Abstract
Smoking is associated with a number of chronic health conditions, including cardiovascular disease and various types of cancer. The decision to smoke can be conceptualized as preference for small, immediate rewards (e.g., relief from withdrawal) over larger, delayed rewards (e.g., good health). Contingency management (CM) takes advantage of this preference for immediate outcomes by delivering incentives, usually financial, for making the healthier choice to abstain from smoking. The current study tested the feasibility of harnessing naturally occurring social contingencies associated with smoking cessation to increase the promise of CM in initiating and sustaining long-term abstinence. Pairs of smokers with an existing relationship (i.e., friends, roommates, family, significant others) were recruited to quit together in the context of a smartphone-delivered, group CM intervention. Approximately 50% of interested participants identified a partner who also met criteria to participate, and five pairs (N = 10) completed the study. Using a within-subject design, participants could earn individual financial incentives for submitting breath carbon monoxide (CO) samples twice daily that met targeted goals for abstinence, and they could earn bonus incentives when both members of the pair met their targets together. Nine participants (90%) successfully reduced their mean breath CO during the intervention relative to baseline conditions. Individuals within a pair performed similarly to one another, for better or worse (i.e., both participants abstained, smoked, or missed samples at the same time). The social contingencies of quitting with someone with whom the smoker has an existing relationship may be helpful, but may also introduce unique challenges, particularly with regard to recruitment and treatment retention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Smoking is the number one cause of preventable death in the United States and is associated with a number of chronic health conditions, including cardiovascular disease, emphysema, and chronic obstructive pulmonary disease (CDC, 2016b). Smoking can cause cancer anywhere in the body, and it is responsible for the vast majority of lung cancer cases (~90%; CDC, 2016a). Despite these staggering health outcomes, approximately 15% of U.S. adults report being current cigarette smokers (Ahmed, King, Neff, et al., 2016). Although 7 out of 10 smokers report a desire to quit, only 4 out of 10 attempt to quit for at least 1 day during each quit attempt (CDC, 2017).
One evidence-based intervention for smoking cessation is contingency management (CM; Dallery, Raiff, & Grabinski, 2013; Higgins, Silverman, & Heil, 2007). CM consists of delivering incentives (typically financial) contingent on objective evidence of smoking abstinence (e.g., breath carbon monoxide [CO] indicative of abstinence), and it is based on the well-established theoretical foundations of behavior analysis and behavioral economics (Bickel & Marsch, 2001; Madden & Bickel, 2010). Behavioral economists have tried to identify the variables that underlie decision making (Borrero et al., 2007; Herrnstein, 1970), where all behavior is viewed as a choice between two or more options. For example, an individual may have a choice between eating chips, going for a bike ride, or smoking a cigarette. The outcomes in each example provide qualitatively different reinforcers, and an individual will choose the outcome with the highest quality reinforcer at that time. The benefits of choosing the healthy options (i.e., going for a bike ride) in the example above are often obscure, probabilistic, and delayed—doing something other than smoking now may help prevent lung cancer or heart disease 10 or 20 years from now. In other words, individuals who smoke choose the unhealthy over the healthy option because it results in an immediate high quality reinforcer (e.g., relief from withdrawal, escape from boredom) compared to the more obscure and probabilistic reinforcer (e.g., improved health) available after an unknown delay (Ortendahl & Fries, 2002). The key to CM is to add immediate reinforcers to the healthy options, thereby “tipping the balance” in favor of healthy choices.
There is evidence that CM can be feasibly and effectively delivered remotely (Dan, Grabinski, & Raiff, 2016; Hertzberg et al., 2013). Dallery, Glenn, and Raiff (2007) developed and tested Internet-based CM interventions that require users to submit web-camera-recorded breath CO samples twice daily over a secure Internet server that are later verified by staff. Automatically delivered monetary incentives are deposited in user accounts based on meeting predetermined breath CO abstinence goals. Mobile CM, delivered via smartphone, has also recently been shown to be both feasible and shows promise for promoting smoking abstinence among individuals diagnosed with posttraumatic stress disorder (Hertzberg et al., 2013) and attention-deficit/hyperactivity disorder (Dan et al., 2016).
