Abstract
The advent of nanotechnology has resulted in a significant number of experimental studies over the past decades into the use of nanoparticles as lubricant additives (also known as nanolubricants). Nanolubricants offer a solution to the environmental problems associated with traditional lubricant additives that contain sulphur, chlorine and phosphorus. Despite their excellent tribological performance, the poor long-term stability of nanolubricants limits their use in real applications. We herein present a review of recent efforts and progress in the preparation of stable nanolubricants, including the evaluation of nanolubricants dispersion stability, factors that affect dispersion stability, and techniques to enhance stability of nanolubricants. This paper also discusses the effects of dispersion stability of nanolubricants on the tribological performance and lubrication mechanisms involved in the enhancement of tribological performance. Finally, research challenges and possible solutions to this problem are discusses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The advent of nanotechnology has allowed the synthesis of nanomaterial in various fields including optics and opto-electronics [1], photocatalysis [2], electrical and sensor devices [3], biomedical applications [4] and so on, due to their excellent chemical, thermal and mechanical properties. Recently, applications of nanomaterial in lubricants have spurred a significant number of experimental research projects for using nanoparticles as lubricant additives (also known as nanolubricants). Nanolubricants can offer a solution to many problems associated with traditional lubricants that contain sulphur and phosphorus, yet promise excellent tribological performance [5,6,7]. Also, nanolubricants show a number of advantages, such as (1) better stability when suspended in a lubricant compared to micro- or macro-sized particles [8], (2) particles are not retained by filters [9], (3) can form films on many different type of surfaces [10], (4) are relatively insensitive to temperature and (5) exhibit limited tribochemical reactions [9].
The preparation of homogeneous and stable nanolubricants is the key issue to limitations in the commercial application of nanolubricants. The main problem associated with the stability of the nanoparticles is agglomeration, where the nanoparticles tend to aggregate due to van der Waals forces [11]. To address this issue, great efforts have been made to improve the stability of nanolubricants with physical treatments [12,13,14], use of surfactants [15,16,17,18] and surface modification of nanoparticles [19,20,21].
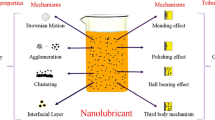
Several mechanisms can be employed to enhance the tribological performance of nanolubricants including rolling mechanism, exfoliation mechanism, self-repairing or mending mechanism, polishing mechanism and tribo-film formation. However, there is still a lack of theoretical understanding of these mechanisms.
This paper offers a systematic summary of research results presented in previously published data, including substantial details of the preparation methods of nanolubricants, factors affecting the dispersion stability, and characterisation techniques of dispersion stability. This overview is intended as a useful guide for researchers to prepare a homogeneous and stable long-term nanolubricant without agglomeration. A detailed review on the theoretical understanding of lubrication mechanisms follows, and finally, this review paper includes a discussion on the challenges and their possible solutions in the nanolubricant field.
2 Dispersion Stability of Nanolubricants
2.1 Evaluation of Nanolubricants Dispersion Stability
Dispersion stability refers to the stability of the suspension of nanoparticles in the base lubricant without sedimentation or settling down of nanoparticles due to a downward body force from cumulative weight [22, 23]. The distribution of nanoparticles in the base lubricant should show long-term stability in order to perform effectively as lubricant additives. Different methods have been developed to evaluate the stability of nanolubricants including the sedimentation method, optical absorbance spectrum/UV–vis spectrophotometer, dynamic light scattering (DLS), zeta potential analysis and the imaging method. Table 1 shows a comparison of the advantages and limitations of available methods used to evaluate the stability of suspended nanolubricants. The details around these evaluation methods will be further discussed below.
2.1.1 Sedimentation Method
The sedimentation method is the simplest and most economical method to analyse the dispersion stability of nanolubricants. Sedimentation is the process of nanoparticles settling down or being deposited as sediment at the bottom of the base lubricant. This process is related to the balance between buoyancy and frictional forces [31]. The sedimentation rate of nanoparticles can be calculated using Stoke’s law (Eq. 1), and this equation is applicable for most spherical nanoparticles [32].
where d is the diameter (cm) of nanoparticle, δ is the density (g/cm3) of nanoparticle, ρ is the density (g/cm3) of base lubricant and µ is the viscosity (Pa s) of base lubricant.
Sedimentation can also be visually analysed using the photography method over a period of time [33,34,35]. A stable dispersion of nanolubricant has minimum sedimentation, where the nanoparticles will remain suspended for a long time. To obtain a quantitative assessment of the stability, the sedimentation ratio can be calculated based on photographs obtained over a specified period. Yin et al. [36] evaluated the dispersion stability of suspensions by measuring the sedimentation ratio against time. The sedimentation ratio was calculated by dividing the height percentage of the sediment nanoparticles against the total suspension height. The larger sedimentation ratio indicated stable dispersion stability.
On the other hand, Haghighi et al. [31] experimentally measured the stability of the suspension through the sedimentation balance method. The weight of sediment nanoparticles on a suspended tray balanced in the suspension was measured by an accurate scale to record the sedimentation rate of nanoparticles. They concluded that the sedimentation rate of nanoparticles obtained by sedimentation balance method are consistent with those from the photography method.
2.1.2 UV–Vis Spectrophotometer
The dispersion stability of nanolubricant can be evaluated using an ultraviolet visible (UV–Vis) spectrophotometer that obeys the Beer-Lambert law. The higher absorbance level of visible light at a certain wavelength by the nanoparticles in the base lubricant indicates excellent stability of the nanolubricant [37]. Table 2 shows the wavelength range and absorbance peak for different nanolubricants taken by previous researchers. As mentioned in Table 1, the use of UV–Vis spectrophotometer is limited to highly concentrated lubricants or base lubricants that are dark in colour. However, Alves et al. [37] suggested that it is necessary to dilute dark coloured nanolubricants before performing the absorption spectra test.
Some researchers reported the absorbance level over different time intervals, after initially identifying the absorbance peak of the nanolubricant. Gulzar et al. [7] performed the absorbance spectra tests of CuO/CMPO and MoS2/CMPO nanolubricants with and without oleic acid over a time duration of 72 h at a wavelength of 429 nm. Thottackad et al. [16] measured the absorbance level of CeO2 nanoparticles in coconut oil, paraffin oil and engine oil at 429 nm at time duration of 1000 h. Koshy et al. [17] identified the absorbance spectra of MoS2/coconut oil and MoS2/paraffin oil nanolubricants with and without SDS at wavelength of 429 nm over a time duration of 120 h. Song et al. [19] measured the dispersion ability of ZnAl2O4 nanoparticles in lubricant oil over a time duration of 140 h. Lee et al. [38] evaluated the dispersion stability of fullerene/mineral oil and CNT/mineral oil nanolubricants over 800 h time duration. The decrease in absorbance level over time indicated the sedimentation of nanoparticles. The nanolubricant that had no gradient variation in absorbance level as a function of time indicates the excellent dispersion stability of the nanolubricant.
2.1.3 Dynamic Light Scattering
Some researchers confirmed the stability of the nanolubricant suspension by using the dynamic light scattering (DLS) technique [20, 46,47,48,49]. This technique was used to measure the particle size distribution of nanoparticles in the lubricant to evaluate the dispersion stability with time. It measures the diffusion of particles moving under Brownian motion and converts it to particle size using the Stoke-Einstein relationship, where the particle size ranges from 0.3 nm to 10 µm [39, 47]. The particle size distribution is obtained based on analysis of the resultant exponential autocorrelation functions. Basically, the dark nanolubricant should be dried out or diluted before taking the measurement, but doing so will change the nature of the microstructure of nanoparticles in the lubricant [50]. Thus, an appropriate method for characterising the particle size of the nanolubricants is critical to avoid changing the nature of the microstructure of the nanolubricant.
2.1.4 Zeta Potential
The dispersion stability of nanolubricants can be measured via zeta potential analysis, which is determined by the surface charge density as characterised by the electro-kinetic potential [34]. The stability of suspensions can be expressed as the zeta potential value, where a higher value of zeta potential (negative or positive) represents better nanolubricant dispersion stability [51, 52]. A high value of zeta potential (electrostatic ability) increases the electrostatic interactions between the particles, thus preventing coagulation and the settling of nanoparticles [34]. The suspensions that have a zeta potential value higher than 30 mV are believed to be stable, suspensions with zeta potential value from 40 to 60 mV have good stability, and suspensions with zeta potential value above 60 mV have excellent stability [53,54,55]. The prepared TiO2/mineral oil nanolubricant by Sabareesh et al. [53] was found to have a zeta potential of 70 mV which showed a stable suspension even 800 h after preparation. Chang et al. [56] found that TiO2 nanolubricant had a good suspension stability with a zeta potential of 35 mV and pH value 7, where the suspension was found to be stable for 7 days.
2.1.5 Microscopy
This section will discuss the dispersion stability of suspensions by characterisation techniques. The stability of suspensions can be characterised using different types of microscopy including optical microscope (OM), scanning electron microscopy (SEM), transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM) and freeze etching replication transmission electron microscopy (FERTEM).
To characterise the samples under microscopy, the samples should be prepared in accordance with sample preparation protocol for the particular microscopy technique. For an optical microscope sample preparation, the sample was dropped on a glass slide, and left at room temperature for a few days [57]. For SEM sample preparation, the nanoparticles were extracted from the oil onto atomically smooth <100> Si wafers by using n-heptane [58]. For the TEM specimen preparation, the nanoparticles can be dispersed in toluene, benzene, chloroform [59] or absolute alcohol [37, 60, 61]. Then the solution was dipped onto the copper grid covered by carbon film and let fully dried at ambient temperature. The use of OM enabled researchers to observe the aggregate state of suspension in micrometer scale, but the observation might not be representative of the whole sample [28]. The observations through SEM and TEM required the samples to undergo pre-treatment which might have changed the actual microstructures of the particles. Some researchers suggested observing the samples using cryogenic electron microscopy (cryo-TEM) or FERTEM, since this method is suitable for characterizing wet samples [50, 62]. The microstructures of the particles will not change during cryoation. This method allow direct visualization of nanoparticles, i.e., size, shape and internal structure, and the aggregate structure of nanoparticles in suspension [63]. For the FERTEM method, the actual microstructure of the nanofluid was cryofixed by freezing the prepared nanofluid using liquid nitrogen. After that, the sample was fractured, etched and replicated with Pt/C coatings, and the particles were then removed from the replica using a hydrochloric acid solution [50].
An optical microscope is a good method to characterise the aggregate state of suspension on a micrometer scale, while electron microscopy is a good method to characterise the nanoparticles in suspension. Compared to the optical microscope, electron microscopy techniques such as SEM, TEM and HRTEM are relatively expensive and have limitations when aiming to obtain statistical results since only a portion of particles—from tens to hundreds—can be measured from among a large number of particles [11]. Previous researchers used the optical microscope method and only obtained a qualitative idea of the stability of nanolubricants. Therefore, it is recommended to combine the use of optical microscope with image analysis to get a quantitative assessment of the stability.
