Abstract
This work investigates the degradation mechanism of Acid Red 17 dye (AR-17) using HPLC-MS/MS as well as it studies the feasibility of applying ozone and ultraviolet to detoxify the wastewater containing AR-17 dye. Different parameters such as pH, initial dye concentration and treatment time were evaluated to obtain the optimal degradation conditions. The initial dye concentration and solution pH were the parameters which affected the color removal and the maximum decolorization was obtained at pH 11. A 100% color removal took place after 25 min of O3/UV treatment (for 100 ppm dye concentration). Kinetic analyses of AR-17 dye removal followed the pseudo-first-order kinetics. When ultraviolet (UV) was applied with ozone simultaneously, the first-order rate constant (kdx[O3]l) increased and the time of dye decolorization shortened to 5.5 min for 500 ppm dye concentration. GC-MS and HPLC-MS/MS studies of pre-treated dye solution which were performed during and at the end of the pre-treatment time, showed complete degradation of the AR-17 dye. The obtained results also demonstrated that the UV-ozone treatment process decreased the zooplankton toxicity of the treated AR-17 dye wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Textile effluents contain extreme amounts of organic fertilizer and inorganic stuff. Wastewater from dye rinsing operations and spent dye baths in the textile industry have unfixed dyes that can be extremely colored and can have detrimental effects on aquatic systems. The latest environmental concerns and guidelines had put stress on textile firms to decrease pollutants and reuse chemicals and water (Mark and Hammer 2011; Reife and Freeman 1996).
The textile industry in Egypt is composed of 31 large public companies. These are divided into 100% spinning, 70% weaving, 40% knitting and 30% finished goods. There are over 3000 private sector manufactures who are members of the Egyptian Textile Manufacturers Federation (ETMF). There are also many small factories and workshops which are not ETMF members, along with informal workers who are not included in any group (EPAP 2003). In general, an extreme amount of the used dyes can be present in manufacturing effluents. The majority of dyes are chemically stable, poisonous and non-biodegradable which make most of the dyes harmful to the ecosystem (Moussavi and Mahmoudi 2009). So, there is a critical need for cost-effective approaches for the elimination of such risky organic pollutants from the industrial wastewaters before discharge (El-Sikaily et al. 2006; Abdelwahab et al. 2006; El Nemr et al. 2008; El Nemr et al. 2009; Khaled et al. 2009; Hassaan et al. 2017a, b; El Nemr et al. 2014).
Azo dyes are commonly employed as reactive dyes (Basiri Parsa et al. 2009). It is assessed that 10 to 15% of the dye mass disappears through the dyeing procedures (Chen et al. 2010). Due to their sluggish degradation rate and high noxiousness, those dyes are categorized as ecologically harmful substances. The conventional approaches for wastewater treatment, for instance, reverse osmosis, bio-treatment, carbon adsorption and ozone oxidation treatments, experience several limits (Kusic et al. 2006; El-Sikaily et al. 2006; Abdelwahab et al. 2006; El Nemr et al. 2008; El Nemr et al. 2009; Khaled et al. 2009; Hassaan et al. 2017b; El Nemr et al. 2014). It has been shown that neither these biological treatments nor the normal chemical methods can result in acceptable amounts of decolorization and chemical oxygen demand (COD) reduction (Chen et al. 2010; El Nemr 2012; Hassaan and El Nemr 2017). The advanced oxidation processes (AOPs) are assumed to be encouraging techniques which treat harmful and noxious organic contaminants in liquid solutions. The production of hydroxyl radicals during AOPs are considered very reactive and are appropriate to oxidize most of the organic compounds existing in wastewater (Kusic et al. 2006). The photoelectrocatalytic degradation of azo dye Acid Red 17 (AR-17) with ammonium persulphate has been reported (Thabet and El-Zomrawy 2016). Ultrasonic enhanced performance of montmorillonite-dodecyltrimethylammonium bromidein decolorization of AR-17 solution through adsorption and sonocatalytic degradation processes has also been reported (Acisli et al. 2016).
Ozonation is one class of advanced oxidation processes (AOPs) which are applied for successful degradation of organic fertilizer chemical compound from effluent. Moreover, it is accepted as an environmentally friendly technique for the elimination of various pollutants such as herbicides, dyes, pesticides and even bio-resistant chemical groups which may persist in some biological treatment methods. O3 has a reduction potential (E°) of 2.07 V (Eq. (1) and O3 is considered as a massive oxidizer of both inorganic and organic compounds (Pillai et al. 2009):
A molecule, which has particular functional groups, for instance C=C bonds and aromatic rings, has the ability to be attacked by ozone and hence create a carbonyl compound (Kurniawan et al. 2006). Ozone also oxidizes the chemicals in two diverse ways, namely indirect and direct (Kishimoto et al. 2005). Moreover, by-products are also formed, and in such circumstances a post-treatment, using activated carbon or biological treatment methods, may be required to remove them (Kishimoto et al. 2005). To obtain better efficiency, some adjustments such as using UV radiation and/or hydrogen peroxide, have also been examined (Javier et al. 2003; Alsheyab and Munaz 2006). The following Eqs. (2) to (5) have been proposed for UV enhanced ozonation treatment (Prat et al. 1990; Shu 2006; Kazemi et al. 2004; Song et al. 2008a, b). The presence of different oxidants in the solution can initiate a synergistic effect which will improve dye degradation in the wastewater.
Regarding Eqs. (2) to (5), when UV was applied to the ozone scheme, the rate of O3 decomposition into hydroxyl radicals raised (Kusic et al. 2006). It has been reported that the major disadvantage of using ozone as oxidizer for dyes is the probability to form toxic by-products (Zhang et al. 2004). Therefore, this makes mandatory the testing of toxicity of the chemical compounds which have been created by the ozonation to determine if the treated effluents are a threat to the aquatic environment at the point of discharge or are innocuous to be reused.