Although CM interventions for smoking cessation can initiate abstinence, relapse is the most likely outcome, as with all smoking cessation interventions (Dallery et al., 2013). CM interventions that rely solely on financial incentives to reinforce abstinence can be too costly to maintain for extended periods of time, and once the incentives are removed, many individuals relapse. Thus, strategies are needed to not only improve the initiation of smoking abstinence but also to maintain abstinence once the treatment is removed. One strategy is to explore what Baer and Wolf (1967) described as “behavioral traps,” which consist of identifying the natural contingencies that can take over and maintain behavior that is changed within the context of treatment.
One behavioral trap might involve harnessing the social nature of smoking. Indeed, Christakis and Fowler (2008) found that smoking abstinence occurred in clusters of people who knew each other (e.g., spouses, friends, siblings). One method for introducing social support to CM interventions is via group contingencies, in which incentives for abstinence are tied to the individual’s own success as well as the success of other members of their group. For example, Meredith, Grabinski, and Dallery (2011) assigned two to three smokers to an online group CM intervention. Participants could earn independent incentives for meeting their own abstinence goals during treatment (e.g., $1.50), and they could earn bonus, interdependent incentives when all members of the group met their abstinence goals for a particular submission (e.g., an additional $3.00). Participants did not know each other prior to participating in the study and all of their interactions occurred via an electronic forum that was moderated by the investigators. In the forum, participants posted an average of 10 posts per person, and the posts were largely positive in nature, involving social support for team members who were struggling to quit or congratulating members when they were successful. The group CM intervention was found to be feasible and promote reductions in smoking (a 56% increase in abstinence samples relative to baseline conditions). Because smokers in Meredith et al. (2011) were only able to interact via the electronic forum, once the intervention ended participants not only lost access to the monetary incentives but they also lost access to the social support that was built in to the group contingencies.
The purpose of the present study was to conduct a systematic replication of Meredith et al. (2011) by recruiting pairs of smokers with preexisting relationships, with the goal of “trapping” abstinence achieved during treatment via these naturally occurring social contingencies. In this early stage research, the primary aim was to evaluate the feasibility of a smartphone-delivered group CM intervention in smokers who already knew each other.
Method
Participants
Participants were recruited through print media posted on the Rowan University campus or in nearby communities, and via e-mails sent to Rowan University students and employees. All participants (N = 10) were English-speaking and between the ages of 19 to 48 years. Participants were required to own a smartphone with Android or iOS operating systems, video recording capabilities, and uninterrupted monthly data access. Participants were also required to smoke at least five cigarettes per day for at least 1 year, have a minimum intake breath CO of ≥10 parts per million (ppm), be in good physical and mental health, and answer affirmatively when asked, “Do you want to quit smoking?” Participants who were exposed to unavoidable ambient CO (e.g., fire, car exhaust) or who smoked anything other than cigarettes (e.g., marijuana, cigars) were excluded. Finally, participants were required to identify one other smoker who wanted to participate and met all of the requirements listed above. Women were excluded if pregnant or breastfeeding. Approval of study procedures was obtained from the Institutional Review Board at the university of the corresponding author, and all participants completed the informed consent procedures before beginning any of the study activities described below.
Procedure
Pairs of interested applicants were screened over the telephone to assess whether preliminary inclusion criteria were met, and a brief description of the study was provided at that time. Qualifying applicants were invited to participate in the study, and an in-person intake appointment was scheduled with both members to attend at the same time. During this intake appointment, the study was described in greater detail and informed consent was obtained, independently, from each participant. Urine samples were collected using the iCup Six-Panel Drug Test (Norfolk, VA) for drug screening, as well as pregnancy testing for women using the MooreBrand hCG Urine Dipstick (Farmington, CT) strips. Participants then completed the Fagerström Test for Nicotine Dependence (FTND) (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991), a contact information sheet, and a psychosocial history that asked questions about smoking history, smartphone accessibility, and mental/physical health. The National Cancer Institute’s “Clearing the Air” booklet was given to all participants to provide guidance on how to track triggers for smoking and to provide suggestions for how to overcome withdrawal and cravings. Instructional manuals describing the treatment conditions, monetary payment schedule, and breath CO submission process were distributed to the participants, who were then quizzed on the information using a multiple-choice format. Finally, two mobile messenger applications, Kakao Talk (an online social support forum for participants and the research assistant to communicate about smoking cessation), and mōtiv8 (our mobile CM application used to submit breath CO videos, monitor quantitative progress, and see monetary earnings, which can be seen in Dan et al., 2016) were installed on participants’ smartphones.