2.2 Factors Affecting Stability of Nanolubricants
Some factors, including the properties of the nanoparticles (particle size, shape and volume fractions) and the properties of the base-lubricants (density, viscosity, pH and polarity), will influence the stability of nanolubricants. According to the theory of colloid chemistry, when the particles reach their critical size (Rc), sedimentation will stop as sedimentation and diffusion enters equilibrium due to the Brownian motion of particles (diffusion) [62]. Nevertheless, smaller nanoparticles are not always stable because smaller nanoparticles have a high surface energy that will enhance the van der Waals attractive forces on the surfaces of the nanoparticles. These forces make the nanoparticles’ surface attractive to other nanoparticles, thereby causing the agglomeration of nanoparticles. In an analysis of the dispersion stability of an Ag/paraffin oil nanolubricant, Choi et al. [64] found that a 7 nm Ag suspension was more stable than that of a 50 nm Ag suspension. Noted that the smaller nanoparticles are more easily suspended in the suspension than the larger nanoparticles since larger nanoparticles will cause instability of nanolubricants.
The influence of the shape of nanoparticles is another factor for consideration on the dispersion stability of nanoparticles. Choi and co-workers [65] found that rod-shaped nanoparticles have a poor dispersion stability compared to spherical-shaped particles due to the increase of the aspect ratio (length/diameter) of nanoparticles. The high aspect ratio of such nanoparticles can increase the possibility of the nanoparticles to agglomerate, thus forming a non-homogeneous suspension. It must be noted that the dispersion of carbon nanotubes (CNT) in a fluid medium is very challenging due to their sheet and rod-shaped in which have high aspect ratio and very large specific surface area lead to strong van der Waals forces between nanoparticle surfaces and agglomeration [66]. Moreover, the hydrophobic nature of CNT enables easy aggregation and the formation of unstable clusters in the CNT suspensions, resulting in poor dispersion stability [67]. Yin et al. [36] studied nitrogen-enriched carbonaceous nanotubes (N-CTs) prepared by the heat treatment of conducting polyaniline (PANI) nanotubes dispersed in silicone oil as an electro-rheological (ER) fluid. By comparing the result with a granular PANI suspension, they found that the N-CTs suspension showed better dispersion stability. They suggested that the observed better dispersion stability of the N-CTs suspension seems to be associated with nanotubes’ smaller size perpendicular to the axis, and the high aspect ratio nanotube shape. They also indicated that the better dispersion stability of the N-CTs suspension could be attributed to their larger diameter and suitable aspect ratio. They also evaluated the dispersion stability of different N-CT suspensions by increasing the heat treatment temperatures. They found improved dispersion stability in N-CT suspensions as the heat treatment temperatures increased due to the increase in density and a slight reduction of the aspect ratio of nanotubes.
Density is proportional to sedimentation rate, and thus higher density of nanoparticles tend towards faster sedimentation rate. Based on the Stokes’ law, the sedimentation rate was depended to the size and density of nanoparticles, as well as the viscosity of base lubricant. A stable nanolubricants have smaller size of nanoparticles, small differences in the density between nanoparticles and the base lubricant, and more viscous base lubricants [31]. Moreover, several researchers have discovered that low volume fractions of nanoparticles can prevent agglomeration or sedimentation. The higher the concentration of nanoparticles, the closer the nanoparticles are to one another, which in turn increases the van der Waals attraction that causes the nanoparticles to agglomerate [68]. A study performed by Sabareesh et al. [53] dispersed TiO2 nanoparticles (with average size of 30-40 nm) with different volume fractions of 0, 0.005, 0.01 and 0.015 vol% in mineral oil using ultrasonic agitation of 300 min duration. Through observation of sedimentation, they found that the nanolubricants were dispersed uniformly even after 700 h. They also confirmed the stability of the nanolubricants by measuring the zeta potential value from the Dynamic Light Scattering System (Malvern) and found that the value was at 70 mV even 800 h after preparation. Choi et al. [64] also proved that a low volume concentration of nanoparticles would have a better degree of dispersion stability. In this case, 55 nm graphite and 7 nm and 50 nm Ag nanoparticles with concentrations of 0.1 and 1.0 vol% were dispersed in paraffin base oil (EP220) using an ultrasonic probe. Alkyl aryl sulphonate and polyoxyethylene alkyl acid ester, a surfactant, were used to ensure a stable dispersion and prevent the agglomeration of nanoparticles. The nanolubricants’ stability was then analysed using a stability analyser (LUMiFuge, L.U.M.) with a centrifugal force of 1.147g. From the plotted graph of transmittance measurements versus time, nanolubricants with 0.1 vol% particles had no gradient variations which represent a better degree of dispersion stability, whereas 1.0 vol% of particles showed a steeper gradient which means they were unstable.
Kotia and Ghosh [39] presented a study of a nanolubricant based on Al2O3 dispersed in gear oil (SAE EP-90) at concentrations of (0.5, 1, 1.5, 2) vol%. The mixtures of nanoparticles and base oil were sonicated for 2 h, and then homogenised using a magnetic stirrer for 1 h. Based on the analysis of density with time intervals, they found that the suspension with 0.5 vol% concentration was the most stable with only a slight variation in density over time. A recent study carried out by Azman et al. [57] dispersed graphene nanoplatelets (GNPs) as an additive in palm oil trimethylolpropane (TMP) ester (5 vol%) blended in polyalphaolefin (PAO) (95 vol%) using a magnetic stirrer, then mixed ultrasonically using an ultrasonic homogenizer (50% amplitude; 2 s pulse; 50 Hz frequency) for 1 h. The concentrations of GNPs were 0.01, 0.03, 0.05, 0.1, 0.2, 0.5, 1 and 3 wt%. Based on the observation stability test, they found that the addition of 3wt% of GNP showed sedimentation in 2 days after sonication, while all other solutions were stable for at least 20 days. They also confirmed the stability of nanolubricants through metallographic micrographs under a micrometre scale to observe the agglomeration state of GNP particles. Through this observation, they found that the agglomeration size for 0.01 and 0.03 wt% of GNP was around 13–18 µm, compared to the higher GNP concentration (> 0.05 wt%) where the agglomeration sizes increase up to 1050 µm.
Another factor that can affect the stability of the nanolubricant is the properties of the base lubricant itself. Kamalgharibi et al. [69] compared the dispersion stability of CuO nanoparticles in water, ethylene glycol (EG) and 50:50 water/EG based fluid. They observed that the dispersion stability of CuO nanoparticles in ethylene glycol is better than that of CuO nanoparticles in water and water/EG base fluid. They confirmed that the viscosity of the base fluid was an influential factor that affected the dispersion stability of nanofluid. The same findings were found by Haghighi et al. [31], where CeO2 showed better dispersion stability EG than that of water/EG base fluid.
Moreover, the variation of pH value in nanofluids also influence the stability of suspension. Zhu et al. [50] investigated the effects of pH value on aggregation behaviour of CaCO3 nanoparticles in distilled water. They found that the optimum pH value was 9–9.5, which provide the best stability. When the pH value is below 9, the stability is not good, whereas further increases in pH value above the optimum pH value results in a less stable nanofluids. The nanofluids with the optimum pH value can keep stable for 1 month. Chang et al. [56] adjusting the pH value of TiO2 nanolubricant to 7, which provide better dispersion stability. This value was depart from the isoelectric point (IEP) of nanoparticles (pH 4), resulting in deagglomeration of nanoparticles. According to DLVO (Derjaguin–Landau–Verwey–Overbeek) theory, when the pH value was equal or near to the IEP, nanoparticles are easily agglomerates resulting in poor dispersion stability [70]. Li et al. [71] reported that the pH value of the reaction medium of nanoparticles affected the reaction rate and the morphology of the final product. They prepared a well-dispersed silver nanoparticles for oil-based nanofluids by contolling the pH of reaction medium to 11. They found that the reaction rate was increased when the reaction medium was adjusted to be neutral or acidic, thus lead to non-uniform morphology of nanoparticles. The pH value of the reaction medium can be reduced by adding acid, or increased by adding base [72].
Sometimes, the polarity of the base fluid itself may affect the dispersion stability of the nanofluid. It is generally accepted that a non-polar molecule will have more solubility in a non-polar base fluid (such as organic and mineral oils) rather than in polar base fluid (such as water, EG and their mixtures). From the experiments conducted by Xing and Wang [73], they found that fullerene C60 nanoparticles which is a non-polar molecules are insoluble in water, but have some solubility in a non-polar refrigerant lubricant oil due to their high symmetry. However, they found that CNT have poor stability due to its higher polarity than C60 which make them easily agglomerated and difficult to disperse in non-polar base fluid. To achieve a stable dispersion stability, the use of surfactant is requires and will discuss in the next section.
2.3 Techniques to Enhance the Stability of Nanolubricants
2.3.1 Physical Method
It is important to prepare a suspension of nanoparticles in lubricant without agglomeration, and for the suspension to have long-term stability before it can be used in actual applications. Nanolubricants can be prepared with either a one- or two-step method. Generally, the two-step method for preparing nanolubricants is the most widely used physical method by previous researchers since it is the most economical method of large-scale nanolubricant production. With this method, nanoparticles are first produced as dry powders by the chemical or physical method, and then dispersed in the base lubricants [74]. Many of the previous researchers purchased the nanoparticles directly from the available market. The dispersion of nanoparticles in the base lubricants can be carried out by several mechanical energy techniques including mechanical stirring, ultrasonication, ball milling and high pressure homogenisation.
Ultrasonication was found to be a more effective physical method to reduce agglomeration of nanoparticles than mechanical stirring. Ultrasonication is a pressure wave that propagates an ultrasonic wave, resulting in a vast energy dissipation and the collapse of unstable cavitation bubbles. During the implosive collapse, the unstable cavitation bubbles will break up aggregates [75, 76]. Mosleh et al. [12] reported that the dispersion stability of molybdenum disulphide (MoS2), tungsten disulphide (WS2) and hexagonal boron nitride (hBN) nanoparticles in the metal working fluid CIMFLO 20 significantly improved by sonication compared with mechanical shaking and stirring. The average size of particle aggregation was significantly reduced from 1000 nm (MoS2), 600 nm (WS2), and 800 nm (hBN) to 600 nm (MoS2), 450 nm (WS2), and 550 nm (hBN). However, nanoparticles still aggregated, and they suggested the use of a surfactant in the formulation of nanolubricants to improve the dispersion stability.
