Abstract
Purpose of Review
This article reviews penetrating cervical vascular injuries, with a focus on the initial control, diagnostic workup, and operative or endovascular management. The review highlights the change in management approach from one based on anatomical zones of the neck to the contemporary approach of using physical exam and computed tomographic angiography to guide decision making.
Recent Findings
The approach to penetrating neck injuries has evolved over the past 40 years with a resultant decrease in the rate of non-therapeutic operations, driven primarily by improvements in imaging technology. The role of endovascular techniques is now established for vertebral artery injuries and continues to evolve for non-emergent carotid injuries.
Summary
Penetrating cervical injuries pose a significant risk to life and triage of patients to the operating room or further imaging by computed tomographic angiography (CTA) must occur promptly based on the physical examination. In the operating room, the management of complex venous injuries consists of simple ligation. Vertebral injuries can be temporized with packing or bone wax and then managed with percutaneous endovascular interventions. Carotid injuries may generally be managed with arteriorrhaphy, primary repair, or reconstruction with venous or PTFE graft. In damage control situations, shunting is preferred.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Penetrating neck trauma, particularly when associated with vascular injury, has always captured the attention of trauma providers. The injured neck is uniquely challenging because of the high density of vital structures combined with a relative lack of bony protection. While injuries to the critical and vulnerable structures of the neck can affect airway, breathing, and circulation, this article will focus on the potential vascular injuries. Penetrating neck trauma is relatively common, making up as much as 5–10% of trauma patients [1]. In patients with shock or overt symptoms, greater than 80% may be found to have a clinically significant injury [2•, 3••]. Of those patients found to have significant injury, 70–80% of these involve vasculature of the neck [2•, 3••]. Even in modern, high-volume trauma centers, mortality for penetrating injuries to these vital structures ranges from 1.3–5% [2•, 3••, 4•, 5]. In recent decades, the management of penetrating neck trauma has evolved significantly, driven by improvements in imaging technology and a growing body of experience with selective non-operative management.
Historical Perspective
During the period of time from the American civil war until World War I, little changed in the management of patients with penetrating neck trauma. During this era, patients with neck wounds were frequently managed expectantly and had mortality rates higher than 10% [5].
Improvements in battlefield care and organized transport to field hospitals during World War II shifted the paradigm from non-operative management to surgical treatment. Patients that survived long enough to make it to a surgeon were generally treated with neck exploration [5]. With more modern anesthesia and surgical techniques, surgeons were given the tools to repair these devastating injuries and, as access to surgical care improved, so too did its importance in the care of penetrating neck injuries.
In 1956, Fogelman et al. reported their experience of 100 penetrating neck wounds and showed that those patients undergoing early operation had a mortality of 6% while those undergoing delayed operation had a mortality of 35%. Along with their negative exploration rate of only 5%, this helped justify an era of mandatory neck explorations. Despite these improvements in treatment capability, the advent of more lethal weapons and munitions brought with them mortality rates that reached 15% for patients with penetrating neck injuries during the Vietnam War [5]. Furthermore, subsequent case series suggested that the rate of non-therapeutic exploration with mandatory exploration was close to 50% [6, 7].
In 1979, after the conflict in Vietnam, Roon and Christensen popularized the still common concept of dividing the neck into anatomical zones, using the external location of the wound to guide management [7]. Zone I spans from the sternal notch to the cricoid cartilage; zone II from the cricoid cartilage to the angle of the mandible; and zone III above the angle of the mandible. Because zones I and III are significantly more difficult to explore surgically, it was advocated that all zone II injuries should undergo exploration while other injuries would be investigated with further imaging. Variations of this paradigm continued to be refined and led to a notable decrease in the number of non-therapeutic surgeries [8, 9].
The rapid advancement in cross-sectional imaging has resulted in another major paradigm shift over the last 20 years towards a selective non-operative approach, sometimes referred to as the “no zone” approach. This approach leans heavily on the diagnostic power of multidetector computed tomographic angiography (CTA) and physical exam to guide initial steps. Concurrently, an expanding array of endovascular tools and techniques has allowed more injuries to be managed non-operatively. As the concepts of damage control surgery and vascular shunting have become widely accepted for other injury patterns, the same approaches have been applied to the vascular structures of the neck.