Participants were loaned a hand-held breath CO meter (Bedfont piCO+ Smokerlyzer, COvita, Haddonfield, NJ) during their participation in the study. They were taught how to correctly submit a breath CO video using the mōtiv8 application on their smartphone (see Dan et al., 2016, for screenshots of the application). To correctly collect a breath CO sample participants opened the mōtiv8 application and began recording a video. With the participants' chest and face visible in the video, they inhaled deeply and held their breath for 15-seconds, after which they emptied their lungs by exhaling slowly into the plastic tube of the piCO+. A number representing the CO level in parts per million (ppm) appeared on the piCO+ screen. This number was captured in the video, and participants manually entered it into the mōtiv8 application. Participants could view their monetary earnings, their quantitative progress, and the progress of their partner via the smartphone application throughout the study. Participants were instructed to submit two breath CO videos daily, with a minimum 8-hour intersubmission interval required between samples, as we have used in the past (Dallery et al., 2013)
The Kakao Talk messenger application installed on participants’ smartphones was used as a group social support forum. The forum allowed participants to read posts from partners regarding personal progress, as well as post comments about their own progress and provide support to one another. Moderators also used Kakao Talk to provide informative comments, such as phase changes in the study or advice about smoking cessation, as well as to provide supportive comments and ensure that communication between partners remained positive and supportive.
A within-subject, nonconcurrent multiple-baseline design was used to evaluate smoking cessation during four conditions: baseline, tapering, abstinence induction, and follow-up. The multiple-baseline design can be used to demonstrate that behavior change occurs when, and only when, the intervention is directed at a particular individual or group (Watson & Workman, 1981). If the intervention is efficacious, the design will show that a change in the independent variable, and not some other variable, resulted in a change in the dependent variable relative to baseline. The influence of other variables such as history or self-monitoring can be ruled out by replicating the effect across multiple individuals or groups with differing baseline durations.
Baseline
This condition lasted between 2 and 4 days, depending on the pair. No monetary earnings were arranged during this condition, but participants had access to the “Clearing the Air” guide, as well as the mōtiv8 and Kakao Talk mobile applications. Two breath CO samples were required daily during baseline.
Tapering
This condition lasted 4 days, and monetary consequences could be earned for meeting twice-daily breath CO goals. Personalized goals were set using the individual’s mean breath CO during baseline, such that a constant step-size reduction was identified for each breath CO sample during the eight samples, and the final CO goal was 4 ppm (the criterion for smoking abstinence; e.g., 26 ppm during baseline minus the 4 ppm final goal = 22 ppm divided by 8 samples during tapering = 2.75 ppm rounded to a 3 ppm step-size reduction per sample). Participants could earn $1.50 for meeting their individual goal (independent contingency) and they could each earn a $1.50 bonus when both partners in the pair met their goal (interdependent contingency), for a total of $12.00 during this 4-day condition. All financial earnings could be requested as soon as they were earned, and were delivered in the form of gift cards.
Abstinence induction
During this 10-day condition, an escalating schedule of monetary consequences could be earned contingent on submission of breath CO samples ≤4 ppm (negative samples). For meeting individual breath CO goals, participants could earn $1.50, and for each consecutive negative sample the value increased by $0.25 (individual contingency). Missed or positive samples resulted in a voucher value of $0.00 and the value of the next voucher reset to $1.50. However, voucher values returned to the amount prior to the reset after two consecutive negative samples. If both members of a team met criteria for a given sample, each member of that team earned an additional $3.00 bonus for that sample (interdependent contingency). As with the Tapering condition, all financial earnings could be requested as soon as they were earned, and were delivered in the form of gift cards.