Another study done by Lee and Rhee [76] also found that ultrasonication was highly effective in reducing agglomeration problems. They prepared an ethylene glycol (EG) based nanolubricant with graphene naoplatelets (GnPs) by using an ultrasonic bath and probe, using mechanical stirring as a benchmark. Based on high-resolution transmission electron microscopy (HRTEM) images, they found that the size of aggregation was reduced significantly after ultrasonication—from 500 to 100 nm. Alazemi et al. [77] synthesised sub-micrometer-sized carbon spheres coated with a MoS2 monolayer (CS-MoS2) via controlled heat treatment. These nanoparticles with concentrations of (0.5, 1.0 and 2.0) wt% were then dispersed in SAE 5W30 engine oil using a piezoelectric ultrasonic probe (750 W) for 120 s at a power amplitude of 20%. The prepared nanolubricants were found by visual inspection to be stable for 2 weeks.
Moreover, other notable studies were carried out by Dubey et al. [75], Jiang et al. [13] and Sharif et al. [41], in which the effect of time duration for dispersing nanoparticles in base lubricants via ultrasonication on the dispersion stability was investigated. Dubey et al. [75] used an ultrasonic probe (with 750 W/cm2 intensity) to disperse nano- and micro-polytetrafluoroethylene (PTFE) particles in a base lubricant. They varied the sonication time at 30, 45, 60 and 90 min, and found that the optimum time required to form a stable solution of nanolubricants using ultrasonic probe was 60 min (stable for 15 days). They also found that a longer ultrasonication time provided no further benefits for the stability of nanolubricants. Jiang et al. [13] prepared a Cu/diisopropyl biphenyl and triisopropyl biphenyl (DBTB) synthetic oil nanolubricant by adding oleic acid using a two-step method with magnetic force agitation for 30 min followed by ultrasonication. They varied the ultrasonication time for 0.5 h and 3 h. They evaluated the nanolubricant stability by measuring the effective thermal conductivity over time and found that there is no significance difference in the thermal conductivity between 0.5 h and 3 h. Another study by Sharif et al. [41] studied the effect of mixing time duration of SiO2 nanoparticles (with an average diameter of 30 nm) in polyalkylene glycol (PAG) lubricant by ultrasonication. The sonication time was varied at 0, 0.5, 1.0, 1.5 and 2 h. They evaluated dispersion stability of nanolubricant using UV–vis spectrophotometer and found that absorbance ratio is decreasing with sedimentation time and strongly dependant to sonication time. The optimum sonication time was found to be 2 h. Thus, the time duration will also influence the dispersion stability of the nanolubricant and it is advisable to discover the optimum time duration for preparing a stable nanolubricant.
In some cases, ultrasonication could not break the aggregated particles. One study by Ettefaghi et al. [78] dispersed copper oxide (CuO) nanoparticles in SAE 20W50 engine oil using three different mechanical methods comprising ultrasonic bath, ultrasonic probe and planetary ball mill. They kept all the solutions for 720 h to observe the stability condition and found less precipitation of the nanoparticles in the sample made by the planetary ball mill method compared to the two other methods. Due to the higher viscosity of engine oil, they found that using a planetary ball mill is the most suitable method to disperse CuO nanoparticles in engine oil since it can supply a great amount of energy to reduce agglomeration problems with CuO nanoparticles. In another study, Jatti and Singh [79] used an ultrasonic shaker for 30 min to mix CuO nanoparticles with the base lubricant before proceeding to the planetary ball mill method to produce a stable nanolubricant. The functional specification of the ball mill used was a speed of 300 rpm for the duration of 3 h, with the ball weighing 200 g and a sample weight (fluid + nanoparticles) of 30 g. They confirmed the dispersion stability of the nanolubricant by UV spectrophotometer and found that the nanolubricant was stable for 125 h at room temperature. However, Choi et al. [80] stated that although the dispersion stability of nanolubricants is excellent using the ball mill method, the product requires high maintenance management. High-pressure homogeniser and ultrasonic pulveriser methods are simpler and easier to use and maintain.
The use of a high shear homogeniser may result in better dispersion since it can break up aggregates by applying extremely strong shear forces. Wan et al. [81] dispersed 0.1 wt%, 0.5 wt% and 1.0 wt% of hexagonal boron nitride (hBN) particles through a high shear homogeniser at 7500 rpm for 30 min into the commercial lubricant SAE 15W40. The prepared nanolubricant exhibited good stability for more than 2 weeks. Fontes et al. [14] produced a MWCNT/transformer oil nanolubricant and a diamond/transformer oil nanolubricant using a high-pressure homogeniser performed up to 400 bar. Through sedimentation analysis, the authors found that the nanolubricants were stable for 24 h. This physical technique also successfully produced a stable suspension of nanofluids (water-based). Fedele et al. [46] prepared a SWCNHs/water nanofluid, a TiO2/water nanofluid and a CuO/water nanofluid using different physical techniques including sonication (performed at 130 W, 20 kHz for 1 h), ball milling (performed at 300 rpm for 2 h) and high pressure homogenisation (at 100 bar with 30 passes). They reported that homogenisation was the most effective method to obtain a stable nanofluid and confirmed the stability of the nanofluid by measuring the size distribution profile using a dynamic light scattering (DLS) process.
2.3.2 Use of Surfactant
Stability of the suspension can be achieved through steric stabilisation and electrostatic stabilisation. Electrostatic stabilization is the mechanism in which the coagulation of nanoparticles is inhibited by the presence of double layer of electric charges that surrounds the nanoparticles. The classic DLVO theory combines the effects of the van der Waals attraction and electrostatic repulsion for describing the stability of a colloidal dispersion [52]. The total of these two measured forces determines whether the net interaction between particles is repulsive or attractive. The repulsive forces between particles must be higher than the van der Waals force to prevent the coagulation and sedimentation of nanoparticles [34]. Generally, electrostatic repulsion is the dominant mechanism of water-based suspension stabilisation. It is less effective in oil-based suspensions with its low electrical conductivity although it still exerts a significant influence [82]. Steric stabilisation is the mechanisms in which the coagulation of nanoparticles is inhibit when the nanoparticles covered with surfactant molecules, which the long loops and tails of surfactant molecules extend out into solution and forming a coating that separates the nanoparticles from another nanoparticles [83]. A surfactant can prevent the suspension from coagulation via either steric stabilisation or a combination of steric and electrostatic stabilisation (known as electrosteric), depending on the types of surfactant used.
There are different classes of surfactant such as anionic (with negatively charged head groups), cationic (with positively charged head groups), non-ionic (without charged groups in its head or neutral) and amphoteric (with zwitterionic head groups) [55]. In a good solvent, steric stabilisation of a suspension is achieved when the surfactant adsorb onto the nanoparticle’s surface, or form a micelle-like structure to provide a physical “cushion” between colliding particles, thus preventing the agglomeration of nanoparticles [16, 40]. In a poor solvent, agglomeration can occur as the surfactant layer disintegrates, causing incremental van der Waals forces. The forces between surfactant onto inorganic and organic surfaces depend on the chemical characteristics of the nanoparticles, the surfactant molecules (whether it is simply adsorbed from solution or transferred onto the surfaces) and the properties of the solvent [84].
In a polar solvent, a suitable surfactant is a water-soluble surfactant where the hydrophobic surfaces of the nanoparticles are modified to become hydrophilic (miscible with water). In a non-polar solvent, the suitable surfactant is an oil-soluble surfactant where the hydrophilic surfaces of the nanoparticles are modified to become hydrophobic (immiscible with water) [72]. It is critical to identify the compatibility of the surfactant for oil-based suspensions as the selection of the surfactants depends on the properties of the particles and base oil. Based on all the literature collected in Table 3, it is clear that the formulation of a nanolubricant with a surfactant represents a significant contribution to the dispersion stability of the suspensions. Most of the researchers added anionic and non-ionic surfactants to nanolubricants. The solubility of a non-ionic surfactant can be evaluated through its hydrophilic-lipophilic balance (HLB) value. The lower the HLB value, the more oil-soluble the surfactant, and vice versa. The HLB value ranges from 1 to 20, where a high HLB surfactant (HLB > 10) is suitable for a water-soluble suspension (hydrophilic surfactant), whereas a low HLB surfactant (HLB < 8) is suitable for an oil-soluble suspension (lipophilic surfactant) [51]. A non-ionic surfactant or polymer can maintain the stability of the suspension via steric stabilisation, thus preventing van der Waals interactions, while ionic surfactants can maintain the stability of the suspension by electrosteric stabilisation [85].
However, the combination of surfactant and nanoparticles in lubricants does not always have positive results. Some of the surfactants cannot withstand higher temperatures; suspensions will lose stability and sedimentation of nanoparticles will occur [86, 87]. For example, Zin et al. [88] reported that poly(vinylpyrrolidone) (PVP) surfactant gave a better dispersion stability at different synthesis temperatures compared to sodium dodecyl sulphate (SDS) surfactant, where they measured the stability based on the reaction yields and nanoparticles size. Li et al. [71] reported that an increase in reaction temperature (25, 50 and 120 °C) did not affect the size of silver nanoparticles, but the oleic acid layer clearly decreased. From TGA/DSC analysis, they found that the content of the organic capping layer are completely decomposed at relatively high temperature (400 °C). So, the functionality of surfactants at different temperatures should be further investigated. The excess surfactant could also form reversible micelles, which leads to reunion among nanoparticles thus affecting the stability of the suspension [89]. The excess surfactant also affect the viscosity, thermal properties and chemical stability of nanolubricants [90].Therefore, the concentration of the surfactant must be controlled with great care to obtain the optimum dispersion stability of suspension.
2.3.3 Surface Modification of Nanoparticles
Other works deal with the stabilization of inorganic nanoparticles in oil-based suspensions by surface modification using either organic modification agents or silane coupling agents. The organic compounds used as modification agents usually comprise polar groups and long alkyl chains that can chemically adsorb on to the inorganic nanoparticles, which enables inorganic nanoparticles to become soluble in an organic solvent [99]. Zhang et al. [59] improved the stability of anatase TiO2 nanoparticles in liquid paraffin by coating its surfaces using stearic acid (SA) in an ethanol solution. From TEM image, it was found that the stability of the coated anatase TiO2 in liquid paraffin was enhanced with no signs of aggregation with a particle size of about 10 nm, which also confirmed with the X-ray diffraction (XRD) analysis. Zhang and co-workers also confirmed that the SA were successfully grafted on the surface of anatase TiO2 nanoparticles through Fourier transform infrared spectroscopy (FTIR) analysis. Chen et al. [100] prepare the oleic acid (OA)-modified graphene oxide (GO) nanoparticles by dispersing GO in ethanol and then add OA. The mixture was stirred under reflux conditions for 0.5 h. Then, the resultant was washed with ultrasound several time to obtain OA-modified GO nanoparticles. The dispersion of modified GO was found to be improved and they confirmed that the surface of GO was coated by OA using FTIR.