Epidemiology
Penetrating neck trauma is overwhelmingly a young and male disease process with most larger studies showing greater than 80% male predominance with mean and median ages typically in the 20s and 30s [2•, 3••, 4•, 5]. The mortality in modern, larger studies ranges from 1.3–5% with a higher mortality rate reported in studies with a greater proportion of gunshot wounds [2•, 3••, 4•, 5]. Regarding mechanism, stab wounds represent from 50 to 74% of injuries depending on the location of the study population and are likely related to the regional availability of firearms [2•, 3••, 4•, 5, 10•]. Gunshot wounds represent 14–47% of injuries with studies that separate out shotgun wounds demonstrating that these are responsible for 1–10% of all ballistic injuries [2•, 3••, 4•, 5, 10•]. It is important to understand the injury pattern caused by the different mechanisms of penetrating injury. Gunshot wounds are more likely to cause an internal injury in an unexpected location and there appears to be a trend towards higher mortality in studies with a higher percentage of gunshot wounds [2•, 3••, 4•].
Initial Management
The initial management for the patient with a penetrating vascular injury with overt life-threatening bleeding remains the same: stop the bleed. While extremity vascular injuries have benefitted from the expanded use of tourniquets, exsanguination from the neck does not have an equivalent temporary solution.
As in all trauma, initial direct digital pressure at the site of bleeding will often provide at least temporary control of bleeding and allow for further assessment of the source. Initial digital pressure may also help differentiate low-pressure venous bleeding from arterial injuries [11].
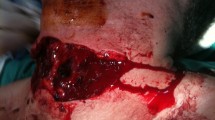
Balloon tamponade is well described for the temporary control of vascular injuries to the carotid. This technique is typically performed by placing one or more Foley or similar balloon catheters into the injury tract and inflating the balloon to achieve hemostasis. This is illustrated in Fig. 1. For peri-clavicular injuries, the catheter may slip into the pleural cavity. If this occurs, it should be inflated then placed on tension to prevent bleeding into the thoracic cavity. A second balloon is then inflated in the wound tract to tamponade the subclavian vascular injury.
Tamponade of vascular injury using a balloon catheter. With permission [11]
A novel tool which may also help provide temporary hemorrhage control is the XSTAT hemostatic device. This consists of multiple compressed, rapidly expanding sponges which are deployed via a large bore syringe-type device directly into the penetrating wound. The XSTAT has been shown to be safe and effective in a small case series [12].
The goal of these temporary hemorrhage control techniques is to stop immediate life-threatening bleeding, allow for rapid assessment for other injuries, give providers time to initiate damage control resuscitation, and enable safe transport to the operating room for definitive hemorrhage control in a better equipped environment.
The “No Zone” Approach
In recent years, the “no zone” approach has become the dominant strategy for establishing next steps in diagnosis and treatment. In this approach, rather than rely upon the anatomical zones, decision making is based principally on whether the patient has hard signs, soft signs, or no signs on physical exam. Vascular hard signs include large or expanding hematomas, severe active bleedings, shock, depressed level of consciousness, and bruits or thrills. Soft signs of vascular injuries include venous oozing and non-expanding or non-pulsatile hematomas [13]. In this approach, all patients with hard signs are taken directly to the operating room for surgical exploration. Those with soft signs undergo further diagnostic testing, usually with CTA given its favorable test characteristics and availability. Asymptomatic patients with no signs of injury to vascular or other structures can be discharged from the hospital or have other concurrent injuries treated [4•, 10•, 14].
This shift in diagnostic approach has been driven partly by studies showing that the location of the external wound often does not predict the location of internal injuries [2•]. At the same time, advances in the resolution and availability of CTA technology have improved the clinician’s ability to discern injuries requiring operative intervention. In a large, prospective multicenter study, CTA was seen to be 100% sensitive and 97.5% specific for detecting clinically significant injuries from penetrating neck trauma [3••]. Several reports have now demonstrated the safety of this approach and its success in decreasing the rate of negative explorations [4•, 10•, 14].
Diagnostic Imaging
Computed Tomographic Angiography
Multidetector computed tomographic angiography has become the gold standard in the initial evaluation of patients with penetrating neck trauma and soft signs who do not require immediate operation. After several series were published in the early 2000s showing the feasibility and safety of CTA for this purpose [15,16,17,18], adoption was rapid and has culminated in several major associations recommending CTA as the imaging modality of choice [8, 13, 19].
