Abstract
Stable isotopes of H and O are the integral parts of water molecule and serve as ideal tracers to understand the recharge processes in groundwater. Hence, a study has been conducted in hard rock aquifers of Madurai District of Tamilnadu to identify the recharge processes using stable isotopes. A total of 54 groundwater samples were collected representing the entire district from various lithounits during post monsoon. Samples were analysed for pH, EC, Ca2+, Mg2+, Na+, K+, Cl− HCO3 −, SO4 2−, PO4 3−, H4SiO4, F−, δ18O and δD. Cl− and HCO3 − were the dominant ions in groundwater samples. Average values of Cl− and HCO3 − ranged from 247 and 244 mg/L in fissile hornblende biotite gneiss, 262 and 268 mg/L in Charnockite, 75 and 185 mg/L in quartzite, 323 and 305 mg/L in granite, 524 and 253 mg/L in floodplain alluvium rock types. Geochemical signatures of groundwater were used to identify the chemical processes that control hydrogeochemistry. Interpretation of δ18O and δD indicates recharge from the meteoric water in charnockite, quartzite, granite and some samples of fissile hornblende biotite gneiss. It is also inferred that recharge take place from evaporated water in floodplain alluvium and fissile hornblende biotite gneiss.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In groundwater hydrology, stable isotopes provide an idea about movement and distribution processes within aquifers. A large number of studies for the past few decades have identified that stable oxygen and hydrogen isotope ratios provide useful tools for hydrological investigations (Clark and Fritz 1997; Mazor 1991; Fontes 1981; Bhattacharya et al. 1985; Yousif et al. 2015). In groundwater studies they are used to: estimate recharges (Gupta 1983); identify the effects of evaporation on groundwater systems (Hendry and Schwartz 1988; Krishnamurthy and Bhattacharya 1991); and also estimate advection/diffusion rates in unsaturated/saturated zones (Gupta 1983). The International Atomic Energy Agency (IAEA) has measured isotopes in precipitation worldwide (Gat 1981). Unfortunately only limited data of δ18O and δD in precipitation is available from a few Indian stations. These isotopic compositions are sensitive tracers and they are widely applied to study the natural water circulation and groundwater movement (Krabbenhoft et al. 1990; Herczeg et al. 1992; Turner et al. 1992). The meteoric processes alter the isotopic composition of water in such a way that precipitation in a particular environment has a similar characteristic as that of stable isotope. This isotopic signature then serves as a natural tracer for identifying the groundwater recharge sources. The isotopic and geochemical analysis of various groundwaters of the Ranga Reddy district with undulating topography, fractured hard rock outcrops and basement, varying thickness of weathered zone with low primary porosity and dominant secondary porosity, identified various recharge processes operative in the study area (Sukhija et al. 1998).
Recharge process of groundwater is mainly replenished by precipitation. In the absence of this process, the groundwater would be completely depleted. Therefore, recharge processes play an important role in groundwater reserve assessment and its determination. They can be studied through various techniques using hydrogeochemical parameters, climatological studies (Jaiswal et al. 2015), and groundwater modeling such as statistical techniques (Viswanath et al. 2015). They are also helpful for the sustainable management and protection. These processes become more important in hard rock aquifers because the available quantity of groundwater is generally lesser. Madurai District is one such region with hard rock aquifers. There have been limited groundwater studies conducted in this region (e.g., Thivya et al. 2013, 2014a, 2014b, 2014c, 2014d, 2014e, 2015a, 2015b, 2015c; Navaraj and Krishnammal 2012; Dharmaraj et al. 2012; Balamurugan and Dheenadayalan 2013; Sivasankar et al. 2013; Padmanaban et al. 2013). The earlier studies have not attempted to investigate the recharge process. Hence, the present study has focused on identifying the recharge processes in hard rock aquifers using stable isotopes of oxygen and hydrogen.