Dye degradation intermediates may be more noxious than the parent dye compounds. Thus, it is highly important to categorize the final degradation compounds produced after the dye degradation process. Liquid chromatography separation techniques coupling with triple quad mass spectrometry (LC-MS/MS) is a good technique established for separation and detection of dyes and their degradation products (Hisaindee et al. 2013). The aim of this study is to investigate the AR-17 dye degradation mechanism using LC-MS/MS technique as well as to study the efficiency of application of O3 and UV/O3 processes for the degradation of the AR-17 dye. The effect of dye concentration, initial pH, kinetic and thermodynamic studies, and toxicity of the treated wastewater when discharged in sea water were also studied. This is the first study which investigates the feasibility of applying ozone (O3) and O3 combined with ultraviolet (UV) to decolorize and detoxify the wastewater containing Acid Red 17 dye. In addition, this work is the first that studies the degradation mechanism of AR-17 dye in the presence of O3 and UV.
2 Materials and Methods
Acid Red 17 was received from ISMA dye company Kafer Eldawar, Egypt (~ 75% of dye content) and was used without further purification. AR-17 chemical structure is shown in Fig. 1. A stock solution of 1000 mg/L of AR-17 was prepared using double distilled water. Other used working aqueous solutions (100, 200, 300, 400 and 500 mg/L) of AR-17 were obtained from the stock solution by dilution to the prerequisite concentration. During the course of this work, dye concentrations in aqueous solution were determined by comparison with known concentration in the visible range of the spectrum. A UV-VIS spectrophotometer (Model No. 45600–02, Cole Parmer Instrument Co., USA) was employed for absorbance measurements. All the experiments were repeated three times for accuracy and only the mean values are reported. The maximum wavelength λmax for AR-17 was determined at 518 nm. Color removal rate of AR-17 was calculated following Eq. (6):
where A0 and At are the initial and the measured absorbances of the samples at different time intervals.
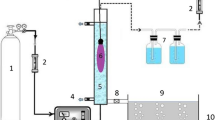
Ozonation took place in a 500 mL glass reactor and all links between the ozonator and the treatment vessels were made of Teflon tubes. AR-17 dye solution (200 mL) of desired concentration (100, 200, 300, 400 and 500 mg/L) was used for each running time. The ozone generator Model N1668A, power 18 W, AC 220 V/50 HZ was used to produce ozone with a flow of 500 mg of O3/h. The ozone which left the glass reactor passed through a glass bottle filled with 2% KI solution. A similar treatment vessel was used for the AOPs (O3/UV) in Horizontal Laminar Flow Cabinet (Bw-LFH1300) with 254 nm UV mercury lamp of 30 W power.
The COD concentration was measured before and after the end of the treatment of AR-17 at concentration 500 mg/L (APHA 2005). The treated AR-17 dye was centrifuged at 10,000 rpm at 20 °C for 10 min. The supernatant was extracted for COD analysis. The same procedures were followed for the untreated samples to gain the initial reading. Furthermore, the supernatant was refluxed with K2Cr2O7, Ag2SO4, HgSO4 and concentrated H2SO4 which was titrated with Ferrous Ammonium Sulfate (FAS) using Ferroin indicator (APHA 2005). COD removal was calculated according to Eq. (7):
The influence of the pH was considered by correcting the reaction mixture to different initial pH (3 to 11) values with 1 M H2SO4 or 1 M NaOH before starting ozonation or UV enhanced O3 processes. The pH measurements were carried out using a JENCO Electronics, LTD pH meter (Model: 6173, Serial No: JC 05345).
GC-Ms analysis was performed using Agilent 7890A GC instrument system linked to single quad 5975C MSD. Extraction of the treated dye solution was performed using benzene as the extractor solvent in the middle and end of the treatment process (USEPA 1996). The extracted solvent was concentrated to 1.0 mL and 1 μL was injected into the GC-MS. An Agilent 7890A GC instrument equipped with a HP5 MS column (30 m × 0.25 mm I’d., 0.25 μm film thickness) was used (USEPA 1996). The injector temperature was 290 °C (splitless) with an initial oven temperature of 90 °C to final oven temperature of 300 °C with a program rate of 8 °C min−1 and stayed 25 min at 300 °C. The carrier gas was He with a column flow of 1.5 mL min−1. The compounds that resulted from the degradation of AR-17 were identified using NIST library connected to the GC-MS.
LC-MS/MS Agilent Technologies 1260 infinity connected with Agilent Technologies 6420A Triple Quad Mass detector and Eclipse Plus C18 column (4.6 × 100 mm, 3.5 μm) was used for degradation mechanism study. Acetonitrile - water with 1% formic acid 50:50 (vol/vol) - was used as mobile phase solvent. The flow rate was 0.5 mL min−1, and 5 μL of sample solution was injected. The mass spectrometer was equipped with an electrospray ionization (ESI) source and operated at negative polarity. The ESI conditions were as follows: capillary voltage, 4.0 kV; the nebulizer pressure, 35 psi; drying gas flow, 10 L min−1; temperature, 325 °C. The mass range was from 90 to 700 m/z.
