Abstract
Background
ImmunoCAP ISAC 112, is a commercially available molecular allergy IgE multiplex test. Data on the comparison of this rather novel test with extract-based as well as molecular ImmunoCAP singleplex IgE tests is missing.
Objective
To perform a comparison between the ISAC multiplex IgE assay and the ImmunoCAP singleplex test results.
Methods
Serum samples of 101 adults with grass pollen allergy were analysed for sIgE to 112 allergenic molecules represented on the ISAC test as well as to common atopy-related extract-based allergy tests with the ImmunoCAP System (house dust mite [d1], cat [e1], dog [e5], cow’s milk [f2], hen’s egg [f1], hazelnut [f17], celery [f85], Alternaria alternate [m6], as well as pollen from birch [t3], hazel [t4], mugwort [w6], and ragweed [w1]). Subsequently statistical analysis was performed with the Spearman rank correlation test and the Clopper-Pearson method in order to compare the ISAC multiplex results with the sIgE singleplex results.
Results
The positive percent agreements (PPA) and negative percent agreement (NPA) of corresponding allergens between the ISAC sIgE test and the extract-based singleplex ImmunoCAP results at cutoff 0.1 kUA/l varied between 60–100 % for PPA and 78–97 % for NPA.
Conclusion
When taking into account corresponding allergens molecular testing with the ISAC multiplex test correlates well with ImmunoCAP singleplex results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, a WAO-ARIA-GA2LEN consensus document [1] was published with the aim to provide the clinician with a practical guide regarding the indications, determination, and interpretation of molecular allergy diagnostics (MA). Currently more than 130 allergenic molecules are available for in-vitro specific immunoglobulin E (sIgE) testing which can be performed on singleplex or multiplex measurement platforms. In this consensus document MA is recommended as subordinate diagnostic (third line-diagnostic), if medical history and extract-based IgE tests, as conventional allergen tests (in-vitro sIgE or skin prick test) are inconclusive. This application as third-line diagnostic in difficult cases leads to the frequent clinical situation, where previous extract-based IgE test results, such as to birch pollen extract, are related to the outcome of subsequent MA testing, e. g. to the allergenic molecule Bet v 1. This approach is complicated due to the fact that IgE results cannot easily be compared between different assay manufacturers [2, 3] and across different analytical platforms. Studies addressing the latter have compared the Immuno-Solid phase Allergen Chip (ImmunoCAP® ISAC), with the ImmunoCAP® singleplex tests (both Thermo Fisher Scientific, Uppsala, Sweden) [4, 5, 6, 7, 8, 9, 10, 11, 12]. However, these studies concerned previous versions of the ISAC test and are not available for the current version with 112 allergens which was brought to market in 2011. For this version only an assessment of test performance is published to date.
It was therefore the aim of this study to perform a comparison between the current version of the ISAC multiplex and the ImmunoCAP singleplex IgE test to provide the practitioner with information on how to best interpret sIgE results as basis for the appropriate clinical conclusions.
Methods
Patients and study design
In this study, sera from 101 adults with allergic rhinoconjunctivitis and diagnosed allergy to timothy grass pollen (median age 27 years; 58.4 % females) were analyzed. Inclusion criteria for the study were a serum level of IgE against timothy grass pollen (Phleum pratense) ≥ 0.35 kUA/l and a positive history of grass pollen allergy. Concomitant disease, previous or ongoing specific immunotherapy (SIT) or pregnancy were exclusion criteria. More details on the data set of this study population were published previously [14, 15].
An informed consent was obtained from all subjects before participating in the study. The study protocol was approved by the local ethics committee prior to the initiation of the study and is in line with the declaration of Helsinki in its latest revision.
IgE Analysis
IgE analysis of serum samples was performed (A) traditional extract-based singleplex-testing with the ImmunoCAP system and (B) with the molecular multiplex test ImmunoCAP® ISAC 112 (both Thermo Fisher Scientific, Uppsala, Sweden).
-
A.