CTA has several important advantages over other diagnostic tools. The equipment is available in nearly all modern trauma centers and emergency departments, it evaluates not only vascular structures but also soft tissues and bony structures, it is not operator dependent, and it is fast to perform.
Multiple studies have established the accuracy of CTA for the diagnosis of vascular injuries. In 2006, a single-center prospective trial followed 91 patients with penetrating neck injury who underwent CTA and compared the findings to an aggregate gold standard assembled from all imaging, surgical procedures, and clinical follow-up. CTA had a specificity of 93.5% and a sensitivity of 100%, missing no injuries that required intervention [20]. In 2012, this was followed by a larger, multicenter prospective study that evaluated a clinical protocol integrating CTA as the initial screening examination of penetrating neck wounds. The 225 patients without immediate indication for surgical exploration all underwent CTA and again compared against an aggregate gold standard, achieving a sensitivity of 100% and a specificity of 97.5% in detecting clinically significant injuries. Only 1.8% of scans were non-diagnostic [3••].
Despite these strengths, CTA has some limitations. The images are at risk of degradation from body habitus, patient motion artefact, and streak artefacts from foreign bodies related to the trauma or from other objects such as amalgam dental implants or spinal hardware. In some situations, these may warrant follow-up with conventional angiography or ultrasound as a complementary imaging technique. Finally, CTA requires a timed contrast bolus. This can lead to risks inherent with the contrast, including allergic reactions and contrast-induced nephropathy, and to loss of accuracy because of suboptimal timing of the contrast load. Overall, these risks are rare, causing limitations in approximately 1–2% of scans [3••, 16, 21, 22].
Catheter-Based Angiography
Digital subtraction, four-vessel angiography was long regarded as the gold standard modality to examine the arterial structures in the neck. Numerous studies showed, however, that the rate of positive findings was generally low and thus many patients were arguably being needlessly subjected to an invasive, expensive procedure [16, 23,24,25]. As the speed and accuracy of CTA have improved, however, the role of angiography has been narrowed to that of a follow-up test if CTA is equivocal or when endovascular therapy is being performed. It may still be more sensitive in situations where metallic foreign bodies create streak artefact on CT scan, as may be seen with bullet fragments or shotgun injuries. Because angiography requires arterial access, complications may also occur, albeit rarely [26]. This includes arterial thrombosis and embolization, dissection, spasm, and puncture site hematoma. Given these disadvantages and the more limited assessment of the other structures in the neck, catheter-based angiography has largely been supplanted by CTA [19].
Ultrasonography
Based on its success for carotid occlusive disease, Doppler ultrasonography became popular as an alternative to angiography in the 1990s but has since largely fallen out of practice. Advantages of ultrasound include that it is non-invasive, fast, repeatable, free of ionizing radiation or IV contrast material, portable, and relatively inexpensive. It is able to detect the presence of vascular injury by examining the vessel wall, patency, and flow characteristics [27, 28].
Multiple studies have shown that, when used under optimal conditions, ultrasound is a sensitive test for cervical vascular injury when compared to conventional angiography. In a series of single-center prospective studies, the sensitivity of Doppler ultrasound was 91–100% and the specificity was 100% [27,28,29,30].
The enthusiasm for this modality has been tempered by its significant limitations. This includes the inability to fully assess the intrathoracic and intracranial portions of the carotid and significant portions of the vertebral artery. Like all ultrasound studies, it is operator dependent and not all centers have consistent availability of qualified personnel, especially after business hours. Subcutaneous air, dressings, and bullet or bone fragments can also obscure the views of the vasculature. Finally, ultrasound is inadequate for assessing the aerodigestive, tracheal, esophageal, or spinal structures in the neck [22].
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) can be used to diagnose blunt cervical vascular injuries but their utility in penetrating injuries remains limited [22]. While they demonstrate good test characteristics for evaluation of the spinal cord, ligamentous, laryngeal cartilaginous, and disk injury, magnetic resonance–based modalities are not practical in the early evaluation of trauma. The significant amount of time it takes to perform the scan and the concern for metallic foreign bodies relegate these studies to specific situations after a CTA has already been performed to evaluate the vasculature [19, 31, 32].
Anatomy
In order to manage penetrating cervical vascular injuries, it is important to understand the vascular anatomy of the neck and its relation to external landmarks.