2.1 Toxicity Assay
The influence of the degradation products of the AR-17 dye on the zooplankton was studied. Rotifer toxicity experiments were made on both the untreated and the 25 min UV enhanced ozonated treated AR-17 dye solutions. The toxicity bioassay was done using rotifer Bruchionus plicatilis and followed standard methods (Standard Methods for the Examination of Water and Wastewater 1985; EPA 1985). Test animals were obtained by hatching cysts, thus eliminating the need for stock cultures. Because the animals which were hatched from the cysts were of similar age, genotype and physiological condition, the test variability was greatly reduced. The controlled hatching of cysts was achieved by transferring to lower salinity, warmer temperature and light. Hatching began and proceeded rapidly after 23 h at 25 °C and 15‰ salinity. By the 28th h, about 90% of the cysts were hatched (Snell and Persooneb 1989). 40 mL of AR-17 dye with concentration (500 mg/L) were completed by 200 mL seawater. Each mL of sea water contained 20 neonates of rotifer Bruchionus plicatilis for treated and untreated samples. The control wells contained the same volume of normal seawater. The number of live and dead neonates in every well was calculated by an inverted trinocular microscope (Nikon Eclipse TS100F) at different time intervals of 0, 15, 30, 45 and 60 min.
3 Results and Discussion
3.1 Effect of pH Value
Wang et al. (2008) mentioned that ozone can mainly decompose to hydroxyl radicals (.OH) under alkaline conditions, which react with the target contaminant. Figure 2a illustrates the color removal of AR-17 dye under different pH values and interval times. The color removal started fast in alkaline condition but after 40 min of ozonation both acidic and alkaline conditions gave almost similar results. Figure 2a shows that the color of the AR-17 dye reflects how it had been reduced from the initial concentration of 100 ppm to less than 30 ppm after the first 5 min at pH 11 after ozone had been provided at 500 mg/h. The highest decolorization rate for AR-17 dye occurred under high alkaline condition (pH 11). This can be attributed to more hydroxyl radical forming at pH 11 (Wang et al. 2008), which is more effective than O3 in the degradation of AR-17. Figure 2b shows the change in the initial pH of AR-17 dye concentration of 500 ppm from 11 to 9.25 resulting in complete color removal. The change in the solution pH from 11 to 9.25 may be attributed to the formation of some organic acid or carbonic acid as a result of dye degradation. In acidic pH, ozone controlled the oxidation process whereas in alkaline pH the hydroxyl radical indicated a more considerable influence (Chu and Ma 2000). As a result of the influence that extra massive reactions of O3 with OH− had on the elevated pH, the stable state concentration of O3 in water decreased while more hydroxyl radicals (.OH) were generated at an elevated pH (Chu and Ma 2000). The above mentioned relationships influence the color removal in the liquid phase in the following two ways: (1) O3 molecule controls the process of oxidation at a lower pH state; and (2) hydroxyl radical (.OH) controls the oxidation process at a higher pH state. It is also worth mentioning that the pH of the solution decreases at the early stage of the experiment, dropping from the initial solution (pH 11 at t = 0 min) to about 9.25 after 1 h of ozonation. Later, the reduction of pH continued slowly until a steady-state was reached. Even though the pH value was falling, it remained still in the alkaline state.
a Color removal of the AR-17 against contact time at different pH (3, 5, 7, 9 and 11) values (200 mL of 100 mg/L dye concentration and 500 mg/h O3); b pH monitoring during ozonation and advanced oxidation processes (O3 = 500 mg/h, treated volume = 200 mL, initial solution pH 11 and 500 mg/L dye concentration)
It has been reported that hydroxyl radicals (.OH) have a higher oxidizing potential and are also less selective than molecular ozone O3 when it comes to attacking the chromophore of dye. This led to a decrease in the rate of decolorization while the rate of mineralization increased at high pH values (Oguz et al. 2006; Beltran 2004; Swaminathan et al. 2005). Thus, the structure of the dye and its solution pH play an important role in the ozonation process and can affect the rate of decolorization by oxidation of hydroxyl radical or ozone, as in the instance of AR-17 where the maximum color removal happened at pH 11. Outcomes from this work revealed that suitable pH regulations might be mandatory after the main oxidation mechanisms were verified.
3.2 Effect of the Initial Dye Concentration
From a practical point of examination, it is important to investigate how the concentration of initial dye influences color removal. Thus, the influence of AR-17 initial concentrations (100–500 mg/L) on color reduction was examined. The effect of AR-17 initial concentration on decolorization efficiency is shown in Fig. 3. The decolorization of AR-17 by UV enhanced ozonation was more efficient than that mentioned by Peternel et al. (2006). The color reduction efficiency using ozonation only for the concentration values of 100, 200, 300, 400 and 500 mg/L in the first 25 min were 97, 91, 87, 78 and 81%, respectively (Fig. 3a). Although the removal efficiency for 500 ppm initial concentration was higher at the first 25 min than that of the 400 ppm concentration, it still took about 30 min more to reach the same removal percentage for 400 ppm (99.85%). By increasing the dye concentration in AOPs, numerous intermediates arose after the parent dye degradation which can interfere with the oxidation process. Such suppression would be more noticeable in the presence of high amount of the degradation intermediate products which had occurred by increasing the dye concentration (Song et al. 2008a, b). Therefore, the time for complete decolorization should be longer for higher initial dye concentrations. Figure 3a shows that the color reduction efficiency for concentration values of 100, 200, 300, 400 and 500 mg/L in the first 25 min were 100, 96, 94, 88 and 86%, respectively, for UV-assisted O3. The color removal efficiency processes were reduced as the initial dye concentration increased due to the stability of the ozone dose. It is seen from Figs. 3a, b that when the dye concentration increased, the color removal percentage was lowered which led to slower complete decolorization. This may be due to the practical conditions of having the ratio of dye molecules to ozone molecules in the solution decreasing while the dye concentration increased (Hassaan et al. 2017b). As mentioned by Sevimli and Kinaci (2002), an increase in the initial dye concentration would cause more ozone to be destroyed.
