Abstract
Purpose of Review
The purpose of this review is to synthesize the current evidence regarding biological changes to the regulation of sleep-wake behavior in adolescence, summarize the impact of environmental factors (e.g., media use, school start times) on sleep, and discuss the implication of these biological and behavioral changes for adolescent emotional, physical, and cognitive development.
Recent Findings
Although our basic understanding of the sleep regulatory process in adolescence has not shifted in recent years, emerging findings highlight the influence of environmental factors (e.g., media use) on sleep behavior. Furthermore, a flurry of recent experimental studies has bolstered our understanding of the influence of short sleep on cognitive and emotional functioning. Despite these advances, longitudinal data elucidating whether there are maturational changes in the impact to sleep loss on adolescent development are largely absent.
Summary
A confluence of biological and environmental factors leads to short and ill-timed sleep among adolescents. Given the importance of sleep for the cognitive, emotional, and physical health in adolescence, the high prevalence of sleep loss in this population represents a public health issue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adolescence, the transitional phase between childhood and adulthood, is characterized by rapid physical, emotional, and cognitive maturation. Interacting with and supporting these processes is sleep. Sleep, however, is not a stable phenomenon during adolescence—sleep behavior and circadian rhythms undergo marked changes during this developmental period. In this review, we give an overview of the changes to sleep behavior and physiology during adolescence with an emphasis on literature published within the last 3 years. We also highlight recent evidence pertaining to the role of sleep in the emotional, cognitive, and physical development of adolescents. Given the focus of this review on literature published in the last 3 years, readers interested in a broader overview of the field are referred to the following two recent reviews [1, 2].
Sleep Behavior
In their seminal paper, Terman and Hocking (1913) summed up the most prominent developmental progression in sleep patterns of adolescence as follows: “It may be that adolescence tends to change the individual from the vesperal to the matinal type of sleeper.” [3]. That is, adolescents become more and more difficult to wake in the morning; they also note that adolescents stay up later in the evening. Terman and Hocking went on to say that “It is more probably due to the fact that…high school pupils…were required to do more evening work than the younger children.” [3]. Furthermore, as had others in the late 19th and early twentieth century [4,5,6], Terman and Hocking identified a reduction in the amount of sleep (consequent to the adolescent delay) and longer sleep on weekends vs. school days. Research in the twenty-first century has not altered the observed patterns of adolescents delaying sleep, reducing sleep, and extending weekend sleep as they mature. Roenneberg, for example, elegantly displayed the delaying pattern across the second decade with his marker of the midsleep time on “free” nights [7]. The logical argument that more evening homework drives the later nights, reduces sleep, and challenges morning arousals has been augmented by findings in the latter years of the twentieth century and early years of this century that identified changes to biological processes as intrinsic factors that help account for this developmental change.
Recent studies report a similar pattern of later, less, and irregular sleep for adolescents in samples from many parts of the world. To identify a few, for example, these findings are seen in Saudi Arabian school children [8]; rural Canadian adolescents from farming communities [9]; Nigerian “school-attending” adolescents [10]; adolescent-age students in Delhi, India [11]; and adolescent students in southern Brazil [12]. Of interest are the kinds of external factors that arise as contributing to adolescent sleep. For example, the study of Saudi Arabian teens showed associations of short sleep to gender, socioeconomic status, daytime naps, and screen time [8]. Not all of these associations were in the expected direction; for example, adolescents reporting television or computer (Internet) screen exposure of 2 or more hours per day were less likely than others to report sleep deprivation. This unexpected finding is a mark of the inconsistencies of findings of associated factors.
The two factors receiving most research in recent reports regarding sleep duration and timing are electronic media use and school start time. The review paper of Cain and Gradisar (2010) provided a well-reasoned model for features of media use that may adversely affect adolescent sleep: (1) media use that directly displaces sleep; (2) media use resulting in increased arousal; and (3) a circadian delaying effect of evening light exposure [13]. The various components of this model have been supported in certain reports and not in others and often are not clearly addressed. That said, Exelmans and Van den Bulck (2017a, 2017b) in two papers address displacement and arousal. As to the issue of displacement, they propose a dual process wherein media use can delay going to bed and further displace sleep by delaying the process of attempting to fall asleep [14]. With regard to presleep arousal, this team compared the impact on sleep of “regular” to “binge” evening TV watching, the latter presumed more arousing and, indeed, showing a more substantial impact on sleep of older adolescents [15]. Finally, Touitou et al. (2016) reviewed the theoretical basis for an impact of evening media use and light exposure on adolescent sleep [16].
When surveying other aspects of recent literature, most reports are cross-sectional and observational; however, a striking note is the increasing global nature of these investigations examining the association of electronic media use with disrupted sleep and/or daytime sleepiness. A study by Reynolds and colleagues (2019) identified an association in female Australian adolescents of social media use (i.e., email and instant messaging) in the hour before bed with increased reports of insufficient sleep (controlling for age) [17]. An Indonesian report [18] highlighted increased insomnia in association with social network site use, as well as academic stress. From Spain [19], we learn that Internet use time of adolescents mediates the association of sleep quality and academic performance. Arrona-Palacios (2017) from Mexico compared the impact of evening electronic media use in students who were enrolled in a school system with split classroom times, a morning (0730–1330) and an afternoon (1320–1900) group [20]. Although the afternoon group (regardless of media use) went to bed and woke up at later times, the overall association of evening media use on bed time and rise time was observed for all students. Gender and school shift interactions were also found for various types of media use in this interesting study. We note as well papers from Saudi Arabia [8], Nigeria [10], Singapore [21], and South Korea [22, 23] examining sleep behavior in the context of media use. One meta-analysis has appeared in recent years [24], identifying some inconsistencies but overall significant associations of evening electronic device use with shortened and disrupted sleep and daytime sleepiness. A final emerging construct that has been linked to social media usage and adolescent sleep outcomes is the construct of “fear of missing out” (FOMO), which Scott and Woods (2016, 2018) have linked to self-esteem and social anxiety [25, 26].
The second general topic commonly reported as affecting adolescent students’ reduced sleep duration is an early start of the school morning, typically earlier than when students were younger. Again, this association has been made in many cohorts from many countries. The USA has produced much of this literature, primarily due to the common use of tiered school bell systems: youngest students scheduled to attend later than oldest students. Recent reports have focused on efforts to improve adolescent sleep by modifying (delaying) the starting bell for school in this age range. These studies often suffer from a lack of control for other factors, often with a limitation of cross-sectional design, order effect, and lack of randomization, and students’ self-report of sleep. One group in the UK attempted to account for such limitations by running a randomized controlled trial [27]. Unfortunately, this study ran into challenges when only two of the 100 schools they approached agreed to participate. The major reason for nonparticipation was the prospect faced by being randomized to the delayed start time (10:00 am).