The piCO+ Smokerlyzers were collected at the end of the abstinence induction phase, and an exit interview was conducted. During this exit interview, participants were asked to complete several questionnaires including (1) a Behavioral Change Inventory that asked nine open-ended questions about recent smoking, withdrawal symptoms, and use of smoking cessation aids and other substances; (2) a Treatment Acceptability Questionnaire that asked open-ended questions about what participants liked/disliked about the intervention and questions that utilized a 100 mm visual analog scale (0 = very strongly disagree, 100 = very strongly agree) that participants could use to rate a number of the intervention components, including the mōtiv8 and Kakao Talk applications, earning vouchers, seeing their quantitative progress, as well as their partner’s progress; (3) a Group Environment Questionnaire that was comprised of Likert-scale questions regarding the nature and importance of interactions between the participant and their partner (0 = very strongly disagree, 5 = very strongly agree), as well as questions about motivation for participating in the study; and (4) an External Communications Questionnaire that consisted of multiple-choice questions regarding the nature of interactions between pairs of participants that did not occur via Kakao Talk. The primary contact author for this manuscript (Raiff) can provide copies of questionnaires upon request.
Follow-up
Participants were invited to attend a follow-up appointment 1 month after the abstinence induction phase concluded, during which they were asked to provide one breath CO sample and complete the Behavioral Change Inventory and the External Communications Questionnaire. Regardless of the breath CO level of the sample, each participant earned a $30.00 voucher for attending the session. All vouchers were exchanged for gift cards at locations chosen by the participants.
Results
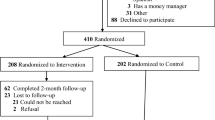
A total of 73 individuals inquired about the study during the recruitment period, but only 31 (42%) completed the screening. Of those who completed the screening, 22 qualified; however, only 16 (52%) were able to identify another smoker who also met all of the criteria. Of the 16 individuals who qualified and knew someone else who qualified, 12 enrolled in the study, for a total of six pairs of participants. One pair of participants dropped out of the study after 7.5 days. Because these two participants did not get to the abstinence induction phase, their data were eliminated from the analyses. Table 1 shows the demographics from the remaining 10 participants. Participants were an average age of 26.2 (range: 19–48), 50% Female, and 70% White. At intake, participants had a mean breath CO of 17 ppm (range: 10–29 ppm), they reported smoking about 9.4 cigarettes per day on average (range: 5–20), they reported smoking daily for an average of about 10 years (range: 2–33 years), and they had an average FTND of 3.4 (range: 2–6). Group 2 withdrew from the study during abstinence induction. Groups 4 and 5 completed the abstinence induction condition but did not complete the exit interview or the 1-month follow-up, whereas Groups 1 and 3 completed all of the conditions. At follow-up, all four participants submitted positive breath CO samples (1A = 10 ppm, 1B = 9 ppm, 3A = 7 ppm, 3B = 8 ppm).
Figure 1 shows the mean and individual participants’ percentages of breath CO samples that were negative for smoking during the baseline (M = 1.25%, SD = 4.0), tapering (M = 13.8%, SD = 18.1), abstinence induction (M = 35.5%, SD = 35.7), and follow-up (M = 0%, SD = 0) conditions. Missed samples, including those from Group 2 participants who withdrew from the study, were labeled positive for smoking. A repeated-measures ANOVA, with Huyn-Feldt corrections, determined that there were significant differences in the mean percentage of breath CO samples across conditions, F(1.6, 14.2) = 6.8, p < .05. Bonferroni post hoc comparisons revealed that the percentage of negative breath CO samples was significantly higher in the abstinence induction condition, compared to the baseline, but not the tapering, condition.
Figure 2 shows all of the breath CO samples submitted by each of the 10 participants during the study. Pairs of participants are labeled with the same number and are organized one above the other in a column (e.g., 1A is above 1B, 2A above 2B, etc). Baseline (A), tapering (B), abstinence induction (C), and follow-up (D) are labeled, and the phase lines are staggered to correspond with the multiple-baseline design. Groups 1 and 2 experienced a 2-day baseline, Groups 3 and 4 experienced a 3-day baseline, and Group 5 experienced a 4-day baseline. Phi coefficient was calculated for data submitted during the abstinence induction phase, and only for periods of active participation for the pair of participants who dropped out before completing this phase, to avoid artificially inflated correlations. The phi coefficient was significant (0.637, p < .05), indicating the presence of a correlation between positive, negative, and missed submissions across pairs of participants. Mean voucher earnings were $47.90 (range: $0–$132.5).