a Effect of ozone on different AR-17 concentrations (100, 200, 300, 400 and 500 mg/L) versus time (O3 = 500 mg/h, treated volume = 200 mL, initial solution pH 11); b Effect of UV-assisted ozone on different AR-17 concentrations (100, 200, 300, 400 and 500 mg/L) versus time. Ozonation (O3 = 500 mg/h, treated volume = 200 mL, initial solution pH 11)
3.3 Promotion Effects of UV on AR-17 Dye Degradation
Figure 3 shows that, in the case of UV/O3 processes rather than O3 alone, the time needed for the complete color removal of AR-17 dye decreased by 15, 45, 45, 80 and 60 min for the respective dye concentration values of 100, 200, 300, 400 and 500 mg/L. This reveals a better performance of dye degradation by UV/O3 process in comparison to that of the ozone alone. UV enhanced O3, when compared to the ozone alone, through the production of more hydroxyl radicals, was found to increase the efficiency of color removal. The ozone is available at acidic pH as molecular O3, while at basic pH, the ozone decomposes to secondary oxidants such as hydroxyl radical which is considered to be the most important one produced (Yasar et al. 2007). The results obtained in this research work for O3 and UV/O3 were much better than those obtained by photoelectrochemical degradation of AR-17 reported by Thabet and El-Zomrawy (2016), i.e., maximum removal for AR-17 of 60% at pH 2. Also, the AOP results presented in this research work were much better than those reported by Acisli et al. (2016) for ultrasound (US) assisted decolorization of AR-17 dye, where the maximum removal was 70% in 30 min time for 100 mg/L concentration of AR-17 dye.
3.4 COD Analysis
COD analysis is used to indirectly measure the amount of organic compounds in the wastewater. After 2 h and 50 min of AOPs and complete color removal, more than 72% of the initial COD concentration was eliminated for 500 mg/L AR-17 initial concentration (Eq. 8). Similarly to Zhao et al. (2004), the ozonation of dyes commonly produces little organic molecular fragments (e.g., ketones, epoxides, aldehydes, etc.) which may be the reason of the remaining COD measured. This remaining COD due to the newly produced compounds can be decreased by biological treatment methods (Lin and Lin 1993).
3.5 Kinetics of Acid Red 17 Dye
The kinetic study of AR-17 dye removal process was studied to evaluate the treatment method. The direct reaction of ozone with the dye is supposed to follow pseudo-first-order kinetics in case of the presence of an excess amount of ozone. During this work, the ozonation kinetics of AR-17 was estimated by drawing Ln(C t /C0) readings against the time of reaction using Eq. (9):
where Ct is the concentration of AR-17 dye with reaction time t during the ozonation; C0 is the initial concentration of AR-17 dye; and kdx[O3]l is the kinetic constant. Figure 4a, b illustrates the plot between Ln(Ct/C0) versus time (min) of the removal of AR-17 dye concentration using ozonation and UV-assisted ozonation. It was noticed that the first-order rate constant (kdx[O3]l) decreased when the initial AR-17 concentration increased in both the ozonation process and AOPs (Fig. 4). The first-order performance was also seen through other ozonation and UV-assisted O3 process studies of azo-dyes (Wu and Wang 2001; Kusvuran et al. 2011; Zhang et al. 2006). A series of kdx[O3]l shown in Table 1 (from 0.304 to 0.048 min−1) remained higher than those (from 0.130 to 0.035 min−1) attained in the ozonation processes.
According to the dynamics theory, t1/2 represents the time of AR-17 decolorized to its half of initial concentration. For the first-order reaction, the readings of t1/2 could be calculated as in following Eq. (10):
Comparing AOPs and ozonation process alone, t1/2 was shortened to 5.5 min at the initial concentration of 500 mg/L and the whole reaction time was decreased by 28% (Table 1). As previously mentioned, the presence or absence of UV radiation hardly affects the decolorization rate (Shu and Chang 2005).
3.6 Identification of the Intermediate by-Products of AR-17 during the AOP
It is desirable that the ozonation-decomposed final products be safe, but the intermediate and end products after ozonation of dyes could have poisonous influences (Vanhulle et al. 2008; Selçuk et al. 2006; Shang et al. 2002). Therefore, ecotoxicological assessment and/or identification of such organic intermediate and final products have to be studied to prevent problems which may provide information on further successive biological treatment. As reported (Kralicek 1995; Pielesz et al. 2002; Alvares et al. 2001), a lot of earlier data indicated that the intermediate and end products of ozonation relied on the chemical structure of the dye’s parent compounds in addition to the ozonation circumstances.
Figure 5a, b show the GC chromatograms of ozonated textile wastewater (pH 9.25) after 25 and 55 min. Figure 5a, b explain that the AOPs considerably lowered the substances of the organic textile wastewater extracts. A primary analysis of the AOPs synthetic wastewater samples displayed a peak at a retention time of 6.016 min. It was recognized using NIST library as being 2-ethyl-1-hexanol. Other peaks were at retention times of 6.137 min (1-methyl-4-(1-methylethenyl)-cyclohexene) and 10.705 min (1,4-naphthalenedione). The peak at retention time 11.745 min is for compound named 1a,7a–dihydro-naphth[2,3-b]oxirene-2,7-dione, as well as the peak at retention time 12.421 min is for diethylphthalate compound and peak at retention time 15.782 min is for hexadecanoic methyl ester compound (Fig. 5a).