The common carotid (CCA) and its branches are the major anterior arteries and together carry approximately 70% of the total cerebral blood flow [33]. The right CCA originates from the brachiocephalic artery near the right sternoclavicular joint while the left CCA originates from the aortic arch in the superior mediastinum, behind the sternum. The CCAs course superiorly in the carotid sheath which is shared with the internal jugular vein anterolaterally and the vagus nerve posteriorly. Near the level of the superior border of the thyroid cartilage, the CCA bifurcates into the internal carotid (ICA) and external carotid (ECA). The ECA can be differentiated from the ICA by its more medial position, smaller caliber, and presence of extracranial branches, which are notably absent from the ICA. At the angle of the mandible, the hypoglossal nerve and the posterior belly of the digastric muscle cross superficially over both arteries. The ECA progresses distally to terminate in the parotid gland while the ICA courses posteromedially to enter the boney carotid canal [11, 34].
The paired vertebral arteries (VA) are the major posterior vessels of the neck. Anatomically, each VA is divided into four segments: V1 is from the origin at the subclavian artery to the transverse foramen of C6, V2 is from the foramen at C6 to the transverse foramen of C2, V3 is from the foramen of C2 to where it pierces the dura, and V4 is from the dura to the basilar artery. The first segment, V1, can be accessed surgically in a triangle bounded by the clavicle and sternal and clavicular heads of the sternocleidomastoid muscle. The external landmark for the C6 vertebrae, distal to which the VA is protected by boney structures, is the cricoid cartilage [11, 35]. These segments are shown in Fig. 2.
Anatomy of the vertebral arteries. With permission [35]
The internal jugular veins (IJV) are the most significant venous structures of the neck and responsible for the majority of cerebral blood drainage. They originate from the jugular sinus near the base of the skull and course anterolaterally and parallel to the carotid arteries. Near the root of the neck, they join the subclavian veins bilaterally to form the brachiocephalic veins. The facial vein serves as a useful landmark as it joins the IJV at the level of the carotid bifurcation [11, 34]. Ligating the facial vein allows unimpeded access to a large portion of the carotid, as illustrated in Fig. 3.
Intraoperative anatomy of the neck as seen through incision along the anterior border of sternocleidomastoid. Highlighted are the IJV and facial vein overlying carotid bifurcation. With permission [34]
Management
Operative Exposure
Penetrating cervical vascular injuries are best approached with an ipsilateral oblique incision along the anterior border of the sternocleidomastoid muscle. The incision can be extended from the sternum inferiorly to the mastoid superiorly, offering excellent exposure along the length of the carotid and internal jugular veins, facilitating proximal and distal control. If bilateral injuries exist or are suspected, the incision can be mirrored on the contralateral side. For central injuries, especially with a preexisting laceration amenable to extension over the injury, a modified collar incision is another option. After the dissection is carried through the platysma, the sternocleidomastoid can be retracted laterally to expose the internal jugular vein. Ligating the facial vein and omohyoid muscle offers broad exposure to the carotid artery which is posteromedial to the internal jugular vein [28, 29].
The incision is extensible, and for inferior injuries near the junction between the thorax and neck, a median sternotomy may be necessary to achieve proximal control. For injuries to the subclavian arteries proximally, a standard clavicular incision may also be required. Patients with injuries near the base of the skull pose a particularly difficult problem. A number of different approaches have been described to control these injuries including transarterial occlusion with a balloon catheter and mandibulotomy [11, 36].
Venous Injury
Injuries to the veins of the neck are relatively common. Several series have shown that approximately 16% of cervical stab wounds that penetrate the platysma will have injury to the IJV [37,38,39]. Injuries to the IJV may be discovered on imaging or at the time of surgical exploration. In patients with no hard signs mandating operation, internal jugular injuries may be treated non-operatively. In a small prospective series examining this question, eight IJV injuries were identified and treated non-operatively without complications [39]. In patients where a venous injury is discovered in the operating room, the options are either to perform a lateral venorrhaphy, if it can be performed without excessive narrowing of the vessel, or ligation. In situations where there is an injury to the bilateral IJVs, an effort should be made to repair one of them as ligation of both may cause elevated intracranial pressure and facial edema [40]. While this has not been well investigated outside of case reports in the trauma population, oncologic series where patients underwent bilateral radical neck dissections and ligation of their internal jugular veins demonstrated that facial swelling and cyanosis were common, abating within a few days or weeks [41, 42].