The stability of SiO2 nanoparticles in liquid paraffin was improved by modification of its surface using oleic acid (OA) in ethanol. This organic modification agent will adsorb on to the surface of the nanoparticles and reduce their surface energy, thus preventing the agglomeration of the nanoparticles. They confirmed that the surfaces of SiO2 nanoparticles were coated with OA through characterisation via FTIR analyser. Nanolubricants prepared using modified SiO2 were found to be stable for at least 30 days with no visible sedimentation [33]. Song et al. [19] modified the surface of monodispersed ZnAl2O4 with OA in cyclohexanol solution to obtain better dispersion stability in lubricant oil. They concluded that the suspension was stable and confirmed that the OA molecules had been successfully modified on the surface of ZnAl2O4 through grafting mechanism. The carboxyl groups of OA molecules interacted with the hydroxyl group on ZnAl2O4 nanoparticles’ surface and formed a chemical reaction modification layer (inter-layer). The physical adsorption modification OA outside of the chemical reaction modification layer was formed due to the hydrophobic driving force and hydrogen bonding. The formation of the physical and chemical adsorption layer outside the surface of ZnAl2O4 led to enhanced stability of ZnAl2O4 in lubricant oil.
The surface of graphite nanoparticles was modified with silane coupling agent KH570 in ethyl alcohol before it was added to naphthenic mineral oil. The prepared nanolubricants in the preparation were found to be stable even after 60 days. The stability of these modified graphite nanoparticles in naphthenic mineral oil were due to steric stabilisation which prevents agglomeration of nanoparticles [20]. Ma et al. [21] also used silane coupling agent KH560 as a surface modification agent to improve the stability of ZrO2 in 20# machine oil. The reaction mechanism of the grafting process of KH560 starts with the hydrolysis of the X group that produces Si–OH. Then, hydrolysed silane will react with the hydroxyl (–OH) group on the surface of nanoparticles, thus forming a surface modified nanoparticle. Luo et al. [6] conducted an in situ-modification method that combines the preparation and surface modification of Al2O3 nanoparticles at the same time. Silane coupling agent KH560 possesses the property of reacting with both organic and inorganic materials. The modified Al2O3 nanoparticles show excellent dispersion stability in lubricating oil. The author confirmed the improvement of the dispersion stability of nanoparticles via: (1) zeta potential value (increased from ~ 18 to 25.1 mV); (2) SEM images (showed a homogeneous dispersion of modified Al2O3 nanoparticles); (3) optical absorbance spectrum with time (modified Al2O3 nanoparticles showed a small change in absorbance level); and (4) photographic (suspension remained about 50 days). Table 4 shows various surface modification agent and nanolubricant combinations reported in literature with classifications, including metals, metal oxides, sulfides, composites, carbon materials, rare earth compounds and others.
3 Tribological Performance of Nanolubricants
3.1 Effects of Dispersion Stability on the Tribological Performance of Nanolubricants
Recently scientists are interested to study the effects of nanoparticles on tribological performance in based lubricants. Different classes of nanoparticles included metals (Fe, Cu, Ag, Pd), metal oxide (TiO2, Al2O3, ZrO2, ZnO, CuO), sulfides (WS2, CuS, MoS2), carbon materials (graphite, grapheme, diamond, CNT, MWNT), rare earth compounds (Y2O3, CeO2, LaF3, CeBO3), nanocomposites (Sc-Cu/GO, Cu/rGO, TiO2/SiO2) and others (ZnAl2O4, SiO2, PTFE, hBN) shows positive effect on tribological performance of nanolubricants. However, numerous researchers found that nanoparticles are tend to agglomerate due to their poor dispersion stability will negatively affects their tribological performance [7, 18, 106,107,108,109,110].
The agglomeration of the nanoparticles inhibits their free motion and make them easily pushed out from the rubbing surfaces, thus prevents the penetration of nanoparticles to rubbing suraces [45, 58]. The results from the experimental work carried out by Xie et al. [111] exhibited that SiO2 nanolubricants possess better friction reduction performance of than MoS2 nanolubricants. They also found that the worn surface of flat sample lubricated by SiO2 nanolubricants homogenously occurred over the rubbing surfaces compared to MoS2 nanolubricants. They indicated that the agglomeration of MoS2 nanoparticles inhibits the penetration to contact surfaces thus resulting in worse friction reduction performance. Zhao et al. [107] compared the effects of different nanoparticles, such as layered grapheme and MoS2 nanosheet on the tribological performance of hydraulic oil-based lubricants. The results suggested that surface roughness of the friction pairs and the temperature affects the tribological performance. From the observation outside of the wear track, they found that multilayer graphene strongly agglomerated and crystal defects, which results in poorer tribological performance than that of MoS2 nanosheet.
Research done by Hwang et al. [109] found that the fibrous nanoparticles agglomerated significantly than the spherical nanoparticles, resulting in higher coefficient of friction. The agglomerated fibrous nanoparticles became larger than the lubricant film that make them did not appear to play the role of ball bearings between the rubbing surfaces. The agglomerated fibrous nanoparticles also produced deeper grooves and severe scratches on the rubbing surfaces, results in an increase in surface roughness. Zareh-Desari et al. [112] stated that the excess amounts of nanoparticles leads to agglomeration, which causes a reverse trend on friction reduction capability and surface roughness. Khalil et al. [113] also agreed that the agglomerated nanoparticles could act as a contaminant and increase the incidence of scratches on the rubbing surfaces.
3.2 Lubrication Mechanisms
Previous researchers have reported several different mechanisms which enable nanoparticles to act as a powerful additive to improve the tribological performance of a lubricant. These include the rolling mechanism, self-repairing or mending mechanism, polishing mechanism and tribo-film formation. These mechanisms are illustrated in Fig. 1. A summary of the lubrication mechanism of nanolubricants and the surface analysis techniques used by previous researchers appears in Table 5. This table divides the reported lubrication mechanism of nanoparticles into six types based on their characteristic chemical elements: carbon and its derivatives, metals, metal oxide, sulphides, rare earth compounds and others. Note that the improvements in the tribological performance by nanolubricants are not always due to just a single lubrication mechanism; sometimes nanoparticles perform two or more lubrication mechanisms. Various surface characterisation techniques were used by previous researchers to further confirm the analysis on lubrication mechanisms, such as scanning electron microscopy (SEM), (FESEM), energy dispersive x-ray spectroscopy (EDS/EDX), atomic force microscope (AFM), x-ray photoelectron spectroscopy (XPS), Raman spectroscopy, electrical contact resistance (ECR), surface profiling and nano-indentation. Lubrication mechanisms that are responsible for improved tribological performance are discussed below.
3.2.1 Rolling Mechanism
The spherical morphology of nanoparticles are believed to provide an effective rolling mechanism between two sliding surfaces and changes the friction mode from sliding to rolling, which allows particles to reduce friction and wear. Stable spherical nanoparticles also improve extreme pressure performance and the load carrying capacity of lubricants [6, 122, 123]. The rolling effect of spherical nanoparticles depends on the film thickness. When the nanoparticle diameter is close to the film thickness, the shape of the nanoparticles would be preserved and the ball-bearing mechanism will dominate [124, 125]. However, when the film thickness is less than the particle diameter of the nanoparticles, a transfer film would be formed. Xie et al. [111] proposed that the lubrication mechanism of platelet nanoparticles is different from that of spherical nanoparticles. Platelet nanoparticles are unlikely to roll at the contact area compared with spherical nanoparticles, as platelet nanoparticles provide low friction and wear due to shearing of the weaker van der Waals bonds between molecular layers.
3.2.2 Self-repairing Effect
The self-repairing or mending effect has also been reported as a lubrication mechanism. In this case, nanoparticles are deposited on the friction surfaces or fill surface grooves to balance mass loss, and form a mono- or multi-layer film on the contact surfaces to reduce surface roughness and asperity contact [19, 93, 96, 126, 127]. Ali et al. [93] have proved the self-repairing effect of Al2O3 and TiO2 nanoparticles in engine oil by measuring the surface roughness of the worn surfaces of a piston ring. They found that a nanolubricant significantly reduced the surface roughness of the piston ring compared to the effect of using engine oil without nanoparticles. Celik et al. [128] indicated that the hBN nanoparticles in engine oil showed a self-repairing effect due to the layered structure and softness of the nanoparticles. Based on surface characterisation using SEM and EDS, they found that the samples exhibited the lowest wear rate when the nanoparticles completely covered the asperities through the mending effect. They further verified this mechanism by measuring the surface roughness values.
3.2.3 Polishing Effect
The abrasiveness of hard nanoparticles can act as a polishing agent to decrease the surface roughness of the rubbing surfaces [129]. In academic research, SiO2 nanoparticles were dispersed in mineral oil. Oxygen and silicon elements were confined in grooves and scars, leading to surface polishing and a reduction in friction temperature [130]. Gulzar et al. [131] investigated TiO2/SiO2 nanocomposites in palm TMP ester and found that the friction reduction of the nanolubricant was due to surface enhancement through the mending and polishing effects of nanocomposites. The same findings were recorded by Kotia et al. [132] who dispersed Al2O3 and SiO2 nanoparticles in SAE15W40 engine oil and proved the effects by FESEM and EDS. Chu et al. [133] investigated the polishing mechanism during oil lubrication using diamond nanoparticles as lubricant additive. It was found that diamond nanoparticles resist scuffing due to the ability to increase surface hardness and decrease friction. The surface hardness was increased by the polishing effect of diamond nanoparticles, which leads to a work hardening effect on the rubbing surface.
3.2.4 Tribo-Film Formation
Tribo-film formation is the most significant mechanism in the reduction of friction and wear of sliding contact. Tribo-film can be formed either through the depositing and adsorption of nanoparticles on the sliding surfaces, or through tribochemical reaction of nanoparticles with the contact surface. It should be noted that under high contact pressure and shear stress, nanoparticles become mechanically unstable and delamination and exfoliation of nanoparticles will be dominant [134]. The exfoliation mechanism occurs more often on particles with a layered structure including graphite-like structures, and inorganic fullerenes of transition metal dichalcogenides nanoparticles, which have weak van der Waals bonds between the molecular layers [118, 135,136,137,138,139]. Material layers of the broken nanoparticles could form as third body material transfer, and adhere to worn surface to form a tribo-film, separating contact surfaces. Moreover, the exfoliated nanoparticles will also fill in the gaps and cover the broken, patchy tribo-film to smooth the surface and provide frictional and wear benefits [118].
Sgroi et al. [139] showed in their work that the exfoliation mechanism of nanoparticles is related to the formation of tribo-film on the contact surfaces. The hypothesis was confirmed by XPS analysis, and they found the presence of molybdenum and an increased quantity of sulphur in the tribo-film. Kalin et al. [138] proposed the involvement of four sub-mechanisms for the adhesion of thin MoS2 nanosheets on the worn surface: (1) exfoliation of individual nanotubes; (2) exfoliation of MoS2 nanosheets from aggregates; (3) exfoliation from the smearing of the nanotubes; and (4) the breaking of the nanotubes and the broken smaller multi-layer MoS2 pieces subsequently further exfoliated. The evidence of this mechanism was found from optical micrographs, SEM, and EDS analysis on the worn surfaces. Ramon-Raygoza et al. [135] reported that the layered structure of multilayer graphene nanosheets and continuous exfoliation between them will allow easier surface sliding which is responsible for the formation of tribo-film.