ImmunoCAP singleplex testing was carried out according to the manufacturer’s guidelines. The quantitation range for sIgE spans from 0.1 to 100 kUA/L and the mean coefficient of variation (CV) is stated as 4 % both inter- and intraassay for values between 0.35 bis 1.5 kUA/L (www.dfu.phadia.com under “alternative search” / ImmunoCAP Specific IgE 0-100). sIgE was measured with ImmunoCAP singleplex (A) against the following common atopy-related allergens: house dust mite Dermatophagoides pteronyssinus (d1), cat (e1), dog (e5), cow’s milk (f2), hen’s egg (f1), hazelnut (f17), celery (f85), Alternaria alternate (m6), as well as pollen from birch (t3), hazel (t4), mugwort (w6), and ragweed (w1).
-
B.
ISAC 112 is a solid-phase immunoassay that allows the simultaneous sIgE detection against 112 allergenic molecules from 51 different allergenic sources was performed as described elsewhere [13]. The measuring range spans from 0.3 to 100 ISU-E and the limit of detection (LoD) is < 0.3 ISU-E for all allergenic molecules. The CV per component within and between ISAC assays is 14 and 8 % respectively for values ranging from 0.3 to 1.0 ISU-E. The ISAC test (B) was used to analyse all patient samples yielding in total 11,312 test results (= 101 samples x 112 allergenic molecules on ISAC) which are all based on triplicate measurements due to the setup of the allergen chip.
Comparison of ISAC multiplex test vs. singleplex sIgE tests
In order to investigate if molecular ISAC-analysis identifies samples, which were tested positive in the corresponding extract-based singleplex ImmunoCAP test the results of both methods were compared.
For this purpose the ISAC allergenic molecules jointly corresponding to the allergen extract, e. g. hazel pollen t4, were identified and the ISU-E values summed up, e. g. rCor a 1.0101 (PR 10-protein) + rBet v 2 (profilin) + rBet v 4 (polcalcin) + MUXF3 (CCD), and the results compared to the extract-based test. As shown in the example of the hazel pollen extract, in addition to the hazel pollen allergen represented on the chip (rCor a 1.0101) also homologue cross-reactive allergens were included for the analysis as substitutes (e. g. rBet v 2 and rBet v 4). This option was chosen if the corresponding allergens from this particular allergenic source were not represented on the ISAC, which would be the profilin and polcalcin from hazel pollen in this case. The same holds true with regard to all extract-based pollen tests such as mugwort pollen (w6), for which the highly cross-reactive profilin and polcalcin were included in the analysis as well. The cross-reactive carbohydrate (CCD) marker MUXF3 was included in the panel for all allergen correlations from the plant kingdom (i. e. foods and pollen). For the house dust mite Dermatophagiodes pteronyssinus Der p 2 and Der p 10 where added to Der p 1 to resemble the house dust mite extract d1. An overview of the ISAC-allergens corresponding to the individual allergen extracts is shown in Tab. 1.
Only ImmunoCAP extract-based tests with at least 20 positive results out of the 101 individuals tested were included in the analysis. Based on this inclusion criterion the analysis was performed for g6, t3, t4, w6, d1, e1, f17, f85, while the following were excluded: ragweed (w1), dog (e5), the Alternaria alternate (m6), hen’s egg (f1), cow’s milk (f2).
Statistical analysis
The statistical analysis was performed in order to compare the sIgE results of the ISAC multiplex test with the outcome of the singleplex assays on the extract-based level. The aim was to test if a sensitized patient could be detected with the limited number of allergenic molecules on the ISAC. For the calculation all values below the ISAC LoD of 0.3 ISU-E were set to zero and values above that level were considered as positive with regard to the given allergen. The ISAC outcome was then correlated using rank based analysis (ρ) to the results of the extract-based tests at different levels. As cutoff the values 0.1 kU/l and 0.35 kU/l were used as well as 1 kU/l, which was proposed in an earlier investigation [7] (Tab. 2). Based on this correlation the percentage of patients showing sensitizations in both methods to the corresponding allergens resp. extracts was calculated as the positive percent agreement (PPA) which is shown in Tab. 1 for the different cutoffs.
In addition, the negative percent agreement (NPA) was calculated as the percentage of patient showing no sensitization in ISAC at 0.3 ISU-E out of all individuals who were negative in the extract-based sIgE-test at 0.1 kUA/l, 0.35 kUA/l and 1 kUA/l respectively.