Carotid Injury
Approximately 25% of patients with vascular hard signs will have an injury to their carotid [4]. After temporary control is achieved with compression or balloon occlusion, patients should be taken to the OR immediately for neck exploration. Once the injury has been evaluated, a decision can be made whether to perform definitive repair or temporary shunting. The surgical team must consider injury-related factors including size and location of the defect; patient-related factors including physiologic state and injury burden; and resource factors including availability of equipment and experienced personnel [34, 36].
Ligation, while rapid, is generally not recommended outside of very specific situations. A 1978 paper by Liekweg and Greenfield suggested that the only indications for ligation of common or internal carotid artery are in the comatose patient with no prograde flow and when technical reasons make repair impossible [43]. Whenever possible, the carotid artery should be repaired or reconstructed [44].
The technique used to repair a carotid artery is dependent mainly on the extent of injury. Small, lateral injuries may be repaired using non-absorbable sutures. For larger injuries or those that involved the carotid bifurcation, a patch angioplasty with saphenous vein or biologic substitute may be considered. If the injury is a transection or near-transection, the edges may be debrided and an end-to-end anastomosis or insertion of an interposition graft can be performed. Either a synthetic graft (usually polytetrafluoroethylene [PTFE]) or venous graft (usually reversed saphenous vein) can be used. All standard principles of vascular anastomosis should be followed including systemic heparinization if bleeding risk allows, distal and proximal balloon thrombectomy, the use of small-gauge non-absorbable monofilament suture, and normal flushing sequence to decrease the risk of embolism [34, 36].
Temporary shunting has been reported as a damage control technique in penetrating neck injuries. Shunts are fast, can be performed with minimal technical skillset, and require only basic materials. Because of this, they have been used extensively in the combat setting, particularly for extremity trauma. Shunt placement in the carotid artery follows the same general principles as elsewhere in the body. This includes selecting the largest shunt that will fit comfortably within the vessel, evacuation of proximal and distal thrombi, minimizing debridement of the vessel edges, securing the shunt in place with a non-absorbable tie, and evaluating for distal perfusion. A common carotid artery shunt, secured in position with silk sutures, is shown in Fig. 4. Because of the risk for embolism, carotid shunts should be removed and definitive repair performed as soon as possible. In a large multicenter evaluation of temporary intravascular shunts, eight of 213 shunts were placed for carotid injuries. Of these, six were removed within 6 h and all within 48 h. All patients survived without neurologic injury [45].
Temporary vascular shunt after a common carotid transection injury. With permission [34]
Although endovascular techniques have been well described for the treatment of blunt carotid injuries, it remains of limited value in penetrating injury because, if intervention is needed, it generally requires emergent operation. However, technological advances and increasing comfort with these techniques have led to a growing body of evidence demonstrating that endovascular management is a useful tool for very proximal and distal injuries as well as those that do not require immediate operation. Reviews on the topic and groups who have reported their experience support the safety of stenting, balloon occlusion, and embolization for cases of traumatic pseudoaneurysm, dissection, and arteriovenous fistulae [46, 47, 48•]. Herrera et al. reported on 36 patients with penetrating cervical vascular injuries, 80% of which were gunshot wounds, who were treated with an endovascular approach. The majority (66%) had pseudoaneurysms and 98% had an uneventful clinical recovery. [47].
Vertebral Injury
Vertebral artery injuries (VAI) are relatively rare and are difficult to manage surgically because they are protected within the bony canal of the transverse foramina for most of their course. Although surgical approaches have been described for each of the segments of the vertebral artery, they have generally been abandoned in favor of temporary control or endovascular intervention [35, 36].
In patients with soft signs, VAI is usually detected on CTA and can be managed using an endovascular approach. If the lesion can be traversed with a guidewire, a stent may be deployed to exclude it. When this is not technically possible, the vessel can be embolized proximally and distally to achieve hemostasis. Several authors have reported good results with this approach [32, 49, 50].
Injury may also be suspected at the time of operation, usually because of profuse, arterial bleeding coming from the posterolateral neck. Although proximal control at V1 can be achieved surgically, the collateral supply to the vertebral artery via the contralateral vessel and circle of Willis means bleeding is unlikely to stop. Instead, the conventional approach is to use bone wax, gauze packing, balloon occlusion, and temporary neck closure to control the bleeding and allow the patient to go for angiography and endovascular treatment [35, 36]. In one large series of penetrating vertebral artery injuries from South Africa, of 92 patients, 39 had occlusions, 11 had AV fistulae, and 36 had pseudoaneurysms. Most patients were treated successfully with endovascular embolization with an extremely low rate of complications [51].
