographically similar to the other response forms and extinction of the target plus alternative may have induced responding toward the inactive control stimulus (however, see Doughty et al., 2007, for results suggesting resurgence might be greater when target and alternative responses differ in topography). Future studies of inactive control responses might include three topographically dissimilar response forms to examine this possibility.
Additional important differences exist between human studies and nonhuman animal studies of resurgence that may contribute to the pattern of indifference responding and response persistence during extinction observed with humans. First, human-laboratory studies commonly use generalized conditioned reinforcers of unknown value (e.g., points exchangeable for gift cards) whereas animal studies typically use unconditioned reinforcers (e.g., food) of high value. Second, human-laboratory studies often have few exposures (e.g., over the course of 1–2 hr) to each condition whereas animal studies often include extended exposure (e.g., across days) to each condition. Third, researchers using nonhuman animals as subjects often impose strict control over the subject’s reinforcement history, whereas this is typically uncontrolled and unaccounted for in human studies. These factors in addition to the role of reinforcement history, verbal mediation, and response induction, could contribute to the behavioral patterns observed in human-laboratory studies of resurgence.
The incorporation of control response options into studies of resurgence was ultimately a way researchers could differentiate between resurgence and extinction-induced variability (i.e., the target response reemerged due to a direct history of reinforcement). Although the use of a control response is common in studies using nonhuman animal subjects (e.g., Podlesnik et al., 2006), its use is fairly uncommon in human-laboratory studies, and even more rare in applied studies of resurgence. Therefore, it is unclear whether resurgence of clinically significant behavior could be separated from extinction-induced variability in applied settings (e.g., Fuhrman et al., 2016) given that participants in applied research are not provided an opportunity to engage in a third, unreinforced response alternative. However, regardless of the type of investigation (i.e., basic, translational, applied), it is unclear whether the control response itself is a useful conduit for isolating the difference between resurgence and extinction-induced variability in nonhuman animal and human animal research (see Lattal & Oliver, 2020, for further discussion). Of some importance is the degree to which the difference between resurgence and extinction-induced variability is significant from a practical standpoint. From a clinical standpoint, the resurgence of a formerly reinforced problem behavior or the emergence of novel topographies of problem behavior (e.g., Sullivan et al., 2020) will both usually require intervention. However, they type of intervention that is used may be influenced by the type of responding that emerges during extinction. It may be the case that resurgence is a type of variability induced by extinction given that extinction is known to induce other types of responding (e.g., novelty, emotional responding). Therefore, future researchers should explore the extent to which distinguishing resurgence as a type of extinction-induced variability from other types of emergent behavior may have clinical implications (see Wathen & Podlesnik, 2018, for further discussion).
Distinguishing between resurgence and extinction-induced variability may be important in our conceptual understanding of relapse phenomenon, and the present investigation provides a potential method for clarifying the differences between these processes. Lattal et al. (2019) reported that more recently reinforced behavior resurges before responses associated with a temporally distant reinforcement history. In other words, the order in which responses are trained is an important factor in determining the order in which a response recurs. In the present study, if target responding recurred before control responding occurred, one could attribute the emergence of responding as resurgence as opposed to extinction-induced variability (and vice versa). Comparing the latency to first response for both the target and control response in the first session of Phase 3 could unequivocally determine whether the results suggest a resurgence effect or extinction-induced variability. Unfortunately, the present investigation is limited by the inability to extract latency data in Experiment 1 and indifferences in latency between target and control responding for a subset of participants in Experiment 2. Therefore, future researchers might consider measurement strategies for target and control responding that would allow for differences between resurgence and extinction-induced variability to be better detected.