2 Study Area
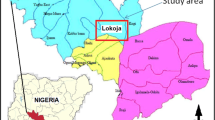
The study area is situated in the southern part of Tamil Nadu State covering an area of about 3741 km2 (Fig. 1), which occupies 2.09 % of the total geographical area of the State. It lies in the north Latitude of 9°33′3.6″ to 10°19′8.4″ and East Longtitude of 77°27′7.2″ to 78°28′58.8″. The district is characterized by Red soil, Black clayey soil and alluvial soil. Geological formations of the study area range from Archean to recent. The lithology of the study area comprises fissile hornblende biotite gneiss, charnockite, granitic intrusions, quartzite and floodplain alluvium. The archean formations comprise khondalite, charnockite, garnitiferous granulite, biotite gniesses and fissile hornblende biotite gniessic group of rocks. Quartzite occurs as ridges and as low lying mounts in the study area (GSI 1995; Thivya et al. 2013). The recent alluvium comprises of clay, sand, gravel and silt along the Vaigai river course. The district is traversed by the Vaigai River along the Northwest – Southeast orientation, dividing the city of Madurai into two parts. The river is the lifeline of the district and its catchment is 1524 m above mean sea level (amsl) in the Western Ghats.
Lithology and location map of the study area (after Thivya et al. 2013)
Groundwater occurs under phreatic conditions in weathered and interconnected shallow fractures and under semi-confined to confined conditions in deeper fractures (CGWB 2010). In Usilampatty and Sedapatty union, the groundwater level is deeper than in other regions. The depth to water level in the district varies from 3.13 to 7.66 m below ground level (bgl) during premonsoon (May 2006) and 1.86 to 5.74 m bgl during post monsoon (CGWB 2010). The variations in the yield of bore wells are very high in the district. Potential fractures with high discharge have been established along Valandur – Usilampatti – Timmarasanayakanur - Thirali - Peraiyur tract and Palkalainagar - Nilayur tract in the district. The higher levels of groundwater are noted in the southern and the northern/northeastern part of the study area, which indicates that the groundwater moves towards the eastern part of the study area. The normal rainfall was 548 mm in year 2012 (Sholavandan Rain Gauge Station) (IMD 2014).
3 Methodology
A total of 54 groundwater samples were collected using drinking water handpumps in post-monsoon (POM) season in January 2012, irrespective of lithology, representing the entire district. 21 samples from fissile hornblende biotite gneiss (FHBG) and charnockite (Char), 3 from quartzite (Qtz), 4 from granite (Grt) and the rest of the samples from floodplain alluvium (FPA). pH and electrical conductivity (EC) were determined in the field using a field analysis kit (Eutech Handheld Instruments). 500 mL of groundwater samples were collected in polyethylene bottles and they were analyzed for major cations and anions using standard procedures (APHA 1995). Calcium, magnesium, bicarbonate and chloride were determined by titrimetric method. Sodium and potassium were analyzed through flame photometry (ELICO CL). The detection limit of flame photometry is 1–100 ppm with an accuracy of ±1 digit. Silica, phosphate, and sulfate were determined by spectrophotometry (ELICO SL 171 minispec). The detection limit of spectrophotometry is 340–1000 ppm with an accuracy of ±2.5 nm. The reliability of the results was determined by the ionic balance of groundwater samples and a 5–10 % of percentage error was noted.
The groundwater samples were collected in 60 mL capacity polyethylene bottles for stable isotope analysis. The sample bottles were filled completely and preserved air tight in order to avoid evaporation and they were measured for δ18O and δD by mass spectrometer (Finnigan Deltaplus Xp, Thermo Electron Corporation, Bermen, Germany) using gas equilibration method with a precision of 0.5 ‰ and 0.1 ‰ (2 σ criterion) accuracy, respectively. Stable isotope results were expressed with respect to Vienna Standard Mean Ocean water (SMOW) in units δ (Permil- ‰), as follows:
where, R = D/H or18O/16O.