Participants’ individual breath CO levels (in ppm) per sample across all conditions. Sample numbers without a data point represent missed samples. Samples at or below the dashed horizontal line indicates smoking abstinence (<4 ppm). Solid vertical lines represent condition change lines (A = baseline, B = tapering, C = abstinence induction, D = follow-up). Participants are arranged by group, and baseline durations increase from 2-day baseline groups (top) to 6-day baseline groups (bottom)
Compliance with video submissions during baseline, tapering, and abstinence induction conditions ranged between 53% (4B) and 94% (3A and 3B), with overall compliance of 78% (272 of 348 samples) across all 10 participants. Kakao Talk messages ranged from zero to 13 posts per participant (M = 4.7, SD = 4.6; see Table 1). All posts were positive and supportive in nature and included simple responses (e.g., “She’s awesome!!!”) and emojicons (e.g., animated high fives). Because only two groups of participants (Groups 1 and 3, n = 4) completed the exit interview and follow-up sessions, data from the Group Environment, External Communications, and Treatment Acceptability Questionnaires were difficult to interpret and have been excluded.
Discussion
The primary goal of this pilot study was to evaluate the feasibility of recruiting pairs of smokers with a preexisting relationship to quit smoking together. Recruitment and retention, two components of feasibility, were challenging. About 48% of participants who showed an interest in participating, and met our criteria for the study, were unable to identify a qualified partner who was also interested in participating. This was a surprising outcome given the social nature of smoking and the high percentage of people who express a desire to quit. Different strategies may be needed to improve these odds, such as recruiting participants from a common workplace, thereby reducing the burden of finding a partner.
With regard to attrition, of the 12 participants who enrolled in the study, four withdrew and an additional four failed to complete the follow up (66% total), leaving only four participants (33%) to complete all of the study activities. It was noteworthy that a significant phi coefficient was found, suggesting a relationship in the performance among pairs of participants. In other words, if one participant submitted a positive sample for smoking, there was a high likelihood that their partner also submitted a positive sample; if one participant submitted a negative sample, there was a high likelihood that their partner also submitted a negative sample; and if one participant missed a sample, there was a similarly high likelihood that their partner missed the same sample. Furthermore, partner participants withdrew from the study together. This finding is consistent with the idea of clustering among preexisting social networks of smokers described by others (Christakis & Fowler, 2008); however, this is the first study to demonstrate clustering on a day-by-day basis. Previous Internet-based group CM studies did not use the same methods that were used in the current study to examine whether associations between partners’ breath CO samples existed (Dallery, Meredith, Jarvis, & Nuzzo, 2015; Meredith & Dallery, 2013; Meredith et al., 2011). Thus, it is unclear whether such clustering occurred because of the preexisting relationships targeted in the current study, or whether similar clustering might occur in any group-CM intervention. Further research is needed to improve retention among participants who have a preexisting relationship, such as arranging a large bonus to be awarded only if both participants remain in the study until the end.
Nevertheless, among the 10 participants who completed the majority of study activities, the results suggest that the intervention was effective at promoting smoking reductions, with seven participants (70%) reducing their mean breath CO levels by about 50% from baseline during the intervention. The percentage of negative samples increased from a mean of 1% during baseline to 36% during treatment. Group 5 is the only group for whom the intervention appeared to have no effect on breath CO. This pair had amongst the highest income of the five pairs, as well as the highest breath CO levels at intake. The only other participant who had similar intake measures was Participant 4A, who also failed to show an effect of the intervention. Although previous research has not found an effect of income on CM outcomes (Rash, Olmstead, & Petry, 2009), more research is needed in this area. Furthermore, the financial incentives used in this feasibility study were lower than previous studies (Dallery et al., 2013; Dallery et al., 2016), which may have reduced motivation to abstain among higher income participants. Of those participants who completed the 1-month follow-up session, breath CO levels remained lower than they had been during baseline (see Fig. 2), suggesting that there may be at least some short-term maintenance of smoking reduction after the intervention is terminated. However, as noted above, there was a high rate of attrition in the study, with only two groups (i.e., 40% of participants) completing the exit interview and follow-up sessions.