Human-laboratory studies are important because the variables affecting resurgence that have direct clinical relevance can be studied without putting individuals at risk of harming themselves or others (Kestner & Peterson, 2017). However, these experiments could suffer from the practical problems of (1) inadvertently evoking long-established behavioral patterns that are a product of extraexperimental reinforcement histories, or (2) participant insensitivity to contingencies due to verbal mediation or rules. Toward that end, the current study underscores potential experimental differences that might influence translational models of relapse. Despite this, human-laboratory studies are one method to facilitate translation between purely experimental and purely applied research on resurgence. Therefore, further research is certainly warranted to uncover the variables that contribute to differences in resurgence studies in human-laboratory and nonhuman-animal research.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Change history
25 May 2021
A Correction to this paper has been published: https://doi.org/10.1007/s40732-021-00473-y
References
Baron, A., Kaufman, A., & Stauber, K. A. (1969). Effects of instructions and reinforcement-feedback on human operant behavior maintained by fixed-interval reinforcement. Journal of the Experimental Analysis of Behavior, 12, 701–712. https://doi.org/10.1901/jeab.1969.12-701.
Bolívar, H. A., Cox, D. J., Barlow, M. A., & Dallery, J. (2017). Evaluating resurgence procedures in a human operant laboratory. Behavioural Processes, 140, 150–160. https://doi.org/10.1016/j.beproc.2017.05.004.
Bolívar, H. A., & Dallery, J. (2020). Effects of response cost magnitude on resurgence of human operant behavior. Behavioural Processes, 178, 104187. https://doi.org/10.1016/j.beproc.2020.104187.
Bruzek, J. L., Thompson, R. H., & Peters, L. C. (2009). Resurgence of infant caregiving responses. Journal of the Experimental Analysis of Behavior, 92, 327–343. https://doi.org/10.1901/jeab.2009-92-327.
Bullock, C. E., Fisher, W. W., & Hagopian, L. P. (2017). Description and validation of a computerized behavioral data program: “BDataPro”. The Behavior Analyst, 40(1), 275–285. https://doi.org/10.1007/s40614-016-0079-0.
Cançado, C. R. X., & Lattal, K. A. (2011). Resurgence of temporal patterns of responding. Journal of the Experimental Analysis of Behavior, 95, 271–287. https://doi.org/10.1901/jeab.2011.95-271.
Cox, D. J., Bolívar, H. A., & Barlow, M. A. (2019). Multiple control responses and resurgence of human behavior. Behavioural Processes, 159, 93–99. https://doi.org/10.1016/j.beproc.2018.12.003.
Craig, A. R., & Shahan, T. A. (2016). Behavioral momentum theory fails to account for the effects of reinforcement rate on resurgence. Journal of the Experimental Analysis of Behavior, 105, 375–392. https://doi.org/10.1002/jeab.207.
Craig, A. R., Sullivan, W. E., & Roane, H. S. (2019). Further evaluation of a nonsequential approach to studying operant renewal. Journal of the Experimental Analysis of Behavior, 112, 210–223. https://doi.org/10.1002/jeab.546.
da Silva, S. P. D., Maxwell, M. E., & Lattal, K. A. (2008). Concurrent resurgence and behavioral history. Journal of the Experimental Analysis of Behavior, 90, 313–331. https://doi.org/10.1901/jeab.2008.90-313.
Diaz-Salvat, C. C., St. Peter, C. C., & Shuler, N. J. (2020). Increased number of responses may account for reduced resurgence following serial training. Journal of Applied Behavior Analysis, 53(3), 1542–1558. https://doi.org/10.1002/jaba.686.
Doughty, A. H., Cash, J. D., Finch, E. A., Holloway, C., & Wallington, L. K. (2010). Effects of training history on resurgence in humans. Behavioural Processes, 83, 340–343. https://doi.org/10.1016/j.beproc.2009.12.001.
Doughty, A. H., da Silva, S. P., & Lattal, K. A. (2007). Differential resurgence and response elimination. Behavioural Processes, 75, 115–128. https://doi.org/10.1016/j.beproc.2007.02.025.
Doughty, A. H., & Oken, G. (2008). Extinction-induced response resurgence: A selective review. The Behavior Analyst Today, 9, 27. https://doi.org/10.1037/h0100644.
Epstein, R. (1983). Resurgence of previously reinforced behavior during extinction. Behaviour Analysis Letters, 3, 391–397.
Fisher, W. W., Saini, V., Greer, B. D., Sullivan, W. E., Roane, H. S., Fuhrman, A. M., Craig, A. R., & Kimball, R. T. (2019). Baseline reinforcement rate and resurgence of destructive behavior. Journal of the Experimental Analysis of Behavior, 111, 75–93. https://doi.org/10.1002/jeab.488.