Conclusion
Penetrating neck trauma with associated vascular injury is a relatively common pathology with potentially devastating consequences. High negative exploration rates and the extremely high sensitivity, specificity, and availability of CTA of the neck have created the modern standard of selective non-operative management of penetrating neck trauma. Patients with penetrating neck injury can be safely classified into three groups with this modern “no zone” management approach. Patients with evidence of shock or hard signs of injury should be taken to the OR for exploration and repair. Patients who are hemodynamically stable with soft signs of injury should be further evaluated with CTA. If a vascular injury is identified on exam or CTA, then it should be explored via a standard oblique neck incision. Venous injuries, if minor, may be repaired. Otherwise, if venous injuries are larger or extensive, they should be ligated. Carotid injuries should be repaired primarily if it will not cause narrowing or patched, if the architecture of the injury is amenable to this. If resection and reconstruction are required, both PTFE and reversed saphenous vein graft are conduit options. Temporary shunting may be of benefit to limit ischemia time and allow for resuscitation in a damage control setting. Carotid ligation should be avoided. Vertebral artery injuries can be challenging and may be best managed with temporizing measures followed by endovascular techniques. Patients with no evidence of injury on CTA or those that are asymptomatic may be safely discharged.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Nason R, Assuras G, Gray P, Lipschitz J, Burns C. Penetrating neck injuries: analysis of experience from a Canadian trauma Centre. Can J Surg. 2001;44(2):122–6.
• Low GM, Inaba K, Chouliaras K, Branco B, Lam L, Benjamin E, et al. The use of the anatomic “zones” of the neck in the assessment of penetrating neck injury. 2014;80(10):970. A study looking critically at traditional neck zones which cast doubt on the validity of using external markings of injury to predict which underlying structures were injured.
•• Inaba K, Branco BC, Menaker J, Alea T, Crane S, DuBose JJ, et al. Evaluation of multidetector computed tomography for penetrating neck injury: a prospective multicenter study. J Trauma Acute Care Surg. 2012;72(3):576 The AAST multi-center trial that most significantly established MDCTA’s superiority as the radiographic method to evaluate penetrating neck injuries. The authors showed this tool to be very sensitive and specific for vascular and aerodigestive structures.
• Ibraheem K, Khan M, Rhee P, Azim A, O’Keeffe T, Tang A, et al. “No zone” approach in penetrating neck trauma reduces unnecessary computed tomography angiography and negative explorations. J Surg Res. 2018;221:113–20 A large, retrospective review of penetrating neck injuries was used to show that the “no zone” approach can decrease rates of negative exploration and that physical exam, not location, should be the principal input to guide management.
Bell BR, Osborn T, Dierks EJ, Potter BE, Long WB. Management of penetrating neck injuries: a new paradigm for civilian trauma. J Oral Maxillofac Surg. 2007;65(4):691–705.
Markey J, Hines J, Nance F. Penetrating neck wounds: a review of 218 cases. Am Surg. 1975;41(2):77–83.
Roon AJ, Christensen N. Evaluation and treatment of penetrating cervical injuries. J Trauma. 1979;19(6):391–7.
Tisherman SA, Bokhari F, Collier B, Cumming J, Ebert J, Holevar M, et al. Clinical practice guideline: penetrating zone II neck trauma. J Trauma. 2008;64(5):1392–405.
Biffl W, Moore E, Rehse D, Offner P, Franciose R, Burch J. Selective management of penetrating neck trauma based on cervical level of injury 1997;174(6):678.
• Prichayudh S, Choadrachata-anun J, Pak-art R, Sriussadaporn S, Kritayakirana K, et al. Selective management of penetrating neck injuries using “no zone” approach. Inj. 2015;46(9):1720 A large series of penetrating neck injuries managed with a “no zone” approach. The authors showed that the there was a low rate of negative exploration and no missed injuries with this updated approach.
Joos E, Inaba K. Neck operations for trauma: general principles. In: Demetriades D, Inaba K, Velmahos G, editors. Atlas of Surgical Techniques in Trauma. Cambridge: 2013. pp. 47–52.