The current study was a systematic replication of Meredith et al. (2011). In that study, 57% of breath CO samples were negative during abstinence induction compared to only 36% in the current study. There are a number of differences between the current study and Meredith et al. (2011) that are worth discussing. First, the most apparent difference, and the one that was targeted in this pilot study, was the social structure of the groups. Meredith et al. (2011) assigned strangers to groups in which their only method of communication was within the context of the intervention. The reason for recruiting smokers in the current study who knew each other outside of the intervention was to harness naturally existing, and more widely accessible, social support, with the goal of behaviorally “trapping” abstinence after the intervention was removed. Unfortunately, this preexisting relationship may have just as easily interfered with abstinence efforts because of the long history between participants regarding smoking as well as the greater ability to communicate without investigator oversight. Participants rarely used the Kakao Talk application to communicate with their partners about smoking abstinence, reporting that their most likely source of communication was face to face (of those who completed the External Communication survey). From a clinical perspective, these face-to-face interactions could be important in helping to promote and maintain abstinence. However, from a research perspective, these unmonitored interactions pose limitations because the nature and content of those interactions are unknown (e.g., we do not observe when two participants attend a party together and one looks at the other and says, “Come on let’s have just ONE cigarette.”). Follow-up studies are needed to investigate the impact of social structure, the nature of interactions inside and outside of the study, and patterns of smoking reduction, abstinence, and relapse among groups of smokers.
Second, the size of the groups in the current study may have impacted the pattern of results. Most of the groups in Meredith et al. (2011) were comprised of three smokers. In the current study, only two participants were assigned to each group due to the difficulty of recruiting more than two smokers who knew each other and met all of the inclusion criteria. Other group CM interventions have had as many as 12 members in the group (Kirby et al., 2008), and to our knowledge the size of the group has not been systematically investigated in the context of group CM interventions. The size of the group may be critically important and is worthy of further investigation.
Third, in Meredith et al. (2011), participants independently contacted researchers because of their own personal interests in quitting smoking, and they were randomly assigned to a group with other similarly qualified participants. In the current study, there was an initial, primary person who showed interest and contacted our research staff about the study, and that person was required to identify a second person they knew who would also want to quit smoking and who would meet all of the inclusion criteria. Although we required all participants to report a desire to quit smoking, it is possible that the latter member of the group was motivated to participate for different reasons than the former member. These differences may have influenced their commitment to quitting, which may have in turn affected relapse or study withdrawal. Thus, as mentioned earlier, in order to reduce attrition it may be necessary to explore different recruitment strategies (e.g., recruiting participants from workplaces where there are existing relationships but where all members volunteer to participate because of their own personal motivation to quit).
Notably, the composition of the groups may also be important to the success of group CM interventions. In the current study, the nature of the relationships was heterogeneous across pairs (see Table 1). Group composition varied by gender (same vs. different), age (similar vs. dissimilar), relationship status (e.g., family, friends, roommates), and smoking history (e.g. heavy smoker vs. light smoker). These and other variables may impact recruitment and retention, as well as treatment outcomes. Indeed, the many variables involved in arranging social contingencies may explain why the literature on the utility of social support for smoking cessation is mixed (Mermelstein, Cohen, Lichtenstein, Baer, & Kamarck, 1986; Murray, Johnston, Dolce, Lee, & O’Hara, 1995; Patten et al., 2012). When Clinical Practice Guidelines (Fiore et al., 2000) were originally published for smoking cessation, social support was a primary component of those recommendations. However, more recently, the role of social support has been minimized (Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff, 2008), and this is likely due to equivocal findings in the scientific literature. The present study tested the feasibility of arranging groups of people with a preexisting relationship; however, future research should be aimed at systematically controlling these sources of variability to identify the most effective arrangement of social support in the context of group CM for smoking cessation.