Flesher, M., & Hoffman, H. S. (1962). A progression for generating variable-interval schedules. Journal of the Experimental Analysis of Behavior, 5, 529–530. https://doi.org/10.1901/jeab.1962.5-529.
Fuhrman, A. M., Fisher, W. W., & Greer, B. D. (2016). A preliminary investigation on improving functional communication training by mitigating resurgence of destructive behavior. Journal of Applied Behavior Analysis, 49(4), 884–899. https://doi.org/10.1002/jaba.338.
Ho, T., Bai, J. Y., Keevy, M., & Podlesnik, C. A. (2018). Resurgence when challenging alternative behavior with progressive ratios in children and pigeons. Journal of the Experimental Analysis of Behavior, 110(3), 474–499. https://doi.org/10.1002/jeab.474.
Kestner, K. M., & Peterson, S. M. (2017). A review of resurgence literature with human participants. Behavior Analysis: Research and Practice, 17, 1–17. https://doi.org/10.1037/bar0000039.
Kuroda, T., Cançado, C. R., & Podlesnik, C. A. (2016). Resistance to change and resurgence in humans engaging in a computer task. Behavioural Processes, 125, 1–5. https://doi.org/10.1016/j.beproc.2016.01.010.
Lambert, J. M., Bloom, S. E., Samaha, A. L., Dayton, E., & Rodewald, A. M. (2015). Serial alternative response training as intervention for target response resurgence. Journal of Applied Behavior Analysis, 48, 765–780. https://doi.org/10.1002/jaba.253.
Lattal, K. A., Cançado, C. R., Cook, J. E., Kincaid, S. L., Nighbor, T. D., & Oliver, A. C. (2017). On defining resurgence. Behavioural Processes, 141, 85–91. https://doi.org/10.1016/j.beproc.2017.04.018.
Lattal, K. A., & Oliver, A. C. (2020). The control response in assessing resurgence: Useful or compromised tool? Journal of the Experimental Analysis of Behavior, 113, 77–86. https://doi.org/10.1002/jeab.570.
Lattal, K. A., & St. Peter Pipkin, C. (2009). Resurgence of previously reinforced responding: Research and application. The Behavior Analyst Today, 10, 254–266. https://doi.org/10.1037/h0100669.
Lattal, K. A., Solley, E. A., Cançado, C. R., & Oliver, A. C. (2019). Hierarchical resurgence. Journal of the Experimental Analysis of Behavior, 112(2), 177–191. https://doi.org/10.1002/jeab.547.
Leitenberg, H., Rawson, R. A., & Bath, K. (1970). Reinforcement of competing behavior during extinction. Science, 169, 301–303.
Mackintosh, I. (1955). Irrelevant responses during extinction. Canadian Journal of Psychology, 9, 183–189. https://doi.org/10.1037/h0083638.
Madden, G. J., Chase, P. N., & Joyce, J. H. (1998). Making sense of sensitivity in the human operant literature. The Behavior Analyst, 21, 1–12. https://doi.org/10.1007/BF03392775.
Marsteller, T. M., & St. Peter, C. C. (2012). Resurgence during treatment challenges. Mexican Journal of Behavior Analysis, 38, 7–23.
Nevin, J. A., Mace, F. C., DeLeon, I. G., Shahan, T. A., Shamlian, K. D., Lit, K., Sheehan, T., Frank-Crawford, M. A., Trauschke, S. L., Sweeney, M. M., Tarver, D. R., & Craig, A. R. (2016). Effects of signaled and unsignaled alternative reinforcement on persistence and relapse in children and pigeons. Journal of the Experimental Analysis of Behavior, 106, 34–57. https://doi.org/10.1002/jeab.213.
Podlesnik, C. A., Jimenez-Gomez, C., & Shahan, T. A. (2006). Resurgence of alcohol seeking produced by discontinuing non-drug reinforcement as an animal model of drug relapse. Behavioural Pharmacology, 17, 369–374. https://doi.org/10.1097/01.fbp.0000224385.09486.ba.