To calculate confidence intervals for the respective PPA and NPA values, the two sided Clopper-Pearson method (assuming beta distributions of probabilities) was used to calculate the respective confidence intervals assuming a beta distribution of the respective probabilities (Tab. 1).
Results
Extract based-sIgE test results
Besides timothy pollen the patients were most frequently sensitized to birch, hazel, Dermatophagoides pteronyssinus and mugwort. On average, a sensitization to 5.5 of the 13 tested extract-based allergens was detected in the study collective (SD 3.1, range 1–10). Additional information regarding the prevalence of sensitizations in this cohort was published earlier and can be assessed online as supporting information via entering the search term “doi: 10.1111/cea” in an internet browser [14].
Comparison of ISAC multiplex test vs. molecular singleplex sIgE tests
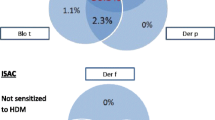
In order to compare the ISAC analysis with the extract-based sIgE test results the individual allergenic molecules contributing to an allergen extract were defined as explained above and are displayed in Tab. 1. For each extract-based ImmunoCAP test the corresponding ISAC allergens are listed in the respective column. The NPA and PPA (with 95% CI) between the relevant ISAC allergens and the extract-based IgE tests are shown for the different cutoff values in Tab. 1. The calculations are based on the data of 101 patients and indicated values between 60–100 % for the PPA and up to 97 % for the NPA respectively.
The correlations are displayed graphically in Fig 1. a-h. All correlations are statistically significant (p<0.0001).
Discussion
This is the first study which compares the ISAC 112 multiplex with the ImmunoCAP singleplex test in regard to extract-based as well as molecular in vitro sIgE analysis.
The main findings are: the correlation analysis revealed the ISAC test to be closely correlated to the ImmunoCAP singleplex results for the corresponding molecular allergens. The sum of the sIgE-values to the single allergens is slightly higher than the results of the extract-based tests. Finally, our data indicates that the test results from ISAC and ImmunoCAP singleplex are not interchangeable due to the different technologies applied. Even though close correlations could be found these have to be considered at the individual allergen level and do not allow defining a general factor for transferring test results from one method to the other.
MA is increasingly used in clinical routine worldwide providing enhanced diagnostic depth in addition to conventionally extract-based sIgE-testing. Especially in food allergy as well as prior to specific immunotherapy in polysensitized individuals with pollinosis an added diagnostic value of MA could be shown promoting its use in clinical routine [9, 16, 17, 18]. Corresponding to the rising use there is a growing need for information on the right applications and how to correctly interpret MA results.
These issues are addressed by the WAO-ARIA-GA2LEN consensus document [1] which provides a practical guide on when to use MA and what conclusions to draw from the results. Regarding the sequence of the diagnostic steps the authors of the document consider MA in general as a third-line approach to be used in the case of inconclusive first- and second-line investigations, which usually provide sufficient information in the majority of patients. For experienced users MA may be used at an earlier stage and could be included in second-line allergy testing. However, ISAC testing often represents a later diagnostic stage and is in general reserved for challenging cases [1]. Due to this sequence of the diagnostic workup in allergy, ISAC testing is usually performed as the last step and is thus frequently compared to previous singleplex MA tests. In the following we will therefore discuss this topic in regard to the comparison of the ISAC vs. the molecular singleplex ImmunoCAP test.
In almost all patients in whom an ISAC test is applied to obtain data on the sIgE sensitization profile previous extract-based testing, e. g. skin prick testing or in-vitro sIgE-testing has been performed already. Thus it is natural that these test results are often compared to each other. In this situation clinicians frequently get the impression, that there is a discrepancy between the test results if the extract-based test is positive, while all of the respective allergenic molecules on the chip are negative or vice versa. This issue is addressed in this study by comparing different technologies (multiplex vs. singleplex), and diagnostic approaches (molecular vs. extract-based). Due to the underlying different methodological backgrounds it is not surprising that differences appear which may for example be caused by ...
-
a.
the triggering allergen not being present on the ISAC,
-
b.
the sensitivity of the ISAC is lower than for singleplex ImmunoCAP tests,
-
c.
the native extract-based test is not identical to the recombinant MA test, or
-
d.
the triggering allergen is not or underrepresented in the allergen extract.