Moreover, most researchers claimed that depositing of nanoparticles on the worn surfaces is responsible for the formation of the tribo-film, as they detected the presence of nanoparticles on the worn surfaces by surface characterisation technique using SEM–EDS/EDX [9, 15, 33, 35, 75, 140,141,142]. Sometimes, EDS/EDX could not detect deposits of nanoparticles on the worn surfaces since the amount of the deposited nanoparticles is almost negligible [143]. Thus, XPS is a suitable characterisation technique to detect the small amounts of elements on the worn surfaces. Employing XPS analysis, Chen and co-workers [116] examined the chemical states of elements on the worn surfaces to confirm the tribo-film formation. They concluded that the two major factors in the improvement of tribological performance of PAO6 base lubricant were the adsorption and depositing of a Ni layer, along with the formation of a boundary lubricating film from as-release organic surface-modifying agents and highly chemically active Ni nanocores.
The tribo-film formation can also be characterised using Raman spectroscopy. Raman peaks (‘G’ and ‘D’ peaks) can be used to determine the sp3/sp2 bonding ratio carbon in the films, which is also closely related to the tribo-film formation [117]. Zheng et al. [35] performed EDX and Raman spectrum analysis inside the wear scars to further confirm the formation of tribo-film. The adsorption of GNP additives on the sliding surfaces forming a tribo-film was demonstrated. Alves et al. [117] performed Raman spectrum analysis after EDX identified only chemical elements and not compounds. Gulzar et al. [7] confirmed formation of the tribo-film by the Raman peaks produced by the cylinder liner specimen treated with the CuO and MoS2 nanolubricant.
On the other hand, some researchers used ECR to verify the film formation generated by lubricant additives where it was a good indicator for qualitative analysis of the contact surfaces [30, 117, 133, 144, 145]. Zhang et al. [144] showed that the ECR value decrease when Cu nanoparticles was added during the friction process. The formation of tribo-film was also verified through surface characterisation techniques using SEM, EDS and XPS. Zin et al. [30] found that the addition of nanohorns reduced the COF value while increased the ECR value, indicating the formation of tribo-film. The same findings were also observed by Alves et al. [117] who used CuO and ZnO nanoparticles as EP additives in the mineral oil and synthetic oil.
On top of that, some researchers evaluate the mechanical properties of the tribo-film to further understand the origin of the tribological performance [118, 146, 147]. Instrumented imaging nano-indentation can be used to estimate the mechanical properties (hardness, H, and reduced elastic modulus, E′) of the tribo-films. Hardness is reflected by penetration depth of the indentation when a fixed load is applied, where a higher value represents a softer surface [146]. Ratoi et al. [118] performed nano-indentation to study the mechanical properties of the generated tribo-film on a steel surface lubricated with PAO oil containing WS2 nanoparticles, and compared it with traditional anti-wear additive (ZDDP) and the ZDDP-FM additive mixture. They found that the tribological performances of PAO oil containing WS2 nanoparticles was excellent due to the formation of WS2 tribo-film, with a higher hardness and elastic modulus than that of ZDDP and ZDDP + OFM. They found that the layered structure of the WS2, with exfoliated nanoparticles that filled the gaps and covered the reacted tribo-film, was responsible for the significant reduction of friction. A study by Zhao et al. [146] found that the excellent tribological performance of sunflower oil containing zinc borate ultrafine powder (ZBUP) was attributed to the increased hardness of the substrate and the formation of tribo-film on the worn surface due to the tribochemical reactions occurring on the worn surfaces. They suggested that the tribo-film composed of Fe, O, C, Zn and B elements have a lower hardness than the steel substrate.
Xie et al. [111] used XPS to ascertain the formation of tribo-film on the contact surface. They indicated that the tribochemical reaction of nano-MoS2 platelets was responsible for the formation of tribo-film, thus providing excellent tribological performance. XPS is a tool that can detect the bond between nanoparticles and other surface elements and can be used to measure the tribochemical reaction of nanoparticles. These tribochemical reactions of nano-MoS2 platelets occur either via the native oxide layer or via the metal Mg atoms, which form MoO3 and MgS. Moreover, the tribochemical reaction of nano-MoS2 platelets with the contact surface becomes easier under a high load application and flash temperature, thus increasing the rate of formation of the tribo-film. Hu et al. [110] reported that both physical adsorption and the tribochemical reaction of MoS2 nanosheets were responsible for the formation of the tribo-film. It starts with the adsorption of the nanosheets on the sliding surface to prevent direct contact. When the adsorbed nanosheets were worn out and oxidised, a complex film composed of MoO3 and FeSO4 formed on the sliding surface, which provided better wear resistance. Using XPS, Boshui et al. [120] characterised the worn surfaces of steel balls to understand the outstanding ability to reduce friction and wear of steric acid-capped cerium borate nanoparticles (SA/CeBO3) in rapeseed oil. They suggested that both SA/CeBO3 and rapeseed oil adsorbed and tribo-reacted to form tribochemical species of B2O3, CeO2 and FeO3 on the sliding surfaces. The interactions among SA/CeBO3, rapeseed oil, tribochemical species and tribomates resulted in the formation of a composite boundary lubrication film that was responsible to reduce friction and wear.
4 Challenges of Nanolubricants
In the literature, nanolubricants promise excellent tribological performance, but there are still challenges to solve. The first challenge is to produce homogeneous nanolubricants without agglomeration, which are stable for long periods. Characteristics such as particle size, shape, volume fractions and the properties of both the particles and the base oil strongly affect the stability of nanolubricants. Physical treatment alone will not improve the stability of nanolubricants; it should be combined with chemical treatments such as the use of surfactants and the surface modification of nanoparticles. However, some surfactants may not function at high temperatures, and thus the selection and functionality of surfactants at different temperature must be taken into account. Moreover, it is necessary to select the optimum concentration of surfactant/surface modifying agent since an excess may lead to instability of the nanolubricants.
The second challenge is the lack of understanding of the lubrication mechanisms responsible for the excellent tribological performance of nanolubricants. More experimental work and theoretical explanations are necessary to comprehend the interaction between nanoparticles and contact surfaces. The tribo-film formation mechanism of nanolubricants should be comprehensively understood, including their tribochemical reactions and the properties of the tribo-film it forms.
The other challenge is the high production cost of nanolubricants. When we solve the challenges associated with the stability of nanolubricants, theoretical understanding and production cost of nanolubricants, we can realise the facilitation of nanolubricants in many applications.
5 Conclusions
This paper presents an inclusive review of the recent developments in the study of nanolubricants, including preparation methods, factors affecting their stability, techniques to enhance their stability, dispersion stability analysis of nanolubricants and the lubrication mechanisms of nanoparticles as an additive to lubricants. Based on a study of the literature, it is still challenging to produce homogeneous and long-term stable nanolubricants without agglomeration. The two-step method to prepare nanolubricants is the most widely used by previous researchers. Homogeneous and long-term stable nanolubricants can be prepared by combining physical and chemical treatment (use of surfactant or surface modification).
There are no recommended standard tests to assess the stability of nanolubricants. Optical absorbance spectrum, dynamic light scattering and zeta potential are the techniques used to obtain a quantitative assessment of stability. The sedimentation method can provide a quantitative assessment of stability, where the sedimentation ratio can be calculated based on photographic evidence. Although the sedimentation method is time consuming, it is the most convenient and cost effective method.
Several lubrication mechanisms have been reported in the literature as being responsible for improved tribological performance, but there is still a lack of theoretical understanding of these mechanisms. Further theoretical and experimental research investigations are necessary to fully understand the lubrication mechanism of nanolubricants. More characterisation is needed using SEM, EDS, XPS, AFM, etc. to further understand the interaction between nanoparticles and contact surfaces. As tribo-film formation is the key mechanism in improving tribological performance, further discussion on these matters should be pursued, including chemical characterisation and properties of the tribo-film formed.
We should not lose the sight of the fact that high production cost is the main challenge to the general use of nanolubricants. Hence, these obstacles should be considered to facilitate the use of nanolubricants in many applications.
References
Sathyaseelan, B., Manikandan, E., Lakshmanan, V., Baskaran, I., Sivakumar, K., Ladchumananandasivam, R., et al. (2016). Structural, optical and morphological properties of post-growth calcined TiO2 nanopowder For opto-electronic device application: Ex-situ studies. Journal of Alloys and Compounds, 671, 486–492.
Reddy, K. R., Gomes, V. G., & Hassan, M. (2014). Carbon functionalized TiO2 nanofibers for high efficiency photocatalysis. Materials Research Express, 1(1), 15012.
Zheng, Z. Q., Yao, J. D., Wang, B., & Yang, G. W. (2015). Light-controlling, flexible and transparent ethanol gas sensor based on ZnO nanoparticles for wearable devices. Scientific Reports, 5, 11070.
Choi, S., Lee, H., Ghaffari, R., Hyeon, T., & Kim, D. H. (2016). Recent advances in flexible and stretchable bio-electronic devices integrated with nanomaterials. Advanced Materials, 28(22), 4203–4218.
Ingole, S., Charanpahari, A., Kakade, A., Umare, S. S., Bhatt, D. V., & Menghani, J. (2013). Tribological behavior of nano TiO2 as an additive in base oil. Wear, 301(1–2), 776–785.
Luo, T., Wei, X., Huang, X., Huang, L., & Yang, F. (2014). Tribological properties of Al2O3 nanoparticles as lubricating oil additives. Ceramics International, 40(5), 7143–7149.
Gulzar, M., Masjuki, H. H., Varman, M., Kalam, M. A., Mufti, R. A., Zulkifli, N. W. M., et al. (2015). Improving the AW/EP ability of chemically modified palm oil by adding CuO and MoS2 nanoparticles. Tribology International, 88, 271–279.
Wang, X. J., Li, X., & Yang, S. (2009). Influence of pH and SDBS on the stability and thermal conductivity of nanofluids. Energy and Fuels, 23(5), 2684–2689.
Chou, R., Battez, A. H., Cabello, J. J., Viesca, J. L., Osorio, A., & Sagastume, A. (2010). Tribological behavior of polyalphaolefin with the addition of nickel nanoparticles. Tribology International, 43(12), 2327–2332.
Spikes, H. (2015). Friction modifier additives. Tribology Letters, 60(5), 1–26.
Devendiran, D. K., & Amirtham, V. A. (2016). A review on preparation, characterization, properties and applications of nanofluids. Renewable and Sustainable Energy Reviews, 60, 21–40.
Mosleh, M., Atnafu, N. D., Belk, J. H., & Nobles, O. M. (2009). Modification of sheet metal forming fluids with dispersed nanoparticles for improved lubrication. Wear, 267(5–8), 1220–1225.
Jiang, H., Li, H., Zan, C., Wang, F., Yang, Q., & Shi, L. (2014). Temperature dependence of the stability and thermal conductivity of an oil-based nanofluid. Thermochimica Acta, 579, 27–30.