Other less ambitious trials have met with better success, though somewhat mixed in terms of impact on sleep. The COMPASS study in Ontario, Canada, that included 49 schools [28] showed that minimal changes in school timing (10 min delay) resulted in reported gains of nearly 25 min in sleep length. By contrast, small advances in earliest school timing (e.g., 8:30 to 8:20 am) resulted in less sleep, but not when school start was later (e.g., 9:00 to 8:50 am). A South Korean study [29] of students’ sleep when school start time was delayed until 9:00 am points out a concern voiced by many that delays of the school day will simply result in later bedtimes. Indeed, Rhie and Chae found an initial lengthening of reported sleep but after a year or two, the sleep duration advantage was lost due to students’ shifting bedtime later. Most observational studies showing increases in sleep following a delayed start have limited follow-up. An alternative design that examines sleeping patterns in students where the starting times are different, i.e., not following a change per se, shows positive sleep outcomes for those with later timing. For example, the report of Nahmod et al. (2017) showed in data collected from a US national cohort study that students attending schools with earliest start times (7:00–7:30 am) compared with those with the latest (8:30 am or later) reported 46 min less sleep at night [30]. Whether the sleep gains are maintained after changing start times remains somewhat uncertain; however, Lo and colleagues (2018) found that when students, teachers, and parents were in support of the modification of the school schedule, the benefits were sustained at least 9 months later. These data were impressive as well because of the cultural framework in the locale (Singapore) that often favors academic success over the need for sleep [31].
We highlight two additional studies where implementation of delayed school start times resulted in changes to sleep as well as changes to academic performance. The 2018 paper of Dunster and colleagues showed that the delay of the school bell resulted in more sleep and better grades [32]. One of the important components of this study was that sleep was measured pre- and post-change with actigraphy. A gain of over 30 min of sleep with a start time delay of 55 min (7:50–8:45 am) was found. Furthermore, the median grades and school attendance were improved a year after the change. One study from the UK [33••] examining several academic outcomes was remarkable for several reasons. First, the start time was delayed to 10:00 am; second, the follow-up was for 2 years; and third, the start time was reversed after the second year and the follow-up continued. Also of interest was the comparison “group,” i.e., nationwide outcomes. This study showed sustained positive outcomes for school absences attributed to illness and school-level academic performance versus the national average. Both trends reverse trajectory following the return to an earlier school start schedule. We note that one of the most fraught issues for school districts in the USA that are attempting to change the starting times is the expense of changing the transportation system to accommodate the schedules. A recent report from a group at the MIT in Boston [34••] is unique in developing an algorithm that would address the school bell scheduling and bus route planning together to derive the most economically viable solution.
The Two Process Model of Sleep Regulation in Adolescence
Understanding the unique sleep behaviors of adolescents requires an understanding of how sleep regulatory systems change across this period. According to our current understanding, two processes, a homeostatic (Process S) and circadian (Process C), regulate the timing and duration of sleep. The homeostatic process builds in a saturating exponential manner during waking and dissipates during sleep. This system favors waking after long periods of sleep and sleep after prolonged waking. The circadian system on the other hand oscillates with a period close to 24 h independent of prior sleep, and favors sleep at certain times of day. The interaction of these two systems determines sleep propensity at any given time. Changes to these two processes during adolescent development provide the context within which sleep behavior occurs during this maturational phase.
Process S
The sleep homeostatic process is reflected in sleep EEG slow-wave activity (SWA; typically defined as low-frequency activity in the range below 4.6 Hz). SWA is sensitive to prior sleep/wake history, accumulating during the waking day and dissipating during sleep. Much of our knowledge about changes to the homeostatic system in adolescence comes from a seminal study conducted in 2005 [35]. In this study, the rate at which sleep pressure built up and was dissipated was modeled using a sleep deprivation (32 h time awake) protocol. The authors observed that sleep pressure builds more slowly in post-pubertal as compared with pre- or early-pubertal adolescents. The maturational slowing of the build-up of sleep pressure led the authors to hypothesize that this change in the homeostatic system allowed more mature adolescents to delay bedtimes as compared with younger adolescents. This hypothesis was supported by a later study which found that younger adolescents (pre- /early-pubertal adolescents; mean age = 11.1 years) fell asleep faster than more mature teens (post-pubertal adolescents; mean age = 13.9 years) after 14.5 and 16.5 h of waking [36]. In line with the idea that sleep pressure builds more quickly in younger animals, a study of adolescent mice found that during a 4-h sleep deprivation phase, younger mice required more frequent intervention (21 times) to stay awake than more mature adolescent mice (7 times) [37].
In the past few years, studies have increasingly used sleep restriction/deprivation protocols in adolescents to further query the sleep homeostatic process. One study in adolescents between the ages of 9.9 and 14 years by Campbell and colleagues examined the impact of four nights consisting of three different doses of sleep (7, 8.5, and 10 h) on SWA [38]. These authors found no differences in SWA among the three sleep opportunity conditions when using the same amount of NREM sleep in the analysis. Another study in somewhat older adolescents (15–19 years) of five nights of 5 h time in bed—markedly less sleep than the Campbell study—found a substantial increase in slow-wave energy, a metric that takes into account the cumulative amount of SWA across the entire sleep period [39]. Whether the differences in findings between the two studies are due to differences in the experimental paradigm (i.e., five nights of 5 h versus four nights of 7 h), or the analysis method (use of SWE versus SWA, comparison to baseline [39] versus comparison across sleep doses [38]) is unclear. Another factor that may account for the disparate findings is the age of the participants studied. In a study of adolescent mice, only those mice at the midpoint of puberty or older showed an increase in SWA after acute sleep restriction (4 h), whereas younger mice did not [37]. This may be due to a ceiling effect that may prohibit the increase in SWA in younger brains, which already exhibit high SWA under well-slept conditions.
In contrast to the build-up of sleep pressure which undergoes a maturational shift during adolescence, evidence from longitudinal [40] and cross-sectional studies [35, 41, 42] to date suggests that the dissipation of sleep pressure does not change across adolescence. The implication of this finding is that sleep need does not change across adolescent development. This conjecture is supported by a series of experimental studies reviewed below in the “Adolescent Sleep Need” section.
Process C
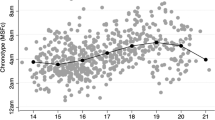
The circadian timing system (Process C) is the second process of the model that works together with the homeostatic sleep system to regulate sleep timing. The approximate 24-h (circadian) timing system, the center of which has been localized to the suprachiasmatic nucleus (SCN) of the hypothalamus, is a self-sustaining, genetically-regulated system. Among many physiological and behavioral rhythms, the circadian system signals fluctuations of more or less sleep propensity across the 24-h day regardless of prior sleep/wake duration. Early studies suggested that adolescents develop more evening-like tendencies (in sleep/wake behavior and peak performance) as they become older and progress through puberty. In now a seminal finding, Carskadon and colleagues confirmed that the central circadian clock measured objectively with salivary melatonin is later in adolescents who are at a more mature puberty stage compared with their less mature peers, despite similar sleep/wake timing conditions [43]. Similar findings are seen in other non-human mammals; the most recent report by Melo and colleagues [44] observed delayed activity rhythms in juvenile marmosets relative to their adult parents.