Within the scope of this study we tackle especially the first of the issues mentioned: Obviously only what is measured can be detected, in other words if a sensitization to e. g. hazelnut (f17) is only due to hazelnut profilin it will not be visible through the hazelnut allergens represented on the chip because there is no hazelnut profilin on ISAC. This is due to the fact that especially highly cross-reactive allergens are not spotted on the ISAC from each source available, but are represented through others of the 112 selected allergens originating in total from 51 allergenic sources. Even though a wide array of allergens are tested simultaneously with the ISAC it is virtually impossible to cover all allergens from all allergenic sources available which could be causing sensitizations in the individual patients. Therefore it is necessary to extrapolate from one allergenic source to the other based on protein homology, to extract the most diagnostic information from the ISAC results. In the case of the hazel nut profilin which is not present on the ISAC other pollen profilins on the chip (Bet v 2 from birch, Phl p 12 from timothy grass and Mer a 1 from Mercury pollen) represent good substitute markers due the high degree of similarity between all profilins from pollen, plant foods and latex. This admittedly requires the clinician to have profound knowledge of MA to make full use of such an approach.
Based on these considerations for each of the extract-based tests performed in our study a good correlation between the extract-based test and the ISAC could be achieved in most of the cases: For the PPA at the cutoff of 0.1 KUA/l values above 90 % were obtained for timothy grass pollen, birch pollen and hazelnut, while hazel pollen and cat were still above 80 %, and only mugwort pollen, celery and Dermatophagoides pteronyssinus resulted in the range between 60–70 %. When analysing the PPA at the cutoff of 0.35 and 1.0 kUA/l, which might have a higher clinical relevance then the sIgE-level of 0.1 kUA/l, five resp. six out of eight tests resulted in values between 90 % and 100 %.
Such a high PPA is also observed for hazelnut, even though the hazelnut storage proteins, which are major allergens in childhood, are missing on the ISAC chip. This finding allows the conclusion, that the storage proteins Cor a 9 and Cor a 14 do not play a major role in our collective of adult patients with allergic rhinoconjunctivits without a history for allergy to hazelnut.
In contrast to the above mentioned findings Dermatophagoides pteronyssinus was lowest with a PPA of 64 (at 0.35 kUA/l) resp. 74 % (at 1.0 kUA/l) indicating that possibly some important house dust mite allergenic molecules which are of relevance for our patient collective may be missing on the chip.
A limitation to this concept is the fact that not all allergenic sources are represented to the same extents on the chip due to technical reasons, e. g. 8 Phl p allergens of timothy grass but only 3 Der p allergens (house dust mite). An additional shortcoming of this approach is that even highly cross-reactive allergens from the same protein family are not fully identical regarding sequence and structure across allergenic sources. Due to this the concept of extrapolation from one allergenic source to the other will always only represent an approximation.
In regard to previous investigations on the comparison between multiplex and singleplex tests our data shows an improved correlation between the two methods [7]. However, for a true comparison of both test systems it is necessary to directly compare allergens on the molecular level with each method and not molecules versus extracts as performed in the current study. The above described approach was chosen after all to explain the principle and give guidance in the comparison of test results originating from different methods in clinical routine. Direct comparison of molecular allergy diagnostics on ImmunoCAP ISAC und ImmunoCAP singelplex was assessed in an earlier investigation and is outside the scope of the current publication [19].
In conclusion, ISAC results show a good correlation to the extract-based ImmunoCAP singleplex tests if the above mentioned principles for the comparison are taken into account. In the allergy workup the ISAC chip is an additional diagnostic tool for the assessment of complex cases and the comparison to other tests has to be performed with care and based on profound knowledge of molecular allergy diagnostics.