Ozone, Ozone-UV and/or the free radicals break the double bonds (–N=N– and –C=C–) in the dye molecule and discoloration is achieved through the electrophilic cleavage of dye chromophoric groups (Khadhraoui et al. 2009). Certain aromatic compounds may react more slowly with ozone during the ozonation process and may need longer treatment times to be degraded (Somensi et al. 2010). During this work, we extended the time of ozonation for another 30 min and we noticed that it was very useful in the degradation of some intermediate compounds. We could only see the absorption peaks of dibutylphthalate (retention time 16.348 min) and diisooctyl-phthalate (retention time 22.556 min) (Fig. 5b). We noticed that most of the fragmentation of AR-17 dye degradation (phenol or other aromatic amines) almost disappeared and the concentration of the alkenes and phethlate compounds which had appeared were very low at ng/L. It was also well-defined that ozonation notably reduced the contents of the organic synthetic wastewater extracts.
3.7 LC-MS/MS Mechanistic Study
The HPLC-MS/MS analysis of water solution of AR-17 dye showed the presence of one peak at retention time of 2.540 with m/z 456.90 (M – H)− and 478.9 (MNa – H)−, which is attributed to the presence of free acid and mono sodium salt of AR-17 dye (Fig. 6). The AR-17 dye decolorization process was analyzed by HPLC-MS/MS at interval times of 15, 30, 45, 60, 120, 160, and 230 min (Fig. 7). The analysis suggested a sequenced oxidation mechanism, in which the O3 and or hydroxyl radical preferentially attacked the chromophore centers of the dye molecules (–N=N–) cleaving them into the lateral substituted naphthalene ring. Azo groups were proposed to be attacked at two positions, namely the C–N single bond between the azo group, and the naphthalene ring, generating N2 gas. The species produced by the cleavage of the azo bond of the dye molecule were the primary reaction intermediates. The compounds were further degraded to give some of the lower-molecular-weight products via a series of reactions. The ring opening of aromatic intermediates led to the formation of organic acids. In this case, smaller aromatic intermediates (e.g., phthalic acid, carboxylic acid derivatives and aliphatic acids as reported in GC-MS analysis). After 15 min of treatment, the peaks of AR-17 dye disappeared and three new peaks appeared: a small peak at retention time 1.554 min with m/z 315.8 and a large peak at retention time 2.020 min with m/z 228.9, and a third peak which appeared at retention time 2.253 min with m/z 232.0 and 228.80. With the increase of the time of treatment many peaks appeared with different m/z values, as presented in Fig. 8, and at the end of the treatment most of the peaks disappeared which reflected the complete mineralization of the treated AR-17 dye. The monitoring of the AR-17 dye after 230 min of treatment showed complete disappearance of the above fragments and no aromatic amine or phenol were detected. The appearance of new minor peaks and disappearance of the major peak in the decolorized dye product elution profile support the biodegradation of AR-17 dye (Fig. 7).
Using this approach, we were able to identify the molecular mass of ten different intermediates produced during AR-17 dye degradation using AOP. The structures of these intermediates were confirmed with product ions study of LC-MS/MS fragmentation. Figure 8 shows a representative intermediate product of the AR-17 dye degradation. It is also interesting to note that the resulting intermediates produced during AR-17 degradation are aromatic compounds, however, they are quickly degraded to smaller and less-compounds which may eventually be mineralized to CO2, H2SO4 and ammonium ions.
3.8 Toxicity Test
3.8.1 Bioassay on Rotifer B. plicatilis
The toxicity rates were evaluated by examining the mortality numbers of rotifer B. plicatilis (Ogunjobi et al. 2012; Furlan et al. 2010). The mortality rates in ozone and UV treated 100 mg/L of AR-17 dye were close to 0, 0, 0, 2 and 5% after 0, 15, 30, 45 and 60 min, respectively (Fig. 9). While the raw dye of alike concentrations exhibited 0, 0, 5, 10 and 20%, respectively. It is also worth mentioning the treated and untreated samples were left for 24 h and the mortality percent still remained the same at levels 20 and 5% for untreated and treated samples, respectively. Figure 9 shows that there were not any mortality rates for the control samples at the first hour.
The mortality levels of untreated dye solution were higher than those of the treated dye effluent. Therefore, the treated dye effluents were less harmful to the aquatic organisms and could be released to the marine ecosystem with more safety. The mortality test obviously revealed that the AOPs had a positive outcome on zooplankton toxicity of the AR-17 untreated solution.
3.9 Application of Ozone and UV-Assisted Ozone on Seawater
The seawater sample was taken in the front of Kayet Bey Castle at Alexandria, Egypt (31°12′49.3”N, 29°53′03.2″E). Physical and chemical parameters were measured and are presented in Table 2.
Stock solution of 1000 mg/L of AR-17 were prepared using seawater and other working aqueous solutions (100, 200, 300, 400 and 500 mg/L) of AR-17 were obtained from the fresh prepared stock solution by dilution in seawater to the required concentration. The experiment was run under the same conditions stated in Table 2 to simulate the discharge of industrial dye wastewaters in coastal areas.