Warriner Z, Lam L, Matsushima K, Benjamin E, Strumwasser A, Demetriades D, et al. Initial evaluation of the efficacy and safety of in-hospital expandable hemostatic minisponge use in penetrating trauma. J Trauma Acute Care Surg. 2018;Publish Ahead of Print:1.
Sperry JL, Moore EE, Coimbra R, Croce M, Davis JW, Karmy-Jones R, et al. Western trauma association critical decisions in trauma. J Trauma Acute Care Surg. 2013;75(6):936–40.
Shiroff A, Gale SC, Martin ND, Marchalik D, Petrov D, Ahmed HM, et al. Penetrating neck trauma: a review of management strategies and discussion of the “No Zone” approach. 2013;79(1):23.
Núñez DB, Torres-León M, Munera F. Vascular injuries of the neck and thoracic inlet: helical CT-angiographic correlation. Radiographics. 2004;24(4):1087–98.
Munera F, Soto JA, Palacio DM, Castañeda J, Morales C, Sanabria A, et al. Penetrating neck injuries: helical CT angiography for initial evaluation. Radiology. 2002;224(2):366–72.
Mazolewski P, Curry J, Browder T, Fildes J. Computed tomographic scan can be used for surgical decision making in zone II penetrating neck injuries. J Trauma. 2001;51(2):315.
Gracias V, Reilly P, Philpott J, Klein W, Lee S, Singer M, et al. Computed tomography in the evaluation of penetrating neck trauma: a preliminary study 2001;136(11):1231.
E, Schroeder JW, Ptak T, Corey AS, Ahmed O, Biffl WL, et al. ACR Appropriateness Criteria® penetrating neck injury. J Am Coll Radiol. 2017;14(11S):S500.
Inaba K, Munera F, McKenney M, Rivas L, de Moya M, Bahouth H, et al. Prospective evaluation of screening multislice helical computed tomographic angiography in the initial evaluation of penetrating neck injuries. J Trauma. 2006;61(1):144–9.
Munera F, Cohn S, Rivas LA. Penetrating injuries of the neck: use of helical computed tomographic angiography 2005;58(2):413.
Steenburg SD, Sliker CW, Shanmuganathan K, Siegel EL. Imaging evaluation of penetrating neck injuries. Radiographics. 2010;30(4):869–86.
Múnera F, Soto JA, Palacio D, Velez SM, Medina E. Diagnosis of arterial injuries caused by penetrating trauma to the neck: comparison of helical CT angiography and conventional angiography. Radiology. 2000;216(2):356–62.
Jarvik J, Philips G, Schwab C, Schwartz J, Grossman R. Penetrating neck trauma: sensitivity of clinical examination and cost-effectiveness of angiography 1995;16(4):647.
Rivers S, Patel Y, Lany H, Veith F. Limited role of arteriography in penetrating neck trauma 1988;8(2):112.
Al-Ameri H, Thomas ML, Yoon A, Mayeda GS, Burstein S, Kloner RA, et al. Complication rate of diagnostic carotid angiography performed by interventional cardiologists. Catheter Cardiovasc Interv. 2009;73(5):661–5.
Ginzburg E, Montalvo B, LeBlang S, Nunez D, Martin L. The use of duplex ultrasonography in penetrating neck trauma 1996;131(7):691.
Fry W, Dort J, Smith R, Sayers D, Morabito D. Duplex scanning replaces arteriography and operative exploration in the diagnosis of potential cervical vascular injury 1994;168(6):693.
Montalvo B, LeBlang S, Nuñez D, Ginzburg E, Klose K, Becerra J, et al. Color Doppler sonography in penetrating injuries of the neck 1996;17(5):943.
Demetriades D, Theodorou D, Cornwell E, Weaver F, Yellin A, Velmahos G, et al. Penetrating injuries of the neck in patients in stable condition. Physical examination, angiography, or color flow Doppler imaging. 1995;130(9):971.
Yaquinto J, Harms S, Siemers P, Flamig D, Griffey R, Foreman M. Arterial injury from penetrating trauma: evaluation with single-acquisition fat-suppressed MR imaging. Am J Roentgenol. 1992;158(3):631–3.
Friedman D, Flanders A, Thomas C, Millar W. Vertebral artery injury after acute cervical spine trauma: rate of occurrence as detected by MR angiography and assessment of clinical consequences. Am J Roentgenol. 1995;164(2):443–7.