The mechanisms underlying this puberty-related phase delay shift in the circadian timing system are still unclear, though works within the past few years have provided some key insights. A long-standing hypothesis posits that the endogenous circadian period (internal day length) lengthens as adolescents transition through puberty. In humans, the endogenous circadian period is not exactly 24 h, and usually runs a little longer than 24 h. A circadian period longer than 24 h favors more evening tendencies, whereas a circadian period shorter than 24 h favors morningness. Therefore, a lengthening of the endogenous circadian period as adolescents mature would be consistent with the evening-like tendencies that are also occurring during this time. Data from animal (McGinnis, Lumia, Tetel, Molenda-Figueira, and Possidente 2007) and human (Carskadon and Acebo 2005) studies provided initial support for this hypothesis showing longer circadian period in adolescents compared with adults. A recent study, however, does not support this hypothesis [45]. When run in the same laboratory protocol during the same season, late- and post-pubertal adolescents (Tanner stage 4 or 5; 14.3–17.8 years) showed similar free-running circadian periods compared to adults (30.8–45.8 years). Both age groups showed an average circadian period of about 24.2 h. Ancestry differences were noted; free-running circadian period was shorter in African-American participants compared with those of other ancestries (mostly White), but this difference was primarily driven by the adult group. Trends for sex differences in both age groups were also reported. These results suggest that late- and post-pubertal adolescents have adult-like circadian periods, and do not provide support for free-running circadian period changing concurrently with sleep behavior during late adolescence. Changes to free-running circadian period may be occurring earlier in development [46], though analyses that consider ancestry and sex differences in these earlier developmental patterns may be needed.
A second area of investigation that has received attention in the past few years is the sensitivity of the adolescent circadian system to light. The circadian system can shift earlier (advance) or later (delay) in response to light exposure, but the direction of shift depends on the time of day that the light is received by the eye. In general, light exposure in the evening or first part of habitual sleep shifts the circadian system later (phase delay) and light exposure in the second half of habitual sleep or shortly after waking shifts circadian rhythms earlier (phase advance). These responses are illustrated as a phase response curve (PRC) to light. The light sensitivity hypothesis posits that a delayed circadian system as seen in older adolescents could develop as a result of a blunted response to morning phase advancing light, an exaggerated response to evening phase delaying light, or a combination of both. A few recent studies have set out to test this hypothesis. In a group of adolescents aged 9.1–15.9 years who spanned the pubertal transition [47], Crowley and colleagues assessed melatonin suppression responses to 0, 15, 150, or 500 lx of light delivered in the evening (between 23:00 and midnight) or the morning (03:00–04:00). Unexpectedly, early- to mid-pubertal adolescents (Tanner stages 1–3) showed greater sensitivity to light compared with the late- and post-pubertal adolescents (Tanner stages 4 and 5) in the evening. These findings contradict the original hypothesis and in fact, the younger group was so sensitive to evening light that melatonin was suppressed in light levels (~ 15 lx) far less than normal room lighting in most homes (~ 100 lx). In the morning, 500 lx of light-suppressed melatonin more in the early- to mid-pubertal group compared with the late- and post-pubertal group, but this difference was only a trend (p = 0.06) and it was not sustained across the 1-h light exposure. Taken together with other studies of circadian sensitivity to light in young children [48] and school-aged children [49], it is more likely that light sensitivity decreases with age and is not related to puberty.
While melatonin suppression provides an index of whether the circadian system can “see” the light stimulus, it does not provide the functional outcome of phase shift—a change in timing of the central circadian clock—to test whether older adolescents delay more to evening light exposure, advance less to morning light exposure, or both. Crowley and Eastman (2017) tested this hypothesis by constructing a phase response curve (PRC) to light in late- and post-pubertal (Tanner stages 4 and 5) adolescents aged 14.3 to 17.8 years [50]. The adolescent phase response curve to bright light showed a predictable pattern with the largest delay shifts occurring in the hours around habitual bedtime, and the largest advance shifts occurring around habitual wake-up time. Unexpectedly, the amplitude of the delay responses and the advance responses were symmetrical, suggesting that late- to post-pubertal adolescents do not show large delay shifts and small advance shifts in response to bright light as was predicted. Also, inconsistent with previous animal data, the older adolescent phase response curve does not differ from a phase response curve constructed in adults (30–45 years) using the same protocol (Crowley, unpublished). Nagare and colleagues also reported no age-related differences in melatonin suppression between adolescents (13–18 years) and individuals who were older (24–55 years) when equalizing characteristics of the light source, like dominant wavelength [51]. Whether phase shift responses of older adolescents differ from younger pre- or early-pubertal adolescents is still unclear.
These recent studies begin to discount the hypothesis that modulation of light sensitivity underlies the puberty-related delay in circadian phase and thus delay of sleep timing. What is more likely is the opportunity for light exposure in the evening increases as adolescents get older. Older adolescents (15–18 years) fall asleep later into their biological night marked by the onset of melatonin compared with younger adolescents (9–13 years) [52]. This difference is likely due to a slowed accumulation of waking homeostatic sleep pressure allowing older adolescents to stay awake later into their biological night. These changes to sleep physiology propping up evening alertness increases the likelihood of light exposure later into the evening, a time when the circadian system is particularly sensitive to phase delaying light [50]. As described in an updated version of Carskadon’s “Perfect Storm” model [1], exposure to light at this time could potentially feedback and reinforce evening alertness and delayed sleep onset in older adolescents.
Adolescent Sleep Need
The “Perfect Storm” model provides a comprehensive overview of factors that limit sleep duration of adolescents, the idea being that there is an optimal amount of sleep—or sleep need—that is curtailed by these intrinsic and extrinsic forces. While sleep need recommendations for adolescents have been offered for generations, empirical research in support of such recommendations has, until recently, been scant [53]. In 1913, Terman and Hocking summarized sleep need recommendations for children and adolescents (see Table 1). They note the “…large number of estimates based upon opinion and loose observation, but no answer based on data of scientific validity” (p. 138) [3]. It would be another 60 years before one of the earliest studies to address this research question would be conducted [54]. Carskadon and colleagues conducted a longitudinal study whereby 19 adolescents spent 3 consecutive days and nights in a sleep laboratory every year for 3 years and were given 10-h sleep opportunities each night. This approach assumes that the amount of sleep obtained in conditions of ample opportunity indicates biological sleep need. Results revealed that adolescents obtained an average of just over 9 h of sleep per night and that this duration did not change across adolescence.
Despite the importance of having a solid evidence-base for sleep recommendations, relevant research directly addressing this question has been largely absent until the last 4 years, during which several divergent approaches have been taken to address the gap in the literature. Expert panels from the American Academy of Sleep Medicine (AASM) and the National Sleep Foundation (NSF) both used the RAND appropriateness method [55] to derive sleep recommendations [56, 57]. The panels reviewed the extant literature on sleep duration and various outcomes and voted on the sleep durations that they deemed as appropriate or not appropriate across the lifespan. Both panels deemed that 8 to 10 h sleep per night was optimal for adolescents aged 13 to 18 years. This approach, however, is subject to a range of potential biases. Further, most of the studies included did not directly assess optimal sleep duration for relevant outcomes, but rather examined linear, cross-sectional relationships in which causation cannot be inferred and “optimal” sleep is not estimated.