Partial pressure of carbon dioxide (pCO2) values were calculated using the WATEQ4F code (Trusdell and Jones 1973). It is the significant factor for enhancing the solubility in carbonate rocks by the dissolution of minerals, such as mainly plagioclase, which increases the pH, resulting in the formation of bicarbonate, thus accumulates more cations in the remaining water (Chidambaram et al. 2011).
4 Results and Discussion
4.1 Hydrogeochemical Processes
The maximum, minimum and average values of stable isotopes are given in Table 1. The average concentrations of major ions are given in Fig. 2 and chemical composition of groundwater is given in Table 2. Cl− is the dominant ion in the region which is observed along the floodplain alluvium in Vaigai river course (Ranjana et al. 2011; Thivya et al. 2013, 2014a). HCO3 − is the next dominant ion, which is observed in granitic terrains, and is mainly due to the weathering process. The higher concentration of Na+ and Ca2+ is observed in floodplain alluvium, which may be due to minerals like calcite, plagioclase and hornblende, as they are the primary sources for calcium in groundwater, and sodium bearing minerals like albite and other members of weathered plagioclase feldspars, nepheline and sodalite, releasing the primary soluble sodium.
The trilinear plot developed by Piper (1944) is used to delineate variability and trends in water quality to understand groundwater flow and geochemistry (Dalton and Upchurch 1978; Wu et al. 2014). The Piper diagram was made in such a way that the milliequivalent percentages of the major cations and anions were plotted in separate triangles. These plotted points in the triangular fields are projected further into the central diamond field, which provides the overall character of the water. The triangular fields are plotted separately with epm values of cations alkali earths (Ca2+, Mg2+), alkali (Na+, K+), weak acid (HCO3 −), and strong acid (SO4 2− and Cl−). The samples fall in Ca2+-Na+-HCO3 − and Ca2+-Cl− type. Most of the samples reflect the mixing type of cations and anions, which may be due to the additional leachate after monsoon (Chidambaram et al. 2012). During this season, ion exchange process/weathering exists and the mixed type of Ca2+-Na+-HCO3 −-Cl− type dominates, and this type may be due to the influence of monsoons (Fig. 3). Some of the samples of FHBG, Qtz and FPA fall in recharge zones with Ca-HCO3 type. Overall, the mixed type of all monsoon and non-monsoon characteristics are reflected in the groundwater samples. The concentrations of Ca2+ in groundwater may decrease while the concentration of Na+ increases during groundwater mixing, due to the ion exchange with the aquifer matrix. The Ca2+ ions are substituted by the Na+ ions on the solid surface, as demonstrated in the equation below:
X is considered to be a soil exchanger, and the groundwater comes into contact with Na+, which originated from seawater or halite minerals (NaCl) or from plagioclase feldspar that precipitated on the aquifer matrix. The Na+ ion pushes away the Ca2+ ion, and thus, it becomes dominant in the groundwater through the process of cation exchange.
4.2 Stable Isotopes (δD & δ18O)
The general meteoric relationship between the oxygen and hydrogen isotopes has been described by the global meteoric water line (GMWL) with a definite equation.
Unfortunately, isotopic data on precipitation are not available for all samples, but it is available only in certain regions of the study area. The plot of δ18O and δD of the rainwater samples of the Tamilnadu, derived for south west monsoon (SWM) precipitation (after Chidambaram et al. 2009), was used for the local meteoric water line (LMWL). A regression of 0.992 was noted for the various samples collected. The LMWL showed an equation of δD = 7.89 δ18O + 10.38, against the GMWL (δD = 8 δ18O + 10) of Craig (1961) and Rozanski et al. (1993). The slope of the LMWL is close to that of the GMWL (Fig. 4). The isotope results obtained were given in terms of δ units (per mil deviation of the isotope ratio from the international standard SMOW); the maximum, minimum and average values are presented in Table 1.