3.9.1 Effect of Initial Dye Concentration
The effect of initial AR-17 (with different concentrations of 100 to 500 mg/L) on color removal from seawater was examined. The effect of AR-17 initial concentration on decolorization efficiency is shown in Figure 10a, b, which show the color removal efficiency of AR-17 in several initial concentration values. The color removal efficiencies for O3 only and for initial concentration values of 100, 200, 300, 400 and 500 mg/L in the first 25 min were 99.6, 92, 84, 87.5 and 88%, respectively (Fig. 10a), which indicates similar action as in freshwater. Figure 10b shows the color removal efficiencies for UV-assisted O3 treatment for initial concentration values of 100, 200, 300, 400 and 500 mg/L, which in the first 25 min were 100, 83.8, 82.5, 76.5 and 65.7%, respectively. Also, it is worth mentioning, as shown in Fig. 10a, b that by increasing the dye concentration in the same concentration levels used above, the decolorization rate decreased and the complete removal of color was attained only slower. Figure 10a, b clearly show that the ozone was more effective than the UV-assisted O3, especially at higher concentrations (200, 300, 400 and 500 mg/L) and after the first 25 min, which was the time needed for decolorization of 100 mg/L concentration using O3 and UV-assisted O3. This result can be explained bearing in mind the scavenging effect of Cl− ion on hydroxyl radicals (.OH), as Cl− ion can consume OH and O3 via serial groups of chemical reaction (Muthukumar and Selvakumar 2005). Sodium chloride and sulfate disrupt the degradation process because of producing a soluble solid in the solution that interferes with ozone–dye reaction (Tehrani et al. 2008). In addition, the negative effect of sodium chloride on decolorization of AR-17 dye in ozonation process is notable in the reduction of ozone decomposition and in the scavenging of hydroxyl radicals by chloride ions (Moussavi et al. 2009). Carbonate and bicarbonate salts are also radical scavengers (Basiri Parsa et al. 2009). Another important reason is the pH (7.75) of the seawater which is not high enough to stimulate the decomposition of O3 or to produce radical. OH in comparison to the pH (11), which was used in the treatment of the freshwater and made the UV-assisted O3 process more efficient than O3 alone (Mallevialle 1982; Muthukumar and Selvakumar 2005).
a Effect of ozone on different AR-17 concentrations (100, 200, 300, 400 and 500 mg/L) versus time. (O3 = 500 mg/h, treated volume = 200 mL, initial solution pH 7.75); b Effect UV-assisted ozone on different AR-17 concentrations (100, 200, 300, 400 and 500 mg/L) versus time. Ozonation (O3 = 500 mg/h, treated volume = 200 mL, initial solution pH 7.75)
As mentioned above, in freshwater treatment, the appropriate pH control might be required after the major oxidation mechanisms are determined. The treatment in seawater is running under slightly alkaline pH which is not high enough to produce hydroxyl radicals using O3 or UV-assisted O3. The presence of salt in the treated solution affected the time required to complete color removal, as the higher the salt content (38.7 ppt) the longer the required time (Muthukumar and Selvakumar 2005). This could be an alternative reaction pathway that depends on UV-assisted O3 reaction that results in extra resistant intermediate products to further oxidation.
It is worth mentioning that it is hard to compare the results of the required time for treatment experiments of color removal in freshwater and in seawater. The absorbances of AR-17 in freshwater and seawater for 100 mg/L concentration were 2.13 and 0.240, respectively. This big difference may be due to the precipitation of the dyes after their preparation in seawater. For this, fresh dye solution of seawater was always prepared. But even with this big difference between the two initial absorbancies, we can notice that the decolorization time for AR-17 behaved in the same way as for UV-assisted O3 in both fresh and seawater after the first 25 min. This was due to the high salt content and the long time required for complete decolorization; these results are in agreement with Khadhraoui et al. (2009).
4 Conclusions
The efficiency of using ozone and the combination of ozone and UV to decolorize and decontaminate an aqueous solution of AR-17 was examined. Regarding the results obtained, O3 treatment demonstrated to be very sufficient in the ultimate removal of color with partial reduction of COD. The enhanced kdx[O3]l indicated that AOP technique was able to shorten T1/2 and reduce the required time for decolorization and detoxification of the dye effluents. The AR-17 dye oxidation process followed the pseudo-first-order kinetics. Color removal efficiency was higher in the case of UV and O3 combination when compared to ozone alone (Wang et al. 2008). HPLC-MS/MS and GC-MS analyses were used to identify AOP treatment process by-products as well as to evaluate the degradation of the dye solution. The degradation mechanism was studied using HPLC-MS/MS analysis during and after the treatment was completed. The toxicity test results also demonstrated that the AOP reduced the zooplankton toxicity of the treated dye wastewater.
References
Abdelwahab O, El Nemr A, El-Sikaily A, Khaled A (2006) Biosorption of direct yellow 12 from aqueous solution by marine green algae Ulva Lactuca. Chem Ecol 22(4):253–266
Acisli O, Khataee A, Karaca S, Sheydaei M (2016) Modification of nanosized natural montmorillonite for ultrasound enhanced adsorption of acid red 17. Ultras Sonochem 31:116–121
Alsheyab MA, Munaz AH (2006) Reducing the formation of THMS by ozone combined with hydrogen peroxide. J Desalination 194:121–126
Alvares ABC, Diaper C, Parsons SA (2001) Partial oxidation by ozone to remove recalcitrance from wastewaters. Environ Technol 22:409–427
APHA, AWWA, WEF (2005) Standard Methods for the Examination of Water and Wastewater. 21st ed., Washington DC. https://trove.nla.gov.au/version/45704677. Accessed 2 Feb 2018
Basiri Parsa J, Rezaei M, Soleymani AR (2009) Electrochemical oxidation of an azo dye in aqueous media investigation of operational parameters and kinetics. J Hazard Mater 168:997–1003
Beltran FJ (2004) Ozone reaction kinetics for water and wastewater systems. Levis Pub. Imprint of CRC Press LLC, Florida
Chen HW, Kuo YL, Chiou CS, You SW, Ma CM, Chang CT (2010) Mineralization of reactive black 5 in aqueous solution by ozone/H2O2 in the presence of magnetic catalyst. J Hazard Mater 174:795–800
Chu W, Ma CW (2000) Quantitative prediction of direct and indirect dye ozonation kinetics. Water Res 34:3153–3160
El Nemr A (2012) Non-conventional textile waste water treatment. Nova science publishers, Inc. Hauppauge, New York
El Nemr A, Abdelwahab O, Khaled A, El-Sikaily A (2009) Removal of direct blue-86 from aqueous solution by new activated carbon developed from orange peel. J Hazard Mater 161:102–110
El Nemr A, El Sadaawy M, Khaled A, El Sikaily A (2014) Adsorption of the anionic dye direct red 23 onto new activated carbons developed from Cynara Cardunculus: kinetics, equilibrium and thermodynamics. Blue Biotechnol J 3(1):121–142
El Nemr A, Khaled A, Abdelwahab O, El-Sikaily A (2008) Treatment of artificial textile dye effluent containing direct yellow 12 by orange peel carbon. Desalination 238:210–232
El-Sikaily A, El Nemr A, Khaled A, Abdelwehab O (2006) Removal of methylene blue from aqueous solution by marine green alga Ulva lactuca. Chem Ecol 22(2):149–157
EPA (1985) Methods for measuring the acute toxicity of effluents to freshwater and marine organisms. 216 pp.