Fontes, D. H., Ribatski, G., & Bandarra Filho, E. P. (2015). Experimental evaluation of thermal conductivity, viscosity and breakdown voltage AC of nanofluids of carbon nanotubes and diamond in transformer oil. Diamond and Related Materials, 58, 115–121.
Wan, Q., Jin, Y., Sun, P., & Ding, Y. (2014). Rheological and tribological behaviour of lubricating oils containing platelet MoS2 nanoparticles. Journal of Nanoparticle Research, 16(5), 2386.
Thottackkad, M. V., Rajendrakumar, P. K., & Prabhakaran, N. K. (2014). Tribological analysis of surfactant modified nanolubricants containing CeO2 nanoparticles. Tribology-Materials, Surfaces and Interfaces, 8(3), 125–130.
Koshy, C. P., Rajendrakumar, P. K., & Thottackkad, M. V. (2015). Evaluation of the tribological and thermo-physical properties of coconut oil added with MoS2 nanoparticles at elevated temperatures. Wear, 330, 288–308.
Taha-Tijerina, J., Peña-Paras, L., Narayanan, T. N., Garza, L., Lapray, C., Gonzalez, J., et al. (2013). Multifunctional Nanofluids with 2D nanosheets for thermal and tribological management. Wear, 302(1–2), 1241–1248.
Song, X., Zheng, S., Zhang, J., Li, W., Chen, Q., & Cao, B. (2012). Synthesis of monodispersed ZnAl2O4 nanoparticles and their tribology properties as lubricant additives. Materials Research Bulletin, 47(12), 4305–4310.
Lou, J. F., Zhang, H., & Wang, R. (2015). Experimental investigation of graphite nanolubricant used in a domestic refrigerator. Advances in Mechanical Engineering, 7(2), 1–9.
Ma, S., Zheng, S., Cao, D., & Guo, H. (2010). Anti-wear and friction performance of ZrO2 nanoparticles as lubricant additive. Particuology, 8(5), 468–472.
Maheswaran, R., & Sunil, J. (2016). Effect of nano sized garnet particles dispersion on the viscous behavior of extreme pressure lubricant oil. Journal of Molecular Liquids, 223, 643–651.
Sadeghinezhad, E., Mehrali, M., Saidur, R., Mehrali, M., Latibari, S. T., Akhiani, A. R., et al. (2016). A comprehensive review on graphene nanofluids: recent research, development and applications. Energy Conversion and Management, 111, 466–487.
Gulzar, M., Masjuki, H. H., Kalam, M. A., Varman, M., Zulkifli, N. W. M., Mufti, R. A., et al. (2016). Tribological performance of nanoparticles as lubricating oil additives. Journal of Nanoparticle Research, 18(8), 1–25.
Ghadimi, A., Saidur, R., & Metselaar, H. S. C. (2011). A review of nanofluid stability properties and characterization in stationary conditions. International Journal of Heat and Mass Transfer, 54(17–18), 4051–4068.
Sharma, S. K., & Gupta, S. M. (2016). Preparation and evaluation of stable nanofluids for heat transfer application: a review. Experimental Thermal and Fluid Science, 79, 202–212.
Anushree, C., & Philip, J. (2016). Assessment of long term stability of aqueous nanofluids using different experimental techniques. Journal of Molecular Liquids, 222, 350–358.
Kim, J. H., Mistry, K. K., Matsumoto, N., Sista, V., Eryilmaz, O. L., & Erdemir, A. (2012). Effect of surfactant on tribological performance and tribochemistry of boric acid based colloidal lubricants. Tribology-Materials, Surfaces and Interfaces, 6(3), 134–141.
Rasheed, A. K., Khalid, M., Rashmi, W., Gupta, T. C. S. M., & Chan, A. (2016). Graphene based nanofluids and nanolubricants—Review of recent developments. Renewable and Sustainable Energy Reviews, 63, 346–362.
Zin, V., Barison, S., Agresti, F., Colla, L., Pagura, C., & Fabrizio, M. (2016). Improved tribological and thermal properties of lubricants by graphene based nano-additives. RSC Advances, 6(64), 59477–59486.
Haghighi, E. B., Nikkam, N., Saleemi, M., Behi, M., Mirmohammadi, S. A., Poth, H., et al. (2013). Shelf stability of nanofluids and its effect on thermal conductivity and viscosity. Measurement Science and Technology, 24(10), 105301.
Zhang, Y., Li, C., Jia, D., Zhang, D., & Zhang, X. (2015). Experimental evaluation of MoS2 nanoparticles in Jet mql grinding with different types of vegetable oil as base oil. Journal of Cleaner Production, 87, 930–940.
Peng, D. X., Chen, C. H., Kang, Y., Chang, Y. P., & Chang, S. Y. (2010). Size effects of SiO2 nanoparticles as oil additives on tribology of lubricant. Industrial Lubrication and Tribology, 62(2), 111–120.
Ivanov, M., & Shenderova, O. (2017). Nanodiamond-based nanolubricants for motor oils. Current Opinion in Solid State and Materials Science, 21(1), 17–24.
Zheng, D., Cai, Z. B., Shen, M. X., Li, Z. Y., & Zhu, M. H. (2016). Investigation of the tribology behaviour of the graphene nanosheets as oil additives on textured alloy cast iron surface. Applied Surface Science, 387, 66–75.
Yin, J., Xia, X., Xiang, L., & Zhao, X. (2010). Conductivity and polarization of carbonaceous nanotubes derived from polyaniline nanotubes and their electrorheology when dispersed in silicone oil. Carbon, 48(10), 2958–2967.
Alves, S. M., Mello, V. S., Faria, E. A., & Camargo, A. P. P. (2016). Nanolubricants developed from tiny CuO nanoparticles. Tribology International, 100, 263–271.
Lee, J., Cho, S., Hwang, Y., Cho, H. J., Lee, C., Choi, Y., et al. (2009). Application of fullerene-added nano-oil for lubrication enhancement in friction surfaces. Tribology International, 42(3), 440–447.
Kotia, A., & Ghosh, S. K. (2015). Experimental analysis for rheological properties of aluminium oxide (Al2O3)/gear oil (SAE EP-90) nanolubricant used in HEMM. Industrial Lubrication and Tribology, 67(6), 600–605.
Clary, D. R., & Mills, G. (2011). Preparation and thermal properties of CuO particles. Journal of Physical Chemistry C, 115(5), 1767–1775.
Sharif, M. Z., Azmi, W. H., Redhwan, A. A. M., Mamat, R., & Yusof, T. M. (2017). Performance analysis of SiO2/PAG nanolubricant in automotive air conditioning system. International Journal of Refrigeration, 75, 204–216.
Ghaednia, H., Hossain, M. S., & Jackson, R. L. (2016). Tribological performance of silver nanoparticle—Enhanced polyethylene glycol lubricants. Tribology Transactions, 59(4), 585–592.
Vakili-Nezhaad, G. R., & Dorany, A. (2009). Investigation of the effect of multiwalled carbon nanotubes on the viscosity index of lube oil cuts. Chemical Engineering Communications, 196, 997–1007.
Wang, A., Chen, L., Xu, F., & Yan, Z. (2014). In situ synthesis of copper nanoparticles within ionic liquid-in-vegetable oil microemulsions and their direct use as high efficient nanolubricants. RSC Advances, 4, 45251–45257.
Ali, M. K. A., Xianjun, H., Mai, L., Qingping, C., Turkson, R. F., & Bicheng, C. (2016). Improving the tribological characteristics of piston ring assembly in automotive engines using Al2O3 and TiO2 nanomaterials as nano-lubricant additives. Tribology International, 103, 540–554.
Fedele, L., Colla, L., Bobbo, S., Barison, S., & Agresti, F. (2011). Experimental stability analysis of different water-based nanofluids. Nanoscale Research Letters, 6(1), 300.
Cremaschi, L., Bigi, A. A., Wong, T., & Deokar, P. (2015). Thermodynamic PROPERTIES of Al2O3 nanolubricants: Part 1—Effects on the two-phase pressure drop. Science and Technology for the Built Environment, 21(5), 607–620.
Kedzierski, M. A. (2013). Viscosity and density of aluminum oxide nanolubricant. International Journal of Refrigeration, 36(4), 1333–1340.
Lee, J., Yoon, Y. J., Eaton, J. K., Goodson, K. E., & Bai, S. J. (2014). Analysis of oxide (Al2O3, CuO, and ZnO) and CNT nanoparticles disaggregation effect on the thermal conductivity and the viscosity of nanofluids. International Journal of Precision Engineering and Manufacturing, 15(4), 703–710.
Zhu, H., Li, C., Wu, D., Zhang, C., & Yin, Y. (2010). Preparation, characterization, viscosity and thermal conductivity of CaCO3 aqueous nanofluids. Science China Technological Sciences, 53(2), 360–368.
Rivera-Solorio, C. I., Payán-Rodríguez, L. A., García-Cuéllar, A. J., Ramón-Raygoza, E. D., Cadena-de-la-Peña, N. L., & Medina-Carreón, D. (2013). Formulation techniques for nanofluids. Recent Patents on Nanotechnology, 7(3), 208–215.
Guo, D., Xie, G., & Luo, J. (2014). Mechanical properties of nanoparticles: basics and applications. Journal of Physics. D. Applied Physics, 47, 1–25.
Sabareesh, R. K., Gobinath, N., Sajith, V., Das, S., & Sobhan, C. B. (2012). Application of TiO2 nanoparticles as a lubricant-additive for vapor compression refrigeration systems–An experimental investigation. International Journal of Refrigeration, 35(7), 1989–1996.
Silambarasan, M., Manikandan, S., & Rajan, K. S. (2012). Viscosity and thermal conductivity of dispersions of sub-micron TiO2 particles in water prepared by stirred bead milling and ultrasonication. International Journal of Heat and Mass Transfer, 55(25–26), 7991–8002.
Yu, W., & Xie, H. (2012). A review on nanofluids: preparation, stability mechanisms, and applications. Journal of Nanomaterials, 2012, 1–17.
Chang, H., Li, Z. Y., Kao, M. J., Huang, K. D., & Wu, H. M. (2010). Tribological property of TiO2 nanolubricant on piston and cylinder surfaces. Journal of Alloys and Compounds, 495(2), 481–484.
Azman, S. S. N., Zulkifli, N. W. M., Masjuki, H., Gulzar, M., & Zahid, R. (2016). Study of tribological properties of lubricating oil blend added with graphene nanoplatelets. Journal of Materials Research, 31(13), 1932–1938.
Kogovšek, J., & Kalin, M. (2014). Various MoS2-, WS2- and C-based micro- and nanoparticles in boundary lubrication. Tribology Letters, 53(3), 585–597.
Zhang, L., Chen, L., Wan, H., Chen, J., & Zhou, H. (2011). Synthesis and tribological properties of stearic acid-modified anatase (TiO2) nanoparticles. Tribology Letters, 41(2), 409–416.