The δD and δ18O data gives an idea about the secondary processes in the water as it moves into the subsurface. The slight deviation of LMWL from GMWL may be due to the effect of climatic factors such as air, temperature, secondary evaporation, seasonality of precipitation and moisture source (Clark and Fritz 1997; Rozanski et al. 1993; Deshpande and Gupta 2012). The groundwater samples fall right on the LMWL, indicating that these samples have undergone some evaporation prior to infiltration (Baskaran et al. 2009). It also indicates that precipitation is not significantly modified by secondary evaporation. Some groundwater samples of floodplain alluvium, granite and charnockite show enriched values indicating evaporation trend. Some samples of fissile hornblende biotite gneiss are recharged by precipitation, and most of the samples are recharged by evaporating water bodies. It is also evident that the study area has more open ponds in addition to agricultural activities (Thivya et al. 2015c), which contribute significant moisture to the atmosphere. There is a clear representation of samples close to the LMWL and the enrichment is due to the strong evaporation (Datta et al. 1991; Mukherjee and Chandrasekharan 1993). Under low or average flow condition or with long residence time, groundwater tends to be isotopically enriched which is a very prominent condition in the hard rock aquifers of the study area. The samples of other lithology are clustered close to LMWL indicating that they are recharged by direct precipitation.
The samples that are recharged by evaporation are compared with Land use and lineament maps (Figs. 5 and 6). It is observed that the isotope signatures of samples from floodplain alluvium and fissile hornblende biotite gneiss vary according to water level, and they are influenced by surface water bodies, like open ponds and rivers (Thivya et al. 2015a). They also spatially fall along the lesser lineament density region because of the higher residence.
4.3 Deuterium Excess
The deuterium excess (d-excess) is generally used to identify the source of vapor source of precipitation (Kondoh and Shimada 1997; Deshpande et al. 2013). Lower d-excess indicates evaporated rainfall and high d-excess is considered as a footprint of recycled moisture (Machavaram and Krishnamurthy 1995). It also helps in identification of the origin of air masses from which precipitation is formed (Rozanski et al. 1993).
If the d-excess values ranges between 8 and 10, it is expected that it may be due to primary precipitation. Lower d-excess values (< 10) imply evaporation of rain water leaving the impact on residual groundwater with d-excess values, while d-excess >10 values indicate that evaporation in the source region takes place under lower humidity (Gat 1981). Samples show d-excess values ranging from −4 to 10 ‰ which could be attributed to contribution from evaporation of surface water bodies like open ponds and rivers (Fig. 7). The value less than 3 per mil reveals the source as evaporative enrichment with certainty (Harvey 2005). An inverse relation is observed between d-excess and δ18O which shows the kinetic evaporation of water before recharging groundwater (Tirumalesh et al. 2007), intent the precipitation dominance. It is observed from the plot that floodplain alluvium and fissile hornblende biotite gneiss samples fall on the enriched part with relatively low d-excess, which again reflect the contribution of evaporated waters, like open ponds or agricultural return flow. It may also be due to the fact that long residence in FHBG may result in enrichment of stable isotopes. The deep fractures lineament with slightly elevated temperatures may also result in variation of stable isotope fractionation.
4.4 Stable Isotopes and Chloride
Chloride is naturally a conservative ion in groundwater which is not participating in geochemical reactions and remains in the system. It is observed that groundwater samples with high chloride contents are relatively enriched (Fig. 8a). The probable reasons may be congruent dissolution of dry chloride deposits in the soil zone or other anthropogenic factors like fertilizer, salts from septic tanks, etc. (Chidambaram et al. 2010).
The higher Cl− is noted with enriched δ18O and δD (Fig. 8b). This enrichment is observed in floodplain alluvium and higher Cl− is noted along the Vaigai river bed (Thivya et al. 2013), suggesting recharge by surface water; this higher Cl− is mainly due to domestic sewage, as it is discharged into the Vaigai River of Madurai city. The majority of samples of FHBG formation show linear relationship between δ18O enrichment and Cl−, indicating the leaching of evaporative salts deposited in the pore spaces during the previous summer (Chidambaram 2000). Higher concentration of chloride with enriched δ18O also indicates recharge from the evaporated water bodies or due to the evaporation taking place along the fractures or due to the diffusive recharge along the flow path. Consequently, low chloride and depleted δ18O represent the areas recharged frequently by rainfall (Baskaran et al. 2009; Prasanna et al. 2010). δ18O and Cl− values gradually increase in other formations reflecting the higher residence time and enrichment of Cl ions.