In 2016, Ojio and colleagues estimated the sleep duration needed for optimal mood using cross-sectional data from 15,637 Japanese adolescents aged 12 to 18 years [58]. They calculated that sleep durations ≥ 8.5 h were optimal for males, while sleep durations ≥ 7.5 h were optimal for females, with adolescents sleeping significantly more or significantly less than these amounts reporting heightened depressed mood and anxiety. This study extended existing knowledge by evaluating non-linear relationships between sleep duration and mood and by using these data to estimate sleep need associated with lowest risk of mood deficits. This approach also has inherent limitations, including the inability to infer causation and the tendency associated with this approach whereby the points of lowest risk tend to approximate the norm for that population [59]. Thus, being an outlier within your own culture seems to be associated with heightened risk [59]. The fact that the sleep durations that define “outliers” vary so widely across cultures is problematic when considering the ability of this method to accurately estimate biological sleep need in a generalizable fashion.
Fuligni and colleagues similarly estimated sleep durations needed for optimal mood functioning [60••]. They examined nightly sleep duration and next-day distress (the sum of depression and anxiety items) for 2 weeks in a group of 419 Mexican American adolescents. Findings supported a non-linear association between sleep duration and mood. The sleep duration associated with lowest levels of next-day distress was 9 h per night, with both too much and too little sleep associated with heightened next-day distress. They also reported that sleep need was higher in younger adolescents and in adolescents with elevated psychopathology.
More recently, Short and colleagues [61••] utilized an experimental design to estimate sleep need using two approaches. First, they estimated sleep need from extended sleep opportunities. They also used a dose-response design from which the amount of sleep adolescents would need to obtain to sustain optimal attention performance across wake was estimated [61••]. These different approaches converged in their findings. Given extended sleep opportunities, adolescents obtained approximately 9 h of sleep per night, consistent with earlier findings [54], while the amount of sleep needed to maintain optimal sustained attention performance was estimated to be an average of 9.35 h of sleep per night.
Overall, the field has made significant advances in our understanding of, and evidence for, adolescent sleep need over recent years (Table 1). Current evidence-based estimates largely concur with those summarized by Terman and Hocking over 100 years prior that were based on “…opinion and loose observation…” [3]. Taken together, these divergent studies support sleep durations of 8 to 10 h per night for adolescents. Estimating sleep need, and the related goal of defining long and short sleep in adolescents, is fraught with challenges, not the least of which is accounting for inter-individual variability [62]. Relying upon naturally occurring sleep durations to estimate sleep need for optimal functioning, or even to examine the relationship between sleep duration and functioning, is problematic because of the paucity of adolescents who regularly obtain sleep in the recommended range [63]. The relative absence of adolescents whose normative sleep falls within the recommended range means that there is often a restriction in the range of sleep durations which potentially limits these studies from accurately estimating optimal sleep. Chronic sleep restriction in the absence of causal medical or psychological conditions is so widespread it has become the “norm” for most adolescents, while long sleep is relatively rare [64]. In normative samples, it is unclear whether long sleep causes poor outcomes or if it is a consequence of poor outcomes. Further dose-response studies including a range of sleep durations are needed to estimate optimal sleep for a range of different outcomes and thus provide a rigorous base on which to provide sleep duration recommendations.
Implications of Adolescent Sleep Restriction
The chronic sleep restriction that many adolescents experience summarized in the above sections negatively impacts multiple domains of functioning. In the below section, we summarize areas where research efforts have focused; however, we note that the impact of sleep loss is far reaching and that many disorders influence and are influenced by sleep loss.
Sleep and Cognition
Adolescence is an important developmental period with regard to the acquisition of cognitive abilities [65] since academic performance in high school influences future career opportunities [66]. Current evidence suggests that sleep supports multiple cognitive domains in adolescents, from boosting attention to promoting long-term memory consolidation and having a positive influence on the development of semantic schemata [67]. Experimental and observational studies have shown that the chronic sleep restriction that is characteristic of adolescent sleep has serious consequences for cognitive function [68], negatively impacting processing speed [69,70,71], sustained attention [68, 69], working memory [68, 69, 72], long-term memory consolidation [67, 68], psychomotor vigilance [68], executive function [68, 69], inhibition [72], and cognitive flexibility [72].
Although the supportive role of sleep with regard to cognitive function has been extensively studied and established in adults, only recently have experimental studies begun to systematically address the impact of sleep restriction and deprivation on cognitive performance in adolescence. These studies attempt to replicate what happens in the “real world” and typically compare multiple nights (4 to 7 nights) of sleep restriction (5 h to 6.5 h TIB) to adequate sleep (9 h to 10.5 h TIB) in order to study the impact of sleep loss on adolescent cognitive functioning. The results of these studies are clear—a few hours of sleep loss over several days significantly impact multiple domains of cognitive functioning, including information processing speed [70, 71], sustained attention [69], working memory [69], executive function [69], declarative memory [73,74,75], and spatial memory [73].
Interestingly, not all domains of cognitive function are equally impacted by sleep loss. In a series of studies using experimental manipulation of sleep combined with measurement of several cognitive domains, the largest effect sizes were found for sustained attention (psychomotor vigilance task; PVT) and processing speed, as compared with higher order cognitive functions, such as executive functions [69,70,71]. Furthermore, higher order cognitive functions may more quickly return to baseline levels after recovery sleep. For example, following 1 week of significant sleep restriction (5 h TIB), two nights of recovery sleep (9 h TIB) were insufficient to achieve a complete recovery in processing speed [69], while performance on working memory and executive function task returned to near-baseline levels.
Napping can in part ameliorate the detrimental effects of sleep restriction on processing speed [71]. In the aforementioned studies, participants were randomly assigned to either a 9-h, 6.5-h, or 5-h TIB combined with a 1.5-h afternoon nap. Thus, total sleep opportunity was the same in the 6.5 h and 5 h + 1.5 h nap conditions. Participants who were given a nap opportunity had better sustained attention [76], working memory and executive function [69], faster speed of processing [71], and declarative memory [73] compared with those whose sleep opportunity was confined to nocturnal sleep. We note that these effects were strongest in the afternoon and evening following the nap as compared to morning performance prior to the nap.
More recent evidence suggests that chronotype may play just as important a role in academic performance as sleep duration. A study comparing school performance in early and late chronotypes found that students with late chronotypes had worse grades [66]. Surprisingly, this study found that chronotype was a better predictor of academic performance than sleep duration, suggesting that the association between these factors may be driven by daytime somnolence or a mismatch between environmental demands and biological predisposition [66]. Along the same lines, a study by Philipps and colleagues which used a novel metric, sleep regularity index (SRI), to quantify the regularity of sleep timing (bed and rise times) using sleep diaries in 61 undergraduate students for 30 days [77] found a positive correlation between sleep regularity and academic performance [77]. In other words, a pattern of more regular sleeping was associated with better academic performance [77]. Therefore, other measures such as chronotype and sleep regularity may also be important in sustaining and supporting academic and cognitive performance.
Sleep and Mental Health
Adolescence is a sensitive period for the emergence of sleep and mood problems [78] in non-psychiatric samples. Previous research has suggested a bidirectional relationship between sleep and mental health, where poor sleep predicts later mental health problems, while disrupted sleep can exacerbate an existing disorder. More recent epidemiological as well as experimental studies continue to show that adolescents who do not get sufficient sleep suffer from diminished mental well-being [79,80,81]. For example, in a study of 27,939 adolescents, 1-h less of weekday sleep was associated with greater odds of feelings of hopelessness, suicidal ideation, and substance use [82].