Zarrinkoob L, Ambarki K, Wåhlin A, Birgander R, Eklund A, Malm J. Blood flow distribution in cerebral arteries. J Cereb Blood Flow Metab. 2014;35(4):648–54.
Kwon E, Grabo D, Velmahos G. Carotid artery and internal jugular vein injuries. In: Demetriades D, Inaba K, Velmahos G, editors. Atlas of surgical techniques in trauma. Cambridge: 2013. pp. 53–68.
Demetriades D, Nash N. Vertebral artery injuries. In: Demetriades D, Inaba K, Velmahos G, editors. Atlas of surgical techniques in trauma. Cambridge: 2013. pp. 88–93.
Feliciano D, Vercruysse G. Management of specific injuries - neck. In: Mattos K, Moore E, Feliciano D, editors. Trauma. 8th Ediction: 2017. pp. 414–429.
Apffelstaedt J, Müller R. Results of mandatory exploration for penetrating neck trauma. 1994;18(6):917.
Gonzalez RP, Falimirski M, Holevar MR, Turk B. Penetrating zone II neck injury: does dynamic computed tomographic scan contribute to the diagnostic sensitivity of physical examination for surgically significant injury? A prospective blinded study. 2003;54(1):61.
Inaba K, Munera F, McKenney MG, Rivas L, Marecos E, de Moya M, et al. The nonoperative management of penetrating internal jugular vein injury. J Vasc Surg. 2006;43(1):77–80.
McGovern P, Swan K. Management of bilateral internal jugular venous injuries. Elsevier Ltd 1985;16(4):259.
Sakata K, Endo Y, Kimura F, Yamamoto I. Effects of bilateral jugular vein ligation on local cerebral blood flow 1999;9(3):207.
Moore O, Frazell E. Simultaneous bilateral neck dissection: experience with 151 patients. Am J Surg. 1964;107(4):565–8.
Liekweg WG, Greenfield LJ. Management of penetrating carotid arterial injury. Ann Surg. 1978;188(5):587–92.
Demetriades D, Skalkides J, Sofianos C, Melissas J, Franklin J. Carotid artery injuries: experience with 124 cases. J Trauma. 1989;29(1):91–4.
Inaba K, Aksoy H, Seamon MJ, Marks JA, Duchesne J, Schroll R, et al. Multicenter evaluation of temporary intravascular shunt use in vascular trauma. J Trauma Acute Care Surg. 2016;80(3):359–65.
Almazedi B, Lyall H, Bhatnagar P, Kessel D, McPherson S, Patel JV, et al. Endovascular management of extra-cranial supra-aortic vascular injuries. Cardiovasc Intervent Radiol. 2014;37(1):55–68.
Herrera DA, Vargas SA, Dublin AB. Endovascular treatment of penetrating traumatic injuries of the extracranial carotid artery. J Vasc Interv Radiol. 2011;22(1):28–33.
• DuBose J, Recinos G, Teixeira PG, Inaba K, Demetriades D. Endovascular stenting for the treatment of traumatic internal carotid injuries: expanding experience. J Trauma. 2008;65(6):1561 A systematic review of endovascular stenting for carotid injuries demonstrating that, as of 2008, the reported experience was mainly for blunt injuries. Stents were generally deployed safely and successfully with no stent-related mortalities reported.
Lam Y-Y, Tsui H-F, Wong H-L, Chow Y-Y. Management approach of penetrating vertebral artery injury with concomitant cervical nerve root injury in regional hospital: report of two cases. J Orthop Traumatol Rehabil. 2018;25:5–10.
Uchikawa H, Kai Y, Ohmori Y, Kuratsu J-I. Strategy for endovascular coil embolization of a penetrating vertebral artery injury. Surg Neurol Int. 2015;6(1):117.
Mwipatayi B, Jeffery P, Beningfield S, Motale P, Tunnicliffe J, Navsaria P. Management of extra-cranial vertebral artery injuries. Eur J Vasc Endovasc Surg. 2004;27(2):157–62.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Trauma to the Neck
Previous Submissions
None. This manuscript has not and will not be submitted to any other journal while it is under consideration by Current Trauma Reports.
Rights and permissions
About this article
Cite this article
Strickland, M., Roedel, E. & Inaba, K. Penetrating Cervical Vascular Injuries. Curr Trauma Rep 5, 40–47 (2019). https://doi.org/10.1007/s40719-019-0161-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40719-019-0161-7