4.5 Relationship between Log pCO2 and δ18O
The Log pCO2 (Raymahashay 1986) for each sample were determined to study its relation to recharge (Prasanna et al. 2007; Chidambaram et al. 2009). Log pCO2 in the groundwater samples ranged between −0.99 to −2.49 irrespective of lithology and these values are higher than the Log pCO2 of the atmosphere (−3.75) which specifies that CO2 is incorporated from the soil during infiltration through the unsaturated zone (Raymahashay 1986) (Fig. 9). Lesser Log pCO2 values and depleted δ18O indicates recent recharge by the local precipitation. The samples with higher Log pCO2 and enriched δ18O values indicates long residence time which is observed in some samples of fissile hornblende biotite gneiss, floodplain alluvium. When depletion of δ18O is noted, there is also a decrease of pCO2 observed in the sample that varies according to lithology. This also suggests that the rainwater charged with atmospheric CO2 has acquired additional CO2 from the soils, and thereby, develops high pCO2 water on its travel to deep unsaturated zone (Chidambaram et al. 2009).
5 Conclusions
The results indicate that the study area is dominated by ion exchange/weathering processes, and predominantly, by mixed type of Ca2+-Na+-HCO3 −-Cl− type due to the influence of monsoons. It is inferred that under long residence time in the hard rock aquifers of the study area, under low/average flow condition, groundwater tends to be isotopically enriched. The samples from floodplain alluvium and few samples from fissile hornblende biotite gneiss show representation from evaporated surface waters, like open ponds or agricultural return flow. Samples collected from floodplain alluvium along the Vaigai river are enriched isotopes due to the recharge by surface water, and higher Cl− is mainly due to domestic sewage. The majority of samples with linear relationship between δ18O and Cl− reflects the leaching of evaporative salts deposited in the pore spaces during the previous summer. Thus, they indicate recharge from evaporated water bodies, or evaporation taking place along fractures, or diffusive recharge along the flow path. In general, few samples from fissile hornblende biotite gneiss showed recharge by precipitation and most of the samples recharge by evaporating water bodies. The study also reflects that low Log pCO2 values and depleted δ18O indicate recent recharge by local precipitation. Hence, the study indicates that recharge of these aquifers takes place by two main processes namely precipitation and surface water sources. The study also shows the influence of the surface water bodies and its significance on the sustainable water resources management, and throws light for construction of artificial recharge structures in this region.
References
APHA (1995) Standard methods for the examination of Water and wastewater, 19th edn. APHA, Washington DC, USASS
Balamurugan C, Dheenadayalan MS (2013) Groundwater quality and its suitability for drinking and agricultural use in Vaigai River basin at Madurai, Tamil Nadu, India. J of Chem, Biol and Phys Sci 2:1073–1078
Baskaran S, Ransley T, Brodie RS, Baker P (2009) Investigating groundwater–river interactions using environmental tracers, Aust J Earth Sci, 56: 13–19
Bhattacharya SK, Gupta SK, Krishnamurthy RV (1985) Oxygen and hydrogen isotopic ratios in ground waters and river waters from India. Proc Indian Acad Sci (Earth Planet Sci) 94:283–294
CGWB (2010) Centre for groundwater board. A report on Madurai district
Chidambaram S (2000) Hydrogeochemical studies of groundwater in Periyar district, Tamilnadu, India. Unpublished Ph.D thesis, Department of Geology, Annamalai University