Not only is sleep duration an important factor with regard to mental health, but self- /parent-reported [81,82,83,84,85] and objectively measured [78, 86] sleep quality is also associated with mood [81, 83, 84, 86], mental well-being [78], behavioral difficulties [78, 82, 87], difficulties regulating emotion [2, 78, 88, 89], psychiatric disorders [79, 81, 82, 90, 91], and aggression [89]. Thus, the higher the sleep quality adolescents experience, the better their emotional, social, and behavioral performance [88].
Chronotype appears to be another important factor when it comes to adolescent mental health. For example, an observational study including 29,635 students between the ages of 10 and 18 years investigated the role of chronotype on mental health, finding that a later chronotype was associated with worse mental health, independent of sleep duration and across internalizing (e.g., anxiety, depression) and externalizing (e.g., aggressive behavior, delinquency) mental health domains [92]. Other studies examining circadian preference found that eveningness is linked to a higher probability of rule-breaking behavior [93] as well as conduct problems [93], lower self-regulation [94], attention deficit/hyperactivity problems [93], affective problems [93], and somatic complaints [93], when compared with a circadian preference towards morningness [93, 94].
While chronotype is largely genetically determined in adolescence [95], there is evidence that environmental factors may mediate this relationship. For example, later school start times were found to be associated with greater psychological health in adolescents [96,97,98,99], although causality could not be inferred. In line with this idea, another study demonstrated that school start times may serve as a moderator in models of adolescent sleep and their daily functioning [98]. In an observational study, 146 adolescents (mean age 16.2; SD = 1.0) wore an actigraph that assessed their bedtime, time in bed, sleep onset latency, and rise time. The measurements took place during a 15-day vacation and offered relatively unconstrained sleep opportunity [86]. In the aforementioned study, more variable time in bed as well as more variable sleep onset latency were found to be linked to poorer self-reported sleep quality, which was in turn associated with more negative mood [86]. This effect was mediated by poorer perceived sleep quality [86]. These findings suggest that objective intra-individual sleep variability is relevant to how adolescents perceive their sleep and their mood [86]. The authors posit that reducing variability in sleep timing and duration and in turn variability of sleep onset latency may decrease sleep complaints and improve mood in adolescents [86].
To summarize, current evidence suggests that sleep duration, quality, and chronotype all impact mental health and mood in non-psychiatric populations. While, the relative contribution of each factor is unclear and somewhat difficult to disentangle, current evidence suggests that each may confer its own unique risk.
Sleep and Physical Health
Although most research has focused on how sleep supports brain function, it is becoming increasingly clear that sleep also benefits the body. During adolescence, a period of rapid bodily growth, sleep supports physical developmental and well-being. For example, negative associations between short sleep duration and obesity [100,101,102,103], adiposity [80], cardiometabolic biomarkers [80], type 1 diabetes mellitus [104], asthma [105], and headache as well as migraine [106] and menstrual problems [107] have been found. We note that while our review focuses on the associations between physical health and sleep in the general population, most medical disorders (e.g., migraine) are accompanied by disruptions in sleep, which may in turn exacerbate the underlying disorder.
Worldwide, obesity rates are increasing, with estimates that approximately one in five children and adolescents are overweight or obese [108]. The chronic sleep restriction observed in adolescents may be one of many factors that contributes to this rising trend. In a systematic review of the relationships between objectively and subjectively measured sleep duration and health indicators in children and youth between 5 and 17 years of age, longer sleep duration was associated with lower obesity levels [80]. Furthermore, a week long cross-sectional actigraphy study in 528 (mean age 14.4; SD = 2.1 years) Mexican youth found that adolescents with sufficient sleep had lower BMIs than those with insufficient sleep duration [109]. Furthermore, among the insufficient sleepers, those with regular sleep timing (e.g., stable sleep) had slightly lower BMI as compared with adolescents with irregular sleep timing.
Along the same lines, chronotype has been found to be linked to physical health (e.g., more headaches, stomach aches, back aches, dizziness, and worse self-related health) [110]. A later chronotype was related to worse physical health and also unhealthy behavior (e.g., daily soft drink consumption, smoking, screen time) in Canadian adolescents aged from 10 to 18 years [110].
Further studies are required to examine the circumstances under which physical health is particularly vulnerable to sleep deprivation and vice versa as well as to deepen knowledge about sleep quality and chronotype and their influence on physical health. To assess physical health problems as well as the sleep complaints of adolescents is crucial and may serve as an important domain for clinical interventions.
Conclusion
During adolescence, sleep duration and timing are constrained by environmental factors, resulting in sleepy teens. While mounting evidence suggests a stable need for about 9 h of sleep, changes to the bioregulatory processes of the homeostatic and circadian timing system during adolescence push sleep later, while school start times pull sleep earlier. This tug of war between biological and societal demands results in a pattern of insufficient and irregular sleep, with consequences for cognitive, mental, and physical health. Though the field has made rapid progress in the past 3 years, many open questions remain. One looming challenge is to make use of longitudinal data to map developmental trajectories and identifying factors that make individuals vulnerable or resilient to irregular or short sleep. Another is to communicate research findings to stakeholders, such as parents, educators, and policy makers, to improve the sleep of adolescents.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Crowley SJ, Wolfson AR, Tarokh L, Carskadon MA. An update on adolescent sleep: new evidence informing the perfect storm model. J Adolesc. 2018;67:55–65.
Tarokh L, Saletin JM, Carskadon MA. Sleep in adolescence: physiology, cognition and mental health. Neurosci Biobehav Rev. 2016;70:182–8.
Terman LM, Hocking A. The sleep of school children, its distribution according to age, and its relation to physical and mental efficiency: part III: the conditions of children’s sleep. J Educ Psychol. 1913;4:269–82.
Claparède E. Theorie Biologique du Sommeil. Arch Psychol. 1905:245–349.
Dukes C. Remedies for the needless injury to children. London; 1899.
Manacéïne M. Sleep: its physiology pathology hygiene and psychology. By Marie de Manaceïne. 1897; Available from: http://archive.org/details/sleepitsphysiolo00mana. Accessed May 20, 2019
Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, et al. A marker for the end of adolescence. Curr Biol. 2004;14:R1038–9.
Nasim M, Saade M, AlBuhairan F. Sleep deprivation: prevalence and associated factors among adolescents in Saudi Arabia. Sleep Med. 2019;53:165–71.
Janssen I, Berg RL, Marlenga B, Pickett W. 2018 Sleep in farm adolescents. The Journal of Rural Health [Internet]. [cited 2019 May 27];0. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/jrh.12341
Olorunmoteni OE, Fatusi AO, Komolafe MA, Omisore A. Sleep pattern, socioenvironmental factors, and use of electronic devices among Nigerian school-attending adolescents. Sleep Health: Journal of the National Sleep Foundation. 2018;4:551–7.