Li, Z., Hou, X., Yu, L., Zhang, Z., & Zhang, P. (2014). Preparation of lanthanum trifluoride nanoparticles surface-capped by tributyl phosphate and evaluation of their tribological properties as lubricant additive in liquid paraffin. Applied Surface Science, 292, 971–977.
Jiang, Z., Zhang, Y., Yang, G., Yang, K., Zhang, S., Yu, L., et al. (2016). Tribological properties of oleylamine-modified ultrathin WS 2 nanosheets as the additive in polyalpha olefin over a wide temperature range. Tribology Letters, 61(3), 24.
Wu, D., Zhu, H., Wang, L., & Liu, L. (2009). Critical issues in nanofluids preparation, characterization and thermal conductivity. Current Nanoscience, 5(1), 103–112.
Mestrom, L., Lenders, J. J., de Groot, R., Hooghoudt, T., Sommerdijk, N. A., & Artigas, M. V. (2015). Stable ferrofluids of magnetite nanoparticles in hydrophobic ionic liquids. Nanotechnology, 26(28), 285602.
Choi, Y., Hwang, Y., Park, M., Lee, J., Choi, C., Jung, M., et al. (2011). Investigation of anti-wear and extreme pressure properties of nano-lubricant using graphite and Ag nanoparticles. Journal of Nanoscience and Nanotechnology, 11(1), 560–565.
Choi, C., Yoo, H. S., & Oh, J. M. (2008). Preparation and heat transfer properties of nanoparticle-in-transformer oil dispersions as advanced energy-efficient coolants. Current Applied Physics, 8(6), 710–712.
Yu, F., Chen, Y., Liang, X., Xu, J., Lee, C., Liang, Q., et al. (2017). Dispersion stability of thermal nanofluids. Progress in Natural Science: Materials International, 27(5), 531–542.
Sadri, R., Ahmadi, G., Togun, H., Dahari, M., Kazi, S. N., Sadeghinezhad, E., et al. (2014). An experimental study on thermal conductivity and viscosity of nanofluids containing carbon nanotubes. Nanoscale research letters, 9(1), 151.
Thottackkad, M. V., Perikinalil, R. K., & Kumarapillai, P. N. (2012). Experimental evaluation on the tribological properties of coconut oil by the addition of CuO nanoparticles. International Journal of Precision Engineering and Manufacturing, 13(1), 111–116.
Kamalgharibi, M., Hormozi, F., Zamzamian, S. A. H., & Sarafraz, M. M. (2016). Experimental studies on the stability of CuO nanoparticles dispersed in different base fluids: Influence of stirring, sonication and surface active agents. Heat and Mass Transfer, 52(1), 55–62.
Wamkam, C. T., Opoku, M. K., Hong, H., & Smith, P. (2011). Effects of pH on heat transfer nanofluids containing ZrO2 and TiO= nanoparticles. Journal of Applied Physics, 109(2), 024305.
Li, D., Hong, B., Fang, W., Guo, Y., & Lin, R. (2010). Preparation of well-dispersed silver nanoparticles for oil-based nanofluids. Industrial and Engineering Chemistry Research, 49, 1697–1702.
Agarwal, D. K., Vaidyanathan, A., & Kumar, S. S. (2013). Synthesis and characterization of kerosene–alumina nanofluids. Applied Thermal Engineering, 60(1–2), 275–284.
Xing, M. B., & Wang, R. X. (2013). Nanorefrigeration oil formed by C60, CNTs and mineral oil for air conditioner. Advances in Materials Research, 629, 247–254.
Jama, M., Singh, T., Gamaleldin, S. M., Koc, M., Samara, A., Isaifan, R. J., et al. (2016). Critical review on nanofluids: preparation, characterization, and applications. Journal of Nanomaterials, 2016, 1–22.
Dubey, M. K., Bijwe, J., & Ramakumar, S. S. V. (2013). PTFE based nano-lubricants. Wear, 306(1–2), 80–88.
Lee, G. J., & Rhee, C. K. (2014). Enhanced thermal conductivity of nanofluids containing graphene nanoplatelets prepared by ultrasound irradiation. Journal of Materials Science, 49(4), 1506–1511.
Alazemi, A. A., Dysart, A. D., Phuah, X. L., Pol, V. G., & Sadeghi, F. (2016). MoS2 nanolayer coated carbon spheres as an oil additive for enhanced tribological performance. Carbon, 110, 367–377.
Ettefaghi, E., Ahmadi, H., Rashidi, A., Mohtasebi, S. S., & Alaei, M. (2013). Experimental evaluation of engine oil properties containing copper oxide nanoparticles as a nanoadditive. International Journal of Industrial Chemistry, 4(1), 28.
Jatti, V. S., & Singh, T. P. (2015). Copper oxide nano-particles as friction-reduction and anti-wear additives in lubricating oil. Journal of Mechanical Science and Technology, 29(2), 793–798.
Choi, C., Oh, J. M. & Jung, M. H. (2013). Preparation method of lubricating oil and lubricating oil produced thereby. U.S. Patent 8,349,774.
Wan, Q., Jin, Y., Sun, P., & Ding, Y. (2015). Tribological behaviour of a lubricant oil containing boron nitride nanoparticles. Procedia Engineering, 102, 1038–1045.
Asadauskas, S. J., Brazinskiene, D., Bikulcius, G., Kreivaitis, R., & Padgurskas, J. (2015). Surfactant influence on stability and properties of metal nanoparticle suspensions in oil. In G. Biresaw & K. L. Mittal (Eds.), Surfactants in tribology (pp. 151–182). New York: Taylor and Francis Group.
Afifah, A. N., Syahrullail, S., & Sidik, N. A. C. (2016). Magnetoviscous effect and thermomagnetic convection of magnetic fluid: A review. Renewable and Sustainable Energy Reviews, 55, 1030–1040.
Zhou, J., Ralston, J., Sedev, R., & Beattie, D. A. (2009). Functionalized gold nanoparticles: Synthesis, structure and colloid stability. Journal of Colloid and Interface Science, 331(2), 251–262.
Timofeeva, E. V., Yu, W., France, D. M., Singh, D., & Routbort, J. L. (2011). Nanofluids for heat transfer: An engineering approach. Nanoscale Research Letters, 6(1), 182.
Halelfadl, S., Estellé, P., Aladag, B., Doner, N., & Maré, T. (2013). Viscosity of carbon nanotubes water-based nanofluids: Influence of concentration and temperature. International Journal of Thermal Sciences, 71, 111–117.
Ilyas, S. U., Pendyala, R., & Narahari, M. (2017). Stability and thermal analysis of MWCNT-thermal oil-based nanofluids. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 527, 11–22.
Zin, V., Agresti, F., Barison, S., Colla, L., Gondolini, A., & Fabrizio, M. (2013). The synthesis and effect of copper nanoparticles on the tribological properties of lubricant oils. IEEE Transactions on Nanotechnology, 12(5), 751–759.
Yang, L., Du, K., Zhang, X. S., & Cheng, B. (2011). Preparation and stability of Al2O3 nano-particle suspension of ammonia–water solution. Applied Thermal Engineering, 31(17–18), 3643–3647.
Jendrzej, S., Gökce, B., & Barcikowski, S. (2017). Colloidal stability of metal nanoparticles in engine oil under thermal and mechanical load. Chemical Engineering and Technology, 40(9), 1569–1576.
Padgurskas, J., Rukuiža, R., Kreivaitis, R., Asadauskas, S. J., & Bražinskienė, D. (2009). Tribologic behaviour and suspension stability of iron and copper nanoparticles in rapeseed and mineral oils. Tribology-Materials, Surfaces and Interfaces, 3(3), 97–102.
Kole, M., & Dey, T. K. (2011). Effect of aggregation on the viscosity of copper oxide-gear oil nanofluids. International Journal of Thermal Sciences, 50(9), 1741–1747.
Ali, M. K. A., Xianjun, H., Elagouz, A., Essa, F. A., & Abdelkareem, M. A. (2016). Minimizing of the boundary friction coefficient in automotive engines using Al2O3 and TiO2 nanoparticles. Journal of Nanoparticle Research, 18(12), 377.
Nallasamy, P., Saravanakumar, N., Nagendran, S., Suriya, E. M., & Yashwant, D. (2015). Tribological investigations on MoS 2—Based nanolubricant for machine tool slideways. Proceedings of the Institution of Mechanical Engineers, Part J: Journal of Engineering Tribology, 229(5), 559–567.
Ali, M. K. A., Xianjun, H., Mai, L., Bicheng, C., Turkson, R. F., & Qingping, C. (2016). Reducing frictional power losses and improving the scuffing resistance in automotive engines using hybrid nanomaterials as nano-lubricant additives. Wear, 365, 270–281.
Gupta, R. N., & Harsha, A. P. (2017). Antiwear and extreme pressure performance of castor oil with nano-additives. Proceedings of the Institution of Mechanical Engineers, Part J: Journal of Engineering Tribology, 0, 1–13.
Abdullah, M. I. H. C., Abdollah, M. F., Tamaldin, N., Amiruddin, H., & Nuri, N. R. M. (2016). Effect of hexagonal boron nitride nanoparticles as an additive on the extreme pressure properties of engine oil. Industrial Lubrication and Tribology, 68(4), 441–445.
Gupta, M. K., Bijwe, J., & Kadiyala, A. K. (2017). Tribo-investigations on oils with dispersants and hexagonal boron nitride particles. Journal of Tribology, 140(3), 31801.
Sánchez-López, J. C., Abad, M. D., Kolodziejczyk, L., Guerrero, E., & Fernández, A. (2011). Surface-modified Pd and Au nanoparticles for anti-wear applications. Tribology International, 44, 720–726.
Chen, T., Xia, Y., Jia, Z., Liu, Z., & Zhang, H. (2014). Synthesis, characterization, and tribological behavior of oleic acid capped graphene oxide. J Nanomater., 2014(3), 1–8.
Xiong, X., Kang, Y., & Yang, G. (2012). Preparation and evaluation of tribological properties of cu nanoparticles surface modified by tetradecyl hydroxamic acid. Tribology Letters, 46, 211–220.
Meng, Y., Su, F., & Chen, Y. (2015). Synthesis of nano-Cu/graphene oxide composites by supercritical CO2—Assisted deposition as a novel material for reducing friction and wear. Chemical Engineering Journal, 281, 11–19.
Jia, Z., Chen, T., Wang, J., Ni, J., Li, H., & Shao, X. (2015). Synthesis, characterization and tribological properties of Cu/reduced graphene oxide composites. Tribology International, 88, 17–24.
Peng, D., Kang, Y., Chen, C., & Shu, S. C. F. (2009). The tribological behavior of modified diamond nanoparticles in liquid paraffin. Industrial Lubrication and Tribology, 61(4), 213–219.