Chidambaram S, Bala Krishna Prasad M, Manivannan R, Karmegam U, Singaraja C, Anandhan P, Prasanna MV, Manikandan S (2012) Environmental hydrogeochemistry and genesis of fluoride in groundwaters of Dindigul District, Tamilnadu (India). Environ Earth Sci 68(2):333–342. doi:10.1007/s12665-012-1741-9
Chidambaram S, John Peter A, Prasanna MV, Karmegam U, Balaji K, Ramesh R (2010) A study on the impact of land use pattern in the groundwater quality in and around Madurai Region. South India—Using GIS Techniques Online J Earth Sci 4:27–31
Chidambaram S, Prasanna MV, Ramanathan AL, Vasu K, Hameed S, Warrier UK, Manivannan R, Srinivasamoorthy K, Ramesh R (2009) Stable isotopic signatures in precipitation of 2006 southwest monsoon of Tamil Nadu. Curr Sci 96:9–10
Chidambaram S, Prasanna MV, Karmegam U, Singaraja C, Pethaperumal S, Manivannan R, Anandhan P, Tirumalesh (2011) Significance of pCO2 values in determining carbonate chemistry in groundwater of Pondicherry region, India. Front Earth Sci 5(2):197–206
Clark ID, Fritz P (1997) Environmental isotopes in hydrogeology. Lewis Publishers, New York
Craig H (1961) Isotopic variations in meterotic waters. Am Assoc For The Adv Of Sci 133:1702–1703
Dalton MG, Upchurch SB (1978) Interpretation of hydrochemical facies by factor analysis. Groundw. 16(4):228–233
Datta PS, Tyagi SK, Chandrasekharan H (1991) Factors controlling stable isotope composition of rainfall in New Delhi. India, J of Hydrol 128:223–236
Deshpande RD, Gupta SK (2012) Oxygen and hydrogen isotopes in hydrological cycle: new data from IWIN National programme. Proc Indian Natl Sci Acad 78:321–331
Deshpande RD, Maurya AS, Angasaria RC, Dave M, Shukla AD, Bhandari N, Gupta SK (2013) Isotopic studies of megacryometeors in Western India. Curr Sci. 104(6):25
Dharmaraj J, Vadivel S, Ganeshkarthick E (2012) Physico-chemical analysis of ground water samples of selected districts of Tamilnadu and Kerala Int J of Sci & Technol Res 1(5):2277–8616
Fontes CH (1981) Environmental isotopes in groundwater hydrology, In: Handbook of Environmental Isotopes Geochemistry, P. Fritz and J.Ch. Fontes (eds.), Vol. 1,The Terrestrial Environment, A,1980; 75–140 Amsterdam: Elsevier Scientific Publishing Co
Gat JR (1981) Stable isotope hydrology: deuterium and oxygen-18 in the water cycle In: J.R. Gat and R. Gonfiantini, (Eds.), IAEA Technical Reports Series, No. 210. Vienna: IAEA (International Atomic Energy Agency)
GSI (Geological Survey of India). (1995). Geological and mineral map of Kerala and Tamil Nadu. Geological survey of India. Source: http://www.portal.gsi.gov.in/gsiImages/information/misc_pub_30_tamilnadu_2006_wm.pdf
Gupta SK (1983) An isotopic investigation of a near-surface groundwater system. Hydrol Sci J 28(2):261–272
Harvey CF (2005) Groundwater arsenic contamination on the Ganges Delta: biogeochemistry, hydrology, human perturbations, and human suffering on a large scale. Comptes Rendus Geosci. 337(1–2):285–296
Hendry MJ, Schwartz FW (1988) An alternative view on the origin of chemical and isotopic patterns in groundwater from the milk River aquifer. Canada Water Resour Res 24:1747–1763
Herczeg A, Lamontagne S, Pritchard J, Leaney F, Dighton J (1992) Groundwater-surface water interactions: testing conceptual models with environmental tracres. 8th Murray Darling Basin groundwater workshop, Victor Harbor, South Australia. p. 6B.3
IMD (2014) Indian meteorological department. Source: http://www.imd.gov.in/section/hydro/dynamic/rfmaps/WeeklyProgress.htm