Singh R, Suri JC, Sharma R, Suri T, Adhikari T. Sleep pattern of adolescents in a school in Delhi, India: impact on their mood and academic performance. Indian J Pediatr. 2018;85:841–8.
Felden ÉPG, Filipin D, Barbosa DG, Andrade RD, Meyer C, Louzada FM. Factors associated with short sleep duration in adolescents. Rev Paul Pediatr. 2016;34:64–70.
Cain N, Gradisar M. Electronic media use and sleep in school-aged children and adolescents: a review. Sleep Med. 2010;11:735–42.
Exelmans L, Van den Bulck J. Binge viewing, sleep, and the role of pre-sleep arousal. J Clin Sleep Med. 2017;13:1001–8.
Exelmans L, Van den Bulck J. “Glued to the tube”: the interplay between self-control, evening television viewing, and bedtime procrastination. Commun Res. 2017;0093650216686877.
Touitou Y, Touitou D, Reinberg A. Disruption of adolescents’ circadian clock: the vicious circle of media use, exposure to light at night, sleep loss and risk behaviors. Journal of Physiology-Paris. 2016;110:467–79.
Reynolds AC, Meltzer LJ, Dorrian J, Centofanti SA, Biggs SN. Impact of high-frequency email and instant messaging (E/IM) interactions during the hour before bed on self-reported sleep duration and sufficiency in female Australian children and adolescents. Sleep Health. 2019;5:64–7.
Nursalam N, Octavia M, Tristiana RD, Efendi F. Association between insomnia and social network site use in Indonesian adolescents. Nurs Forum. 2019;54:149–56.
Adelantado-Renau M, Diez-Fernandez A, Beltran-Valls MR, Soriano-Maldonado A, Moliner-Urdiales D. The effect of sleep quality on academic performance is mediated by Internet use time: DADOS study. J Pediatr. 2018; Available from: http://www.sciencedirect.com/science/article/pii/S0021755718300135.
Arrona-Palacios A. High and low use of electronic media during nighttime before going to sleep: a comparative study between adolescents attending a morning or afternoon school shift. J Adolesc. 2017;61:152–63.
Nasirudeen AMA, Lee Chin Adeline L, Wat Neo Josephine K, Lay Seng L, Wenjie L. Impact of social media usage on daytime sleepiness: a study in a sample of tertiary students in Singapore. Digit Health. 2017;3:2055207617699766.
Mortazavi SMJ, Mortazavi SAR, Paknahad M. Late use of electronic media and its association with sleep, depression, and suicidality among Korean adolescents. Sleep Med. 2017;32:275–6.
Seo WH, Kwon JH, Eun S-H, Kim G, Han K, Choi BM. Effect of socio-economic status on sleep. J Paediatr Child Health. 2017;53:592–7.
Carter B, Rees P, Hale L, Bhattacharjee D, Paradkar MS. Association between portable screen-based media device access or use and sleep outcomes: a systematic review and meta-analysis. JAMA Pediatr. 2016;170:1202–8.
Scott H, Woods HC. Fear of missing out and sleep: cognitive behavioural factors in adolescents’ nighttime social media use. J Adolesc. 2018;68:61–5.
Woods HC, Scott H. #Sleepyteens: social media use in adolescence is associated with poor sleep quality, anxiety, depression and low self-esteem. J Adolesc. 2016;51:41–9.
Illingworth G, Sharman R, Jowett A, Harvey C-J, Foster RG, Espie CA. Challenges in implementing and assessing outcomes of school start time change in the UK: experience of the Oxford Teensleep study. Sleep Med. 2018.
Patte KA, Qian W, Cole AG, Faulkner G, Chaput J-P, Carson V, et al. School start time changes in the COMPASS study: associations with youth sleep duration, physical activity, and screen time. Sleep Med. 2019;56:16–22.
Rhie S, Chae KY. Effects of school time on sleep duration and sleepiness in adolescents. PLoS One. 2018;13:e0203318.
Nahmod NG, Lee S, Buxton OM, Chang A-M, Hale L. High school start times after 8:30 am are associated with later wake times and longer time in bed among teens in a national urban cohort study. Sleep Health. 2017;3:444–50.
Lo JC, Lee SM, Lee XK, Sasmita K, Chee NIYN, Tandi J, et al. Sustained benefits of delaying school start time on adolescent sleep and well-being. Sleep. 2018;41 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5995199/.
Dunster GP, de la L I, Ben-Hamo M, Nave C, Fleischer JG, Panda S, et al. Sleep more in Seattle: later school start times are associated with more sleep and better performance in high school students. Science Advances. 2018;4:eaau6200.
•• Kelley P, Lockley SW, Kelley J, Evans MDR. Is 8:30 a.m. still too early to start school? A 10:00 a.m. school start time improves health and performance of students aged 13–16. Front Hum Neurosci. 2017;11. Available from: https://www.frontiersin.org/articles/10.3389/fnhum.2017.00588/full. This paper tests the efficacy of a start time switch to 10:00 am in a 4-year study that used an A-B-A design and measured results in comparison to concurrently collected national norms. This unique study design had many strengths not found in most studies. In addition, it tested an even later start time than most. The study also used outcomes that are not based on sleep duration per se, including showing improvements in absences due to illness and academic performance, both of which reversed when the school start time was reversed.
•• Bertsimas D, Delarue A, Martin S. Optimizing schools’ start time and bus routes. PNAS. 2019;116:5943–8 This paper provides a strategy for minimizing the cost of school transportation when districts change school schedules. The paper describes an algorithm for setting start times and routing the busses, which was shown to save cost in the city of Boston, USA.
Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in adolescents. Sleep. 2005;28:1446–54.
Taylor DJ, Jenni OG, Acebo C, Carskadon MA. Sleep tendency during extended wakefulness: insights into adolescent sleep regulation and behavior. J Sleep Res. 2005;14:239–44.
Nelson AB, Faraguna U, Zoltan JT, Tononi G, Cirelli C. Sleep patterns and homeostatic mechanisms in adolescent mice. Brain Sci. 2013;3:318–43.
Campbell IG, Kraus AM, Burright CS, Feinberg I. Restricting time in bed in early adolescence reduces both NREM and REM sleep but does not increase slow wave EEG. Sleep. 2016;39:1663–70.
Ong JL, Lo JC, Gooley JJ, Chee MWL. EEG changes across multiple nights of sleep restriction and recovery in adolescents: the need for sleep study. Sleep. 2016;39:1233–40.
Campbell IG, Darchia N, Higgins LM, Dykan IV, Davis NM, de Bie E, et al. Adolescent changes in homeostatic regulation of EEG activity in the delta and theta frequency bands during NREM sleep. Sleep. 2011;34:83–91.
Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep. 2004;27:774–83.
Tarokh L, Carskadon MA, Achermann P. Dissipation of sleep pressure is stable across adolescence. Neuroscience. 2012;216:167–77.
Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R. An approach to studying circadian rhythms of adolescent humans. J Biol Rhythms. 1997;12:278–89.
Melo PR, Gonçalves BSB, Menezes AAL, Azevedo CVM. Circadian activity rhythm in pre-pubertal and pubertal marmosets (Callithrix jacchus) living in family groups. Physiol Behav. 2016;155:242–9.