Yu, L., Zhang, L., Ye, F., Sun, M., Cheng, X., & Diao, G. (2012). Preparation and tribological properties of surface-modified nano-Y2O3 as additive in liquid paraffin. Applied Surface Science, 263, 655–659.
Srinivas, V., Thakur, R. N., & Jain, A. K. (2017). Antiwear, antifriction, and extreme pressure properties of motor bike engine oil dispersed with molybdenum disulfide nanoparticles. Tribology Transactions, 60(1), 12–19.
Zhao, J., He, Y., Wang, Y., Wang, W., Yan, L., & Luo, J. (2016). An investigation on the tribological properties of multilayer graphene and Mos2 nanosheets as additives used in hydraulic applications. Tribology International, 97, 14–20.
Peña-Parás, L., Taha-Tijerina, J., García, A., Maldonado, D., González, J. A., Molina, D., et al. (2014). Antiwear and extreme pressure properties of nanofluids for industrial applications. Tribology Transactions, 57(6), 1072–1076.
Hwang, Y., Lee, C., Choi, Y., Cheong, S., Kim, D., Lee, K., et al. (2011). Effect of the size and morphology of particles dispersed in nano-oil on friction performance between rotating discs. Journal of Mechanical Science and Technology, 25(11), 2853–2857.
Hu, K. H., Liu, M., Wang, Q. J., Xu, Y. F., Schraube, S., & Hu, X. G. (2009). Tribological properties of molybdenum disulfide nanosheets by monolayer restacking process as additive in liquid paraffin. Tribology International, 42(1), 33–39.
Xie, H., Jiang, B., He, J., Xia, X., & Pan, F. (2016). Lubrication performance of MoS2 and SiO2 nanoparticles as lubricant additives in magnesium alloy-steel contacts. Tribology International, 93, 63–70.
Zareh-Desari, B., Abaszadeh-Yakhforvazani, M., & Khalilpourazary, S. (2015). The effect of nanoparticle additives on lubrication performance in deep drawing process: Evaluation of forming load, friction coefficient and surface quality. International Journal of Precision Engineering and Manufacturing, 16(5), 929–936.
Khalil, W., Mohamed, A., Bayoumi, M., & Osman, T. A. (2016). Tribological properties of dispersed carbon nanotubes in lubricant. Fullerenes, Nanotub Carbon Nanostructures, 24(7), 479–485.
Choi, Y., Lee, C., Hwang, Y., Park, M., Lee, J., Choi, C., et al. (2009). Tribological behavior of copper nanoparticles as additives in oil. Current Applied Physics, 9(2), e124–e127.
Zareh-Desari, B., & Davoodi, B. (2016). Assessing the lubrication performance of vegetable oil-based nano-lubricants for environmentally conscious metal forming processes. J Clean Prod., 135, 1198–1209.
Chen, Y., Zhang, Y., Zhang, S., Yu, L., Zhang, P., & Zhang, Z. (2013). Preparation of nickel-based nanolubricants via a facile in situ one-step route and investigation of their tribological properties. Tribology Letters, 51, 73–83.
Alves, S. M., Barros, B. S., Trajano, M. F., Ribeiro, K. S. B., & Moura, E. (2013). Tribological behavior of vegetable oil-based lubricants with nanoparticles of oxides in boundary lubrication conditions. Tribology International, 65, 28–36.
Ratoi, M., Niste, V. B., & Zekonyte, J. (2014). WS2 nanoparticles—Potential replacement for ZDDP and friction modifier additives. RSC Advances, 4(41), 21238.
Zhang, M., Wang, X., & Liu, W. (2013). Tribological behavior of LaF3 nanoparticles as additives in poly-alpha-olefin. Industrial Lubrication and Tribology, 65(4), 226–235.
Boshui, C., Kecheng, G., Jianhua, F., Jiang, W., Jiu, W., & Nan, Z. (2015). Tribological characteristics of monodispersed cerium borate nanospheres in biodegradable rapeseed oil lubricant. Applied Surface Science, 353, 326–332.
Sia, S. Y., Bassyony, E. Z., & Sarhan, A. A. D. (2014). Development of SiO2 nanolubrication system to be used in sliding bearings. International Journal of Advanced Manufacturing Technology, 71(5–8), 1277–1284.
Aldana, P. U., Dassenoy, F., Vacher, B., Le Mogne, T., & Thiebaut, B. (2016). WS2 nanoparticles anti-wear and friction reducing properties on rough surfaces in the presence of ZDDP additive. Tribology International, 102, 213–221.
Ku, B. C., Han, Y. C., Lee, J. E., Lee, J. K., Park, S. H., & Hwang, Y. J. (2010). Tribological effects of fullerene (C60) nanoparticles added in mineral lubricants according to its viscosity. International Journal of Precision Engineering and Manufacturing, 11(4), 607–611.
Bao, Y. Y., Sun, J. L., & Kong, L. H. (2017). Tribological properties and lubricating mechanism of SiO2 nanoparticles in water-based fluid. IOP Conference Series: Materials Science and Engineering, 182, 12025.
Azman, N. F., Syahrullail, S., & Sot, M. N. H. M. (2018). Investigation of tribological properties of CuO/palm oil nanolubricant using pin-on-disc tribotester. Green materials, 6, 30–37.
Lee, C., Hwang, Y., Choi, Y., Lee, J., Choi, C., & Oh, J. (2009). A study on the tribological characteristics of graphite nano lubricants. International Journal of Precision Engineering and Manufacturing, 10(1), 85–90.
Rahmati, B., Sarhan, A. A. D., & Sayuti, M. (2014). Morphology of surface generated by end milling AL6061-T6 using molybdenum disulfide (Mos2) nanolubrication in end milling machining. Journal of Cleaner Production, 66, 685–691.
Çelik, O. N., Ay, N., & Göncü, Y. (2013). Effect of nano hexagonal boron nitride lubricant additives on the friction and wear properties of AISI 4140 Steel. Particulate Science and Technology, 31(5), 501–506.
López, T. D. F., González, A. F., Del Reguero, Á., Matos, M., Díaz-García, M. E., & Badía-Laíño, R. (2015). Engineered silica nanoparticles as additives in lubricant oils. Science and Technology of Advanced Materials, 16(5), 1–11.
Sia, S. Y., & Sarhan, A. A. D. (2014). Morphology investigation of worn bearing surfaces using SiO2 nanolubrication system. International Journal of Advanced Manufacturing Technology, 70, 1063–1071.
Gulzar, M., Masjuki, H. H., Kalam, M. A., Varman, M., Zulkifli, N. W. M., Mufti, R. A., et al. (2017). Dispersion stability and tribological characteristics of TiO2/SiO2 nanocomposite-enriched biobased lubricant. Tribology Transactions, 60(4), 670–680.
Kotia, A., Borkakoti, S., & Ghosh, S. K. (2018). Wear and performance analysis of a 4-stroke diesel engine employing nanolubricants. Particuology, 37, 54–63.
Chu, H. Y., Hsu, W. C., & Lin, J. F. (2010). Scuffing mechanism during oil-lubricated block-on-ring test with diamond nanoparticles as oil additive. Wear, 268(11–12), 1423–1433.
Dai, W., Kheireddin, B., Gao, H., & Liang, H. (2016). Roles of nanoparticles in oil lubrication. Tribology International, 102, 88–98.
Ramon-Raygoza, E. D., Rivera-Solorio, C. I., Gimenez-Torres, E., Maldonado-Cortes, D., Cardenas-Aleman, E., & Cue-Sampedro, R. (2016). Development of nanolubricant based on impregnated multilayer graphene for automotive applications: Analysis of tribological properties. Powder Technology, 302, 363–371.
Rasheed, A. K., Khalid, M., Javeed, A., Rashmi, W., Gupta, T. C. S. M., & Chan, A. (2016). Heat transfer and tribological performance of graphene nanolubricant in an internal combustion engine. Tribology International, 103, 504–515.
Lahouij, I., Dassenoy, F., de Knoop, L., Martin, J. M., & Vacher, B. (2011). In situ TEM observation of the behavior of an individual fullerene-like MoS2 nanoparticle in a dynamic contact. Tribology Letters, 42(2), 133–140.
Kalin, M., Kogovšek, J., & Remškar, M. (2012). Mechanisms and improvements in the friction and wear behavior using MoS2 nanotubes as potential oil additives. Wear, 280, 36–45.
Sgroi, M., Gili, F., Mangherini, D., Lahouij, I., Dassenoy, F., Garcia, I., et al. (2015). Friction reduction benefits in valve-train system using IF-MoS2 added engine oil. Tribology Transactions, 58(2), 207–214.
Talib, N., Nasir, R. M., & Rahim, E. A. (2017). Tribological behaviour of modified jatropha oil by mixing hexagonal boron nitride nanoparticles as a bio-based lubricant for machining processes. J Clean Prod., 147, 360–378.
Bhaumik, S., & Pathak, S. D. (2017). Effect of nano and micro friction modifier based lubricants on wear behavior between steel–steel contacts. Tribology in Industry, 39(1), 136–143.
Wang, Y., Li, C., Zhang, Y., Yang, M., Li, B. K., Dong, L., et al. (2018). Processing characteristics of vegetable oil-based nanofluid MQL for grinding different workpiece materials. International Journal of Precision Engineering and Manufacturing-Green Technology, 5(2), 327–339.
Thottackkad, V. M., Rajendrakumar, P. K., & Prabhakaran Nair, K. (2014). Experimental studies on the tribological behaviour of engine oil (SAE15W40) with the addition of CuO nanoparticles. Industrial Lubrication and Tribology, 66(2), 289–297.
Zhang, M., Wang, X., Liu, W., & Fu, X. (2009). Performance and anti-wear mechanism of Cu nanoparticles as lubricating oil additives. Industrial Lubrication and Tribology, 61(6), 311–318.
Trajano, M. F., Moura, E. I. F., Ribeiro, K. S. B., & Alves, S. M. (2014). Study of oxide nanoparticles as additives for vegetable lubricants. Materials Research, 17(5), 1124–1128.
Zhao, C., Jiao, Y., Chen, Y. K., & Ren, G. (2014). The tribological properties of zinc borate ultrafine powder as a lubricant additive in sunflower oil. Tribology Transactions, 57(3), 425–434.
Zeng, Z., & Tian, B. (2015). A study n wear and worn surfaces of grey cast iron affected by a novel silicate additive. Lubrication Science, 27(8), 479–487.
Acknowledgements
The authors would like to express their thanks to the Research Management Centre (RMC) of Universiti Teknologi Malaysia for the Research University Grant, GUP (17H96, 15J28, 20H29), Faculty of Mechanical Engineering, UTM and Ministry of Education of Malaysia for their support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Azman, N.F., Samion, S. Dispersion Stability and Lubrication Mechanism of Nanolubricants: A Review. Int. J. of Precis. Eng. and Manuf.-Green Tech. 6, 393–414 (2019). https://doi.org/10.1007/s40684-019-00080-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40684-019-00080-x