In conclusion, the current study suggests a number of areas for future research to improve both the feasibility and efficacy of using group contingency management for smoking cessation, whether that be with partners who have a preexisting relationship or not. Future research should investigate novel recruitment strategies and methods for reducing attrition (e.g., workplace recruitment with highly motivated participants, contingent bonuses for study completion) as well as systematically explore whether group composition improves outcomes (e.g., the nature of the relationship, smoking status of group members, size of the group, gender and age composition of the group).
References
Ahmed, J., King, B. A., Neff, L. J., Whitmill, J., Babb, S. D., & Graffunder, C. M. (2016). Current cigarette smoking among adults — United States, 2005–2015. Morbidity and Mortaility Weekly Report (MMWR), 65(44), 1205-1211.
Baer, D. M., & Wolf, M. M. (1967). The entry into natural communities of reinforcement. Washington, DC: Office of Education, U.S. Department of Health, Education, & Welfare.
Bickel, W. K., & Marsch, L. A. (2001). Toward a behavioral economic understanding of drug dependence: Delay discounting processes. Addiction, 96(1), 73–86.
Borrero, J. C., Crsolo, S. S., Tu, Q., Rieland, W. A., Ross, N. A., Francisco, M. T., & Yamamato, K. Y. (2007). An application of the matching law to social dynamics. Journal of Applied Behavior Analysis, 40, 589–601.
CDC. (2016a). Fact sheet: Health effects of cigarette smoking—Smoking & tobacco use. Retrieved from http://www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/effects_cig_smoking/index.htm
CDC. (2016b) Fact sheet: Tobacco-related Mortaility—Smoking and tobacco use. Retrieved from https://www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/tobacco_related_mortality/index.htm
CDC. (2017). Fact sheet: Quitting smoking—smoking & tobacco use. Retrieved from http://www.cdc.gov/tobacco/data_statistics/fact_sheets/cessation/quitting/
Christakis, N. A., & Fowler, J. H. (2008). The collective dynamics of smoking in a large social network. New England Journal of Medicine, 358(21), 2249–2258. doi:10.1056/NEJMsa0706154.
Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. (2008). A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. public health service report. American Journal of Preventive Medicine, 35(2), 158–176. doi:10.1016/j.amepre.2008.04.009.
Dallery, J., Glenn, I. M., & Raiff, B. R. (2007). An internet-based abstinence reinforcement treatment for cigarette smoking. Drug and Alcohol Dependence, 86(2/3), 230–238. doi:10.1016/j.drugalcdep.2006.06.013.
Dallery, J., Meredith, S., Jarvis, B., & Nuzzo, P. A. (2015). Internet-based group contingency management to promote smoking abstinence. Experimental and Clinical Psychopharmacology, 23(3), 176–183. doi:10.1037/pha0000013.
Dallery, J., Raiff, B. R., & Grabinski, M. J. (2013). Internet-based contingency management to promote smoking cessation: A randomized controlled study. Journal of Applied Behavior Analysis, 46(4), 750–764. doi:10.1002/jaba.89.
Dallery, J., Raiff, B. R., Kim, S. J., Marsch, L. A., Stitzer, M., & Grabinski, M. J. (2016). Nationwide access to an Internet-based contingency management intervention to promote smoking cessation: A randomized controlled trial. Addiction. doi:10.1111/add.13715.
Dan, M., Grabinski, M. J., & Raiff, B. R. (2016). Smartphone-based contingency management for smoking cessation with smokers diagnosed with attention-deficit/hyperactivity disorder. Translational Issues in Psychological Science, 2(2), 116–127. doi:10.1037/tps0000062.
Fiore, M. C., Bailey, W. C., Cohen, S. J., Dorfman, S. F., Goldstein, M. G., Gritz, E. R.,…Mecklenburg, R. E. (2000). Treating tobacco use and dependence: Clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services.
Heatherton, T. F., Kozlowski, L. T., Frecker, R. C., & Fagerstrom, K. O. (1991). The fagerström test for nicotine dependence: A revision of the fagerstrom tolerance questionnaire. Addiction, 86(9), 1119–1127.