Crowley SJ, Eastman CI. Free-running circadian period in adolescents and adults. J Sleep Res. 2018;27:e12678.
Carskadon M, Barker D, Crowley SJ, Rupp T, Van Reen E 2017. Changes to the circadian timing system may arise in early adolescence. 9th biennial Pediatric Sleep Medicine Conference, Amelia Island, Florida
Crowley SJ, Cain SW, Burns AC, Acebo C, Carskadon MA. Increased sensitivity of the circadian system to light in early/mid-puberty. J Clin Endocrinol Metab. 2015;100:4067–73.
Akacem LD, Wright KP, LeBourgeois MK. Sensitivity of the circadian system to evening bright light in preschool-age children. Physiol Rep. 2018;6.
Higuchi S, Nagafuchi Y, Lee S, Harada T. Influence of light at night on melatonin suppression in children. J Clin Endocrinol Metab. 2014;99:3298–303.
Crowley SJ, Eastman CI. Human adolescent phase response curves to bright white light. J Biol Rhythms. 2017;32:334–44.
Nagare R, Rea MS, Plitnick B, Figueiro MG. Nocturnal melatonin suppression by adolescents and adults for different levels, spectra, and durations of light exposure. J Biol Rhythms. 2019;34:178–94.
Crowley SJ, Van Reen E, LeBourgeois MK, Acebo C, Tarokh L, Seifer R, et al. A longitudinal assessment of sleep timing, circadian phase, and phase angle of entrainment across human adolescence. PLoS ONE. 2014;9:e112199.
Matricciani LA, Olds TS, Blunden S, Rigney G, Williams MT. Never enough sleep: a brief history of sleep recommendations for children. Pediatrics. 2012;129:548–56.
Carskadon MA, Harvey K, Duke P, Anders TF, Litt IF, Dement WC. Pubertal changes in daytime sleepiness. Sleep. 1980;2:453–60.
Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle, JR, Lazaro P, et al. The RAND/UCLA appropriateness method user’s manual. 2001. Available from: https://www.rand.org/pubs/monograph_reports/MR1269.html Accessed November 20, 2019
Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1:233–43.
Paruthi S, Brooks LJ, D’Ambrosio C, Hall WA, Kotagal S, Lloyd RM, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016;12:785–6.
Ojio Y, Nishida A, Shimodera S, Togo F, Sasaki T. Sleep duration associated with the lowest risk of depression/anxiety in adolescents. Sleep. 2016;39:1555–62.
Beebe DW. The cumulative impact of adolescent sleep loss: next steps. Sleep. 2016;39:497–9.
•• Fuligni AJ, Bai S, Krull JL, Gonzales N. Individual differences in optimum sleep for daily mood during adolescence. J Clin Child Adolesc Psychol. 2017;53:1–11 This paper makes a unique contribution to the extant literature by using a within subjects design to determine the sleep duration needed or optimal next-day mood.
•• Short MA, Weber N, Reynolds C, Coussens S, Carskadon MA. Estimating adolescent sleep need using dose-response modeling. Sleep. 2018;41 This study estimated adolescents sleep need by examining the time course as well as the severity of sleep-related deficits using a dose-dependent design. The results showed that 9.35 h of sleep are needed to maintain optimal sustained attention performance.
Sawyer E, Heussler H, Gunnarsson R. Defining short and long sleep duration for future paediatric research: a systematic literature review. J Sleep Res. 2019:e12839.
Short MA, Blunden S, Rigney G, Matricciani L, Coussens S, C MR, et al. Cognition and objectively measured sleep duration in children: a systematic review and meta-analysis. Sleep Health. 2018;4:292–300.
Galland BC, Short MA, Terrill P, Rigney G, Haszard JJ, Coussens S, et al. Establishing normal values for pediatric nighttime sleep measured by actigraphy: a systematic review and meta-analysis. Sleep. 2018;41.
Andersen SL. Commentary on the special issue on the adolescent brain: adolescence, trajectories, and the importance of prevention. Neuroscience & Biobehavioral Reviews. 2016;70:329–33.
Zerbini G, van der Vinne V, Otto LKM, Kantermann T, Krijnen WP, Roenneberg T, et al. Lower school performance in late chronotypes: underlying factors and mechanisms. Sci Rep [Internet]. 2017 [cited 2019 Apr 18];7. Available froem: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5491513/
Prehn-Kristensen A, Göder R. Schlaf und Kognition bei Kindern und Jugendlichen. Zeitschrift für Kinder- und Jugendpsychiatrie und Psychotherapie. 2018;46:405–22.
de Bruin EJ, van Run C, Staaks J, Meijer AM. Effects of sleep manipulation on cognitive functioning of adolescents: a systematic review. Sleep Med Rev. 2017;32:45–57.
Lo JC, Ong JL, Leong RLF, Gooley JJ, Chee MWL. Cognitive performance, sleepiness, and mood in partially sleep deprived adolescents: the need for sleep study. Sleep. 2016;39:687–98.
Cohen-Zion M, Shabi A, Levy S, Glasner L, Wiener A. Effects of partial sleep deprivation on information processing speed in adolescence. J Int Neuropsychol Soc. 2016;22:388–98.
Lim J, Lo JC, Chee MWL. Assessing the benefits of napping and short rest breaks on processing speed in sleep-restricted adolescents. J Sleep Res. 2017;26:219–26.
Tee JYH, Gan WY, Tan K-A, Chin YS 2018. Obesity and unhealthy lifestyle associated with poor executive function among Malaysian adolescents. PLoS One [Internet]. [cited 2019 Apr 17];13. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5903659/
Cousins JN, Van Rijn E, Ong JL, Chee MWL. A split sleep schedule rescues short-term topographical memory after multiple nights of sleep restriction. Sleep. 2019;42.
Huang S, Deshpande A, Yeo S-C, Lo JC, Chee MWL, Gooley JJ. Sleep restriction impairs vocabulary learning when adolescents cram for exams: the need for sleep study. Sleep. 2016;39:1681–90.
Lo JC, Bennion KA, Chee MWL. Sleep restriction can attenuate prioritization benefits on declarative memory consolidation. Journal of Sleep Research. 2016;25:664–72.
Lo JC, Twan DCK, Karamchedu S, Lee XK, Ong JL, Van Rijn E, et al. 2019 Differential effects of split and continuous sleep on neurobehavioral function and glucose tolerance in sleep-restricted adolescents. Sleep [Internet [cited 2019 May 10]; Available from: https://academic.oup.com/sleep/advance-article/doi/10.1093/sleep/zsz037/5316239
Phillips AJK, Clerx WM, O’Brien CS, Sano A, Barger LK, Picard RW, et al. Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Scientific Reports. 2017;7:3216.
Boafo A, Armitage R, Greenham S, Tavakoli P, Dale A, Nixon A, et al. 2019 Sleep architecture in adolescents hospitalized during a suicidal crisis. Sleep Medicine [Internet]. [cited 2019 Apr 23]; Available from: http://www.sciencedirect.com/science/article/pii/S138994571930005X
Berger A, Wahlstrom K, Widome R. Relationships between sleep duration and adolescent depression: a conceptual replication. Sleep Health. 2019;5:175–9.