EPAP/ EEAA, (2003) The Egyptian Pollution Abatement Project and Egyptian Environmental Affiars Agency, (Self-Monitoring Manual for textile industry report)
Furlan FR, Da Silva LG, Morgado AF, De Souza AAU, Guelli UD (2010) Removal of reactive dyes from aqueous solutions using combined coagulation/flocculation and adsorption on activated carbon. Resour Conserv Recy 54:283–290
Hassaan MA, El Nemr A (2017) Advanced oxidation processes for textile wastewater treatment. Inter J Photochem Photobiol 2(3):85–93
Hassaan MA, El Nemr A, Madkour FF (2017a) Testing the advanced oxidation processes on the degradation of direct blue 86 dye in wastewater. Egypt J Aquat Res 43:11–19
Hassaan MA, El Nemr A, Madkour FF (2017b) Advanced oxidation processes of mordant violet 40 dye in freshwater and seawater. Egypt J Aquat Res 43:1–9
Hisaindee S, Meetani MA, Rauf MA (2013) Application of LC-MS to the analysis of advanced oxidation process (AOP) degradation of dye products and reaction mechanisms. Trends Anal Chem 49:31–44
Javier RF, Beltran F, Gimeno O, Acedo B, Carvalho F (2003) Stabilized leachates: ozone activated carbon treatment and kinetics. J Water Res 37:4823–4834
Kazemi M, Soltan MJS, Khoshfetrat AB, Kaynejad MA (2004) Decolorization of RR- 120 dye using ozone and ozone/UV in a semi-batch reactor. Can J Chem Eng 82:1284–1288
Khadhraoui M, Trabelsi H, Ksibi M, Bouguerra S, Elleuch B (2009) Discoloration and detoxicification of a Congo red dye solution by means of ozone treatment for a possible water reuse. J Hazard Mater 161:974–981
Khaled A, El Nemr A, El Sikaily A, Abdelwahab O (2009) Adsorption isotherm and kinetics of direct Navy Blue-106 removal from artificial textile dye effluent using activated carbon from orange peel. J Hazard Mater 165:100–110
Kishimoto N, Morita Y, Tsuno H, Oomura T, Mizutani H (2005) Advanced oxidation effect of ozonation combined with electrolysis. J Water Res 39:4661–4672
Kralicek P (1995) Detection of carcinogenic amines that can be released from certain azoic dyes. Chimia 49:222–225
Kurniawan TA, Lo WH, Chan GYS (2006) Radicals-catalyzed oxidation reactions for degradation of recalcitrant compounds from landfill leachate. J Chem Eng 125:35–57
Kusic H, Koprivanac N, Bozic AL (2006) Minimization of organic pollutant content in aqueous solution by means of AOPs. UV and ozone based technologies. J Chem Eng 123:127–137
Kusvuran E, Gulnaz O, Samil A, Yildirim O (2011) Decolorization of malachite green, decolorization kinetics and stoichiometry of ozone-malachite green and removal of antibacterial activity with ozonation processes. J Hazard Mater 186(1):133–143
Lin SH, Lin CM (1993) Treatment of textile wastewater by ozonation and chemical coagulation. Water Res 27:1743–1748
Mallevialle J (1982) Ozonation manual for water and wastewater treatment, Vol. 53. John Wiley and Sons, New York
Mark J, Hammer S (2011) Water and wastewater technology, 7th edn. Prentice Hall, New Jercy, 528 pages
Moussavi GM, Khavanin A, Alizadeh R (2009) The investigation of catalytic ozonation and integrated catalytic ozonation/biological processes for the removal of phenol from saline wastewaters. J Hazard Mater 171:171–175
Moussavi G, Mahmoudi M (2009) Degradation and biodegradability improvement of the reactive red 198 azo dye using catalytic ozonation with MgO nanocrystals. Chem Eng J 152(1):1–7
Muthukumar M, Selvakumar N (2005) Discoloration of acid dye effluent with ozone: effect of pH, salt concentration and treatment time. Coloration Technol 121:7–12
Ogunjobi AA, Ademola EA, Sanuth HA (2012) Toxicity and bacterial decolourization of textile dyes. Elec J Env Agricult Food Chem 11:415–423
Oguz E, Keskinler B, Celik C, Celik Z (2006) Determination of the optimum conditions in the removal of bomaplex red CR-L dye from the textile wastewater using O3, H2O2, HCO3 − and PAC. J Hazard Mater 131:66–72
Peternel I, Koprivanac N, Kusic H (2006) UV-based processes for reactive azo dye mineralization. Water Res 40:525–532
Pielesz A, Baranowska I, Rybak A, Wlochowicz A (2002) Detection and determination of aromatic amines as products of reductive splitting from selected azo dyes. Ecotoxicol Environ Safety 53:42–47
Pillai KC, Kwon TO, Shik MI (2009) Degradation of wastewater from terephthalic acid manufacturing process by ozonation catalyzed with Fe2+, H2O2 and UV light: direct versus indirect ozonation reactions. J Appl Catal B–Environ 91:319–328