Jaiswal RK, Lohani AK, Tiwari HL (2015) Statistical analysis for change detection and trend assessment in climatological parameters. Environ Process 2(4):729–749. doi:10.1007/s40710-015-0105-3
Kondoh A, Shimada J (1997) The origin of precipitation in Eastern Asia by deuterium excess. J Japan Soc Hydrol & Water Resour 10(6):627–629
Krabbenhoft DP, Bowser CJ, Anderson MP, Valley JW (1990) Estimating groundwater exchange with lakes: the stable isotope mass balance method. Water Resour Res 26:2445–2453
Krishnamurthy RV, Bhattacharya SK (1991) Stable oxygen and hydrogen isotope ratios in shallow groundwaters from India and a study of the role of evapotranspiration in the Indian monsoon. The Geochem Soc, Spec Publ 3:187–193
Machavaram MV, Krishnamurthy (1995) Earth surface evaporation process: a case study from the Great Lakes region of the United States based on deuterium excess in precipitation. Geochim Cosmochim Acta 59:4279–4283
Mazor E (1991) Chemical and isotopic groundwater hydrology. Marcel Dekker Inc., New York
Mukherjee TK, Chandrasekharan H (1993) Environmental stable isotopes on rainfall over Delhi and Bombay- some observations. J Nucl Agric Biol 22:34–41
Navaraj PS, Krishnammal S (2012) Investigation of water quality and its quotient factor in Thiruppalai Village, Madurai, India. IOSR J of Pharm and Biol Sci 2(6):40–46
Padmanaban R, Dharmendira Kumar M, Sakthivel PB, Elangovan NS (2013) A case study on chemical properties of ground water in Madurai District, Tamil Nadu, India. Int J of Eng And Adv Technol 2(4):2249–8958
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. Am Geophys Union 25(6):914–928
Prasanna MV, Chidambaram S, Srinivasamoorthy K, John Peter A, Anandhan P (2007) Hydrogeochemical characterization of groundwater in Gadilam River basin, through statistical analysis. Int Q J of Environ and Soc Sci 2(1):21–26
Prasanna MV, Chidambaram S, Shahul Hameed A, Srinivasamoorthy K (2010) Study of evaluation of groundwater in gadilam basin using hydrogeochemical and isotope data. Environ Monit Assess 168:63–90
Ranjana UK, Piyadasa, Champa Naverathna M (2011) River sand mining in Southern Sri- Lanka and its effect on environment. 11th International River symposium on “A Future of Extremes” Brisbane, Australia, 2008
Raymahashay BC (1986) Geochemistry of bicarbonate in river water. J of Geol Soc of India. 27:114–118
Rozanski K, Araguas-Araguas L, Gonfiantini R (1993) Isotopic patterns in modern global precipitation. In: Climate Change in Continental Isotopic Records, American Geophysical Union Monograph, vol 78, pp. 1–36
Sivasankar V, Omine K, Msagati TAM, Senthil kumar M, Chandramohan A (2013) Evaluation of groundwater quality in Madurai City, South India for drinking, irrigation and construction purposes. Arabian J Geosci 7:3093–3107. doi:10.1007/s12517-013-0994-2
Sukhija BS, Reddy DV, Nagabhushanam P (1998) Isotopic fingerprints of paleoclimates during the last 30,000 years in deep confined groundwaters of Southern India. Quat Res 50:252–260
Thivya C, Chidambaram S, Singaraja C, Thilagavathi R, Prasanna MV, Jainab I (2013) A study on the significance of lithology in groundwater quality of Madurai district, Tamil Nadu (India) Environment. Dev and Sustain 15:1365–1387. doi:10.1007/s10668-013-9439-z
Thivya C, Chidambaram S, Thilagavathi R, Prasanna MV, Singaraja C, Nepolian M, Sundarrajan M (2014a) Identification of the geochemical processes in groundwater by factor analysis in hard rock aquifers of Madurai district, South India. Arabian J Geosci 7:3767–3777. doi:10.1007/s12517-013-1065-4