Podlesnik, C. A., & Kelley, M. E. (2015). Translational research on the relapse of operant behavior. Mexican Journal of Behavior Analysis, 41, 226–251.
Podlesnik, C. A., & Shahan, T. A. (2009). Behavioral momentum and relapse of extinguished operant responding. Learning & Behavior, 37, 357–364. https://doi.org/10.3758/LB.37.4.357.
Podlesnik, C. A., & Shahan, T. A. (2010). Extinction, relapse, and behavioral momentum. Behavioural Processes, 84, 400–411. https://doi.org/10.1016/j.beproc.2010.02.001.
Pritchard, D., Hoerger, M., & Mace, F. C. (2014). Treatment relapse and behavioral momentum theory. Journal of Applied Behavior Analysis, 47, 814–833. https://doi.org/10.1002/jaba.163.
Reed, P., & Morgan, T. A. (2006). Resurgence of response sequences during extinction in rats shows a primacy effect. Journal of the Experimental Analysis of Behavior, 86, 307–315. https://doi.org/10.1901/jeab.2006.20-05.
Saini, V., & Roane, H. S. (2018). Technological advances in the experimental analysis of human behavior. Behavior Analysis: Research & Practice, 18, 288–304. https://doi.org/10.1037/bar0000124.
Schwartz, B. (1980). Development of complex, stereotyped behavior in pigeons. Journal of the Experimental Analysis of Behavior, 33, 153–166. https://doi.org/10.1901/jeab.1980.33-153.
Sullivan, W. E., Saini, V., DeRosa, N. M., Craig, A. R., Ringdahl, J. E., & Roane, H. S. (2020). Measurement of nontargeted problem behavior during investigations of resurgence. Journal of Applied Behavior Analysis, 53, 249–264. https://doi.org/10.1002/jaba.589.
Sweeney, M. M., & Shahan, T. A. (2015). Renewal, resurgence, and alternative reinforcement context. Behavioural Processes, 116, 43–49. https://doi.org/10.1016/j.beproc.2015.04.015.
Sweeney, M. M., & Shahan, T. A. (2016). Resurgence of target responding does not exceed increases in inactive responding in a forced-choice alternative reinforcement procedure in humans. Behavioural Processes, 124, 80–92. https://doi.org/10.1016/j.beproc.2015.12.007.
Trask, S., Schepers, S. T., & Bouton, M. E. (2015). Context change explains resurgence after the extinction of operant behavior. Mexican Journal of Behavior Analysis, 41(2), 187–210.
Volkert, V. M., Lerman, D. C., Call, N. A., & Trosclair-Lasserre, N. (2009). An evaluation of resurgence during treatment with functional communication training. Journal of Applied Behavior Analysis, 42, 145–160. https://doi.org/10.1901/jaba.2009.42-145.
Wathen, S. N., & Podlesnik, C. A. (2018). Laboratory models of treatment relapse and mitigation techniques. Behavior Analysis: Research & Practice, 18(4), 362–387. https://doi.org/10.1037/bar0000119.
Winterbauer, N. E., Lucke, S., & Bouton, M. E. (2013). Some factors modulating the strength of resurgence after extinction of an instrumental behavior. Learning and Motivation, 44, 60–71. https://doi.org/10.1016/j.lmot.2012.03.003.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Informed Consent
All procedures performed involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments. This study was reviewed and received clearance from the SUNY Upstate Medical University Institutional Review Board [#960296-2 and #1641106-1]. Informed consent and publication of findings was provided in writing by participants. The authors have no conflict of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The last names of 2 co-authors are misspelled and should be corrected as follows:
"Kate Derrenback" should be "Kate Derrenbacker".
"Arohan Rima" should be "Arohan Rimal".
Rights and permissions
About this article
Cite this article
Saini, V., Sullivan, W.E., Craig, A.R. et al. Responding Fails to Extinguish During Human-Laboratory Experiments of Resurgence. Psychol Rec 71, 325–336 (2021). https://doi.org/10.1007/s40732-021-00469-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40732-021-00469-8