Herrnstein, R. J. (1970). On the law of effect. Journal of the Experimental Analysis of Behavior, 13, 243–266. doi:10.1901/jeab.1970.13-243.
Hertzberg, J. S., Carpenter, V. L., Kirby, A. C., Calhoun, P. S., Moore, S. D., Dennis, M. F.,…Beckham, J. C. (2013). Mobile contingency management as an adjunctive smoking cessation treatment for smokers with posttraumatic stress disorder. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 15(11), 1934–1938. doi:10.1093/ntr/ntt060
Higgins, S. T., Silverman, K., & Heil, S. H. (Eds.). (2007). Contingency management in substance abuse treatment. New York, NY: Guilford Press.
Kirby, K. C., Kerwin, M. E., Carpenedo, C. M., Rosenwasser, B. J., Gardner, R. S., & Silverman, K. (2008). Interdependent group contingency management for cocaine-dependent methadone maintenance patients. Journal of Applied Behavior Analysis, 41(4), 579–595. doi:10.1901/jaba.2008.41-579.
Madden, G. J., & Bickel, W. K. (Eds.). (2010). Impulsivity: The behavioral and neurological science of discounting. Washington DC: American Psychological Association. doi:10.1037/12069-000
Meredith, S. E., & Dallery, J. (2013). Investigating group contingencies to promote brief abstinence from cigarette smoking. Experimental and Clinical Psychopharmacology, 21(2), 144–154. doi:10.1037/a0031707.
Meredith, S. E., Grabinski, M. J., & Dallery, J. (2011). Internet-based group contingency management to promote abstinence from cigarette smoking: A feasibility study. Drug and Alcohol Dependence, 118(1), 23–30. doi:10.1016/j.drugalcdep.2011.02.012.
Mermelstein, R., Cohen, S., Lichtenstein, E., Baer, J. S., & Kamarck, T. (1986). Social support and smoking cessation and maintenance. Journal of Consulting and Clinical Psychology, 54(4), 447–453. doi:10.1037/0022-006X.54.4.447.
Murray, R. P., Johnston, J. J., Dolce, J. J., Lee, W. W., & O’Hara, P. (1995). Social support for smoking cessation and abstinence: The lung health study. Addictive Behaviors, 20(2), 159–170. doi:10.1016/S0306-4603(99)80001-X.
Ortendahl, M., & Fries, J. F. (2002). Time-related issues with application to health gains and losses. Journal of Clinical Epidemiology, 55(9), 843–848. doi:10.1016/S0895-4356(02)00447-X.
Patten, C. A., Hughes, C. A., Lopez, K. N., Thomas, J. L., Brockman, T. A., Smith, C. M.,…Offord, K. P. (2012). Web-based intervention for adolescent nonsmokers to help parents stop smoking: A pilot feasibility study. Addictive Behaviors, 37(1), 85–91. doi:10.1016/j.addbeh.2011.09.003
Rash, C. J., Olmstead, T. A., & Petry, N. M. (2009). Income does not affect response to contingency management treatments among community substance abuse treatment-seekers. Drug and Alcohol Dependence, 104, 249–253.
Watson, P. J., & Workman, E. A. (1981). The non-concurrent multiple baseline across-individuals design: An extension of the traditional multiple baseline design. Journal of Behavior Therapy and Experimental Psychiatry, 12(3), 257–259. doi:10.1016/0005-7916(81)90055-0.
Acknowledgements
The authors would like to thank Moran Dan and Jaime Pierce for their help with recruiting participants, and Jesse Dallery for his help reviewing an earlier version of this manuscript. This research was supported with start-up funds at Rowan University awarded to the corresponding author.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure of Potential Conflicts of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Research Involving Human Participants
All procedures involving human subjects participation were approved by the Institutional Review Board at the corresponding author’s institution.
Informed Consent
All participants completed the informed consent process prior to completing research activities.
Rights and permissions
About this article
Cite this article
Raiff, B.R., Arena, A., Meredith, S.E. et al. Feasibility of a Mobile Group Financial-Incentives Intervention Among Pairs of Smokers With a Prior Social Relationship. Psychol Rec 67, 231–239 (2017). https://doi.org/10.1007/s40732-017-0238-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40732-017-0238-z