Chaput J-P, Gray CE, Poitras VJ, Carson V, Gruber R, Olds T, et al. Systematic review of the relationships between sleep duration and health indicators in school-aged children and youth. Appl Physiol Nutr Metab. 2016;41:S266–82.
Zhang J, Paksarian D, Lamers F, Hickie IB, He J, Merikangas KR. Sleep patterns and mental health correlates in US adolescents. J Pediatr. 2017;182:137–43.
Winsler A, Deutsch A, Vorona RD, Payne PA, Szklo-Coxe M. Sleepless in Fairfax: the difference one more hour of sleep can make for teen hopelessness, suicidal ideation, and substance use. J Youth Adolesc. 2015;44:362–78.
Brand S, Kalak N, Gerber M, Clough PJ, Lemola S, Pühse U, et al. During early and mid-adolescence, greater mental toughness is related to increased sleep quality and quality of life. J Health Psychol. 2016;21:905–15.
Hestetun I, Svendsen MV, Oellingrath IM. Sleep problems and mental health among young Norwegian adolescents. Nord J Psychiatry. 2018;72:578–85.
Zhang L, Yang Y, Liu Z-Z, Jia C-X, Liu X. Sleep disturbance mediates the association between intrafamily conflict and mental health problems in Chinese adolescents. Sleep Med. 2018;46:74–80.
Bei B, Manber R, Allen NB, Trinder J, Wiley JF. Too long, too short, or too variable? Sleep intraindividual variability and its associations with perceived sleep quality and mood in adolescents during naturalistically unconstrained sleep. Sleep. 2017;40.
Gregory AM, Sadeh A. Sleep, emotional and behavioral difficulties in children and adolescents. Sleep Med Rev. 2012;16:129–36.
Brand S, Lemola S, Mikoteit T, Holsboer-Trachsler E, Kalak N, Sadeghi Bahmani D, et al. Sleep and psychological functioning of children and adolescents – a narrative review. Praxis der Kinderpsychologie und Kinderpsychiatrie. 2019;68:128–45.
Wang B, Eastwood PR, Becker A, Isensee C, Wong JWY, Huang R-C, et al. 2019 Concurrent developmental course of sleep problems and emotional/behavioral problems in childhood and adolescence as reflected by the dysregulation profile. Sleep [Internet]. [cited 2019 Apr 18];42. Available from: https://academic.oup.com/sleep/article/42/3/zsy243/5231982
Conklin AI, Yao CA, Richardson CG. Chronic sleep deprivation and gender-specific risk of depression in adolescents: a prospective population-based study. BMC Public Health. 2018;18:724.
Yeo SC, Jos AM, Erwin C, Lee SM, Lee XK, Lo JC, et al. Associations of sleep duration on school nights with self-rated health, overweight, and depression symptoms in adolescents: problems and possible solutions. Sleep Med. 2018.
Gariépy G, Riehm KE, Whitehead RD, Doré I, Elgar FJ. Teenage night owls or early birds? Chronotype and the mental health of adolescents. J Sleep Res. 2018:e12723.
Merikanto I, Pesonen A-K, Kuula L, Lahti J, Heinonen K, Kajantie E, et al. Eveningness as a risk for behavioral problems in late adolescence. Chronobiol Int. 2017;34:225–34.
Owens JA, Dearth-Wesley T, Lewin D, Gioia G, Whitaker RC. Self-regulation and sleep duration, sleepiness, and chronotype in adolescents. Pediatrics. 2016;138.
Inderkum AP, Tarokh L. High heritability of adolescent sleep-wake behavior on free, but not school days: a long-term twin study. Sleep. 2018;41.
Berger AT, Widome R, Troxel WM. School start time and psychological health in adolescents. Curr Sleep Med Rep. 2018;4:110–7.
Marx R, Tanner-Smith EE, Davison CM, Ufholz L-A, Freeman J, Shankar R, et al. Later school start times for supporting the education, health, and well-being of high school students. Cochrane Database Syst Rev. 2017;7:CD009467.
Peltz JS, Rogge RD, Connolly H, O’Connor TG. A process-oriented model linking adolescents’ sleep hygiene and psychological functioning: the moderating role of school start times. Sleep Health. 2017;3:465–71.
Wahlstrom KL, Berger AT, Widome R. Relationships between school start time, sleep duration, and adolescent behaviors. Sleep Health. 2017;3:216–21.
Cespedes Feliciano EM, Quante M, Rifas-Shiman SL, Redline S, Oken E, Taveras EM. Objective sleep characteristics and cardiometabolic health in young adolescents. Pediatrics. 2018;142.
Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;9:151–61.
Miller MA, Kruisbrink M, Wallace J, Ji C, Cappuccio FP. Sleep duration and incidence of obesity in infants, children, and adolescents: a systematic review and meta-analysis of prospective studies. Sleep. 2018;41.
Pacheco SR, Miranda AM, Coelho R, Monteiro AC, Bragança G, Loureiro HC. Overweight in youth and sleep quality: is there a link? Arch Endocrinol Metab. 2017;61:367–73.
Monzon A, McDonough R, Meltzer LJ, Patton SR. Sleep and type 1 diabetes in children and adolescents: proposed theoretical model and clinical implications. Pediatric Diabetes. 2019;20:78–85.
Lawless C, Turner EM, LeFave E, Koinis-Mitchell D, Fedele DA. Sleep hygiene in adolescents with asthma. J Asthma. 2018:1–9.
Lateef T, Witonsky K, He J, Ries MK. Headaches and sleep problems in US adolescents: findings from the National Comorbidity Survey – Adolescent Supplement (NCS-A). Cephalalgia. 2019;0333102419835466.
Wang Z-Y, Liu Z-Z, Jia C-X, Liu X. Age at menarche, menstrual problems, and daytime sleepiness in Chinese adolescent girls. Sleep [Internet]. [cited 2019 Apr 23]; Available from: https://academic.oup.com/sleep/advance-article/doi/10.1093/sleep/zsz061/5373063
WHO | Overweight and obesity [Internet]. WHO. 2017 [cited 2019 May 10]. Available from: http://www.who.int/gho/ncd/risk_factors/overweight_obesity/obesity_adolescents/en/
Jansen EC, Dunietz GL, Chervin RD, Baylin A, Baek J, Banker M, et al. Adiposity in adolescents: the interplay of sleep duration and sleep variability. J Pediatr. 2018;203:309–16.
Gariépy G, Doré I, Whitehead RD, Elgar FJ. More than just sleeping in: a late timing of sleep is associated with health problems and unhealthy behaviours in adolescents. Sleep Med. 2018.
Funding
This study was financially supported by the Interfaculty Research Cooperation grant “Decoding Sleep: From Neurons to Heath & Mind” from the University of Bern (to LT), and National Institute of Health (R01 HL105395 and R01 HL112756 to SC; DK101046 to MAC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Sleep and Development
Rights and permissions
About this article
Cite this article
Tarokh, L., Short, M., Crowley, S.J. et al. Sleep and Circadian Rhythms in Adolescence. Curr Sleep Medicine Rep 5, 181–192 (2019). https://doi.org/10.1007/s40675-019-00155-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40675-019-00155-w