Thivya C, Chidambaram S, Tirumalesh K, Prasanna MV, Thilagavathi R, Nepolian M (2014b) Occurrence of the radionuclides in groundwater of crystalline hard rock regions of Central Tamil Nadu, India. J of Radio-Analytical and Nucl Chem 302:1349–1355
Thivya C, Chidambaram S, Thilagavathi R, Prasanna MV, Nepolian M, Tirumalesh K, Jacob N (2014c) Spatio-temporal identification of regions with anomalous values of 222Rn in groundwater of Madurai district, Tamilnadu, India. Environ Process 1:353–367
Thivya C, Chidambaram S, Thilagavathi R (2014d) Evaluation of drinking water quality index (DWQI) and its seasonal variations in hard rock aquifers of Madurai district, Tamilnadu. Int J of Adv Geosci 2(2):48–52
Thivya C, Chidambaram S, Thilagavathi R, Adithya VS, Nepolian M, Singaraja C (2014e) A study on the seasonal variations of groundwater quality in hard rocks aquifer of Madurai district. Tamilnadu Enviro Geo Chimica Acta 1(4):243–248
Thivya C, Chidambaram S, Thilagavathi R, Prasanna MV, Singaraja C, Adithya VS, Nepolian M (2015a) A multivariate statistical approach to identify the spatio-temporal variation of geochemical process in a hard rock aquifer. Environ Monit & Assess, 187 (552): 1–19
Thivya C, Chidambaram S, Rao MS, Thilagavathi R, Prasanna MV, Manikandan S (2015b) Assessment of fluoride contaminations in groundwater of hard rock aquifers in Madurai district, Tamil Nadu (India), Appl Water Sci, 5: 1–13. doi. 10.1007/s13201-015-0312-0
Thivya C, Chidambaram S, Keesari T, Prasanna MV, Thilagavathi R, Adithya VS, Singaraja C (2015c) Lithological and hydrochemical controls on distribution and speciation of uranium in groundwaters of hard-rock granitic aquifers of Madurai District, Tamil Nadu (India). Environ Geochem Health 37:1–13. doi:10.1007/s10653-015-9735-7
Trusdell A H, Jones B F (1973) WATEQ: A computer program for calculating chemical equilibria of natural waters. J of Res US Geol Surv, 2(2): 233–248
Tirumalesh K, Shivanna K, Jalihal AA (2007) Isotope hydrochemical approach to understand fluoride release into groundwaters of Ilkal area, Bagalkot District, Karnataka, India. Hydrogeol J 15:589–598
Turner JV, Townlcy LR, Rosen MR, Sklash MK (1992) Coupling the spatial distribution of solute concentration and stable isotope enrichments to hydrological processes in hypersaline palcochannel aquifers. In: 7th international symposium on Water-Rock interaction (Park City, Utah, USA, July 1992), 217–222
Viswanath CV, Dileep Kumar PG, Ammad KK, Usha Kumari ER (2015) Groundwater quality and multivariate statistical methods, Environ Process, 2(2): 347–360
Wu J, Liu W, Zeng W, Ma L, Bai R (2014) Water quantity and quality of six lakes in the arid Xinjiang region, NW China, Environ Process, 1(2): 115–125
Yousif M, Oguchi T, Anazawa K (2015) Framework for investigation of karst aquifer in an arid zone, using isotopes, remote sensing and GIS applications: the Northwestern Coast of Egypt, Environ Processes, 2(1): 37–60
Acknowledgments
The authors wish to express their thanks to University Grants Commission (UGC), India, for providing the necessary financial support to carry out this study with vide reference to UGC letter No. F: 39-143/2010 (SR) dated 27.12.2010. The authors are also grateful to the editor and anonymous referees for their constructive comments and suggestions, which led to significant improvements to the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thivya, C., Chidambaram, S., Rao, M.S. et al. Identification of Recharge Processes in Groundwater in Hard Rock Aquifers of Madurai District Using Stable Isotopes. Environ. Process. 3, 463–477 (2016). https://doi.org/10.1007/s40710-016-0137-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40710-016-0137-3