Prat C, Vicente M, Esplugas S (1990) Ozone andozone/UV decolorization of bleaching waters of the paper industry. Ind Eng Chem Res 29:349–355
Reife A, Freeman HS (1996) Environmental chemistry of dyes and pigments. John Wiley & Sons, Inc., New York, pp 43–60
Selçuk H, Eremektar G, Meriç S (2006) The effect of pre-ozone oxidation on acute toxicity and inert soluble COD fractions of a textile finishing industry wastewater. J Hazard Mater 37:254–260
Sevimli MF, Kinaci C (2002) Decolorization of textile wastewater by ozonation and Fenton's process. Water Sci Technol 45:279–286
Shang NC, Yu YH, Ma HW (2002) Variation of toxicity during the ozonation of monochlorophenolic solutions. J Environ Sci Health 37A:261–271
Shu HY (2006) Degradation of dyehouse effluent containing C.I. Direct blue 199 by processes of ozonation. UV/H2O2 and in sequence of ozonation with UV/H2O2. J Hazard Mater 133:92–98
Shu HY, Chang MC (2005) Decolorization effects of six azo dyes by O3, UV/O3 and UV/H2O2 processes. Dyes Pigments 65:25–31
Snell TW, Persooneb G (1989) Acute toxicity bioassays using rotifers. I. A test for brackish and marine environments with Brachionu splicatilis. Aquat Toxicol 14:65–80
Somensi CA, Simionatto EL, Bertoli SL, Wisniewski A Jr, Radetski CM (2010) Use of ozone in a pilot-scale plant for textile wastewater pre-treatment: Physico-chemical efficiency, degradation by-products identification and environmental toxicity of treated wastewater. J Hazard Mater 175:235–240
Song S, Xu X, Xu L, He Z, Ying H, Chen J, Yan B (2008a) Mineralization of CI reactive yellow 145 in aqueous solution by ultraviolet-enhanced ozonation. Ind Eng Chem Res 47:1386–1391
Song S, Xu X, Xu L, He Z, Ying H, Chen J, Yan B (2008b) Mineralization of CI reactive yellow 145 in aqueous solution by ultraviolet-enhanced ozonation. Ind Eng Chem Res 47:1386–1391
Standard Methods for the Examination of Water and Wastewater (1985) American Public Health Association. Washington DC:689–823. https://trove.nla.gov.au/version/4081157. Accessed 2 Feb 2018
Swaminathan K, Pachhade K, Sandhya S (2005) Decomposition of a dye intermediate, (H-acid) 1 amino-8-naphthol-3, 6 disulfonic acid in aqueous solution by ozonation. Desalin 186:155–164
Tehrani AR, Mahmoodi NM, Arami M (2008) Study of the efficiency of effective parameters on decolorization of CI. Reactive black 5 wastewater by ozonation. J Color Sci Technol 2:67–75
Thabet M, El-Zomrawy AA (2016) Degradation of acid red 17 dye with ammonium persulphate in acidic solution using photoelectrocatalytic methods. Arab J Chem 9:S204–S208
USEPA (1996) Test methods for evaluating solid waste. SW-846, USEPA Office of Solid Waste and Emergency Response, Washington, DC
Vanhulle S, Trovaslet M, Enaud E, Lucas M, Taghavi S, Van DLD, Van AB, Foret M, Onderwater RCA, Wesenberg D, Agathos SN, Schneider YJ, Corbisier AM (2008) Decolorization, cytotoxicity, and genotoxicity reduction during a combined ozonation/fungal treatment of dye-contaminated wastewater. Environ Sci Technol 42:584–589
Wang XJ, Chen SL, Gu XY, Wang KY, Qian YZ (2008) Biological aerated filter treated textile washing wastewater for reuse after ozonation pretreatment. Water Sci Technol 58(4):919–923
Wu J, Wang T (2001) Ozonation of aqueous azo dye in a semi-batch reactor. Water Res 35:1093–1099
Yasar A, Ahmad N, Amanat AKA, Yousaf A (2007) Decolorization of blue CL-BR dye by AOPs using bleach wastewater as source of H2O2. J Environ Sci 19:1183–1188
Zhang F, Yediler A, Liang X, Kettrup A (2004) Effect of dyes additives on the ozonation process and oxidation by-products: a comparative study using hydrolyzed C.I. Reactive red 120. Dyes Pigments 60:1–7
Zhang H, Duan L, Zhang D (2006) Decolorization of methyl orange by ozonation in combination with ultrasonic irradiation. J Hazard Mater 138(1):53–59
Zhao W, Shi H, Wang D (2004) Ozonation of cationic red X-GRL in aqueous solution: degradation and mechanism. Chemosphere 57:1189–1199
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El Nemr, A., Hassaan, M.A. & Madkour, F.F. Advanced Oxidation Process (AOP) for Detoxification of Acid Red 17 Dye Solution and Degradation Mechanism. Environ. Process. 5, 95–113 (2018). https://doi.org/10.1007/s40710-018-0284-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40710-018-0284-9