Abbreviations
- CCD:
-
Cross-reactive carbohydrate determinant
- CI:
-
Confidence interval
- CV:
-
Coefficient of variation
- IgE:
-
Immunglobulin E
- ISAC:
-
Immuno solid-phase allergen chip
- ISU:
-
ISAC standardized unit
- ISU-E:
-
ImmunoCAP specific IgE
- kUA:
-
Kilounits (corresponding allergens)
- LoD:
-
Limit of detection
- MA:
-
Molecular allergy diagnostics
- NPA:
-
Negative percent agreement
- PPA:
-
Positive percent agreement
- SD:
-
Standard deviation
- sIgE:
-
Specific Immunglobulin E
- SIT:
-
Specific Immunotherapy
References
Canonica GW, Ansotegui IJ, Pawankar R, Schmid-Grendelmeier P, van Hage M, Baena-Cagnani CE, Melioli G, Nunes C, Passalacqua G, Rosenwasser L, Sampson H, Sastre J, Bousquet J, Zuberbier T. A WAO - ARIA - GA(2)LEN consensus document on molecular-based allergy diagnostics. World Allergy Organ J 2013;6:17
Szecsi PB, Stender S. Comparison of immunoglobulin E measurements on IMMULITE and ImmunoCAP in samples consisting of allergen-specific mouse-human chimeric monoclonal antibodies towards allergen extracts and four recombinant allergens. Int Arch Allergy Immunol 2013;162:131–4
Wood RA, Segall N, Ahlstedt S, Williams PB. Accuracy of IgE antibody laboratory results. Ann Allergy Asthma Immunol 2007 Jul;99:34–41
Cabrera-Freitag P, Goikoetxea MJ, Beorlegui C, Gamboa P, Gastaminza G, Fernandez-Benitez M, Ferrer M, Blanca M, Sanz ML. Can component-based microarray replace fluorescent enzimoimmunoassay in the diagnosis of grass and cypress pollen allergy? Clin Exp Allergy 2011 Oct;41:1440–6
Gadisseur R, Chapelle JP, Cavalier E. A new tool in the field of in-vitro diagnosis of allergy: preliminary results in the comparison of ImmunoCAP(c) 250 with the ImmunoCAP(c) ISAC. Clin Chem Lab Med 2011 Feb;49:277–80
Lizaso MT, Garcia BE, Tabar AI, Lasa E, Echechipia S, Alvarez MJ, Anda M, Gomez B. Comparison of conventional and component-resolved diagnostics by two different methods (Advia-Centaur/Microarray-ISAC) in pollen allergy. Ann Allergy Asthma Immunol 2011 Jul;107:35–41
Melioli G, Bonifazi F, Bonini S, Maggi E, Mussap M, Passalacqua G, Rossi ER, Vacca A, Canonica GW. The ImmunoCAP ISAC molecular allergology approach in adult multi-sensitized Italian patients with respiratory symptoms. Clin Biochem 2011 Aug;44:1005–11
Ott H, Baron JM, Heise R, Ocklenburg C, Stanzel S, Merk HF, Niggemann B, Beyer K. Clinical usefulness of microarray-based IgE detection in children with suspected food allergy. Allergy 2008 Nov;63:1521–8
Sastre J, Landivar ME, Ruiz-Garcia M, Andregnette-Rosigno MV, Mahillo I. How molecular diagnosis can change allergen-specific immunotherapy prescription in a complex pollen area. Allergy 2012 May;67:709–11
Twaroch TE, Focke M, Fleischmann K, Balic N, Lupinek C, Blatt K, Ferrara R, Mari A, Ebner C, Valent P, Spitzauer S, Swoboda I, Valenta R. Carrier-bound Alt a 1 peptides without allergenic activity for vaccination against Alternaria alternata allergy. Clin Exp Allergy 2012 Jun;42:966–75
Wang J, Godbold JH, Sampson HA. Correlation of serum allergy (IgE) tests performed by different assay systems. J Allergy Clin Immunol 2008 May;121:1219–24
Ebo DG, Hagendorens MM, De Knop KJ, Verweij MM, Bridts CH, De Clerck LS, Stevens WJ. Component-resolved diagnosis from latex allergy by microarray. Clin Exp Allergy 2010 Feb;40:348–58
Martinez-Aranguren R, Lizaso MT, Goikoetxea MJ, Garcia BE, Cabrera-Freitag P, Trellez O, Sanz ML. Is the determination of specific IgE against components using ISAC 112 a reproducible technique? PLoS One 2014;9:e88394
Darsow U, Brockow K, Pfab F, Jakob T, Petersson CJ, Borres MP, Ring J, Behrendt H, Huss-Marp J. Heterogeneity of molecular sensitization profiles in grass pollen allergy implications for immunotherapy? Clin Exp Allergy 2014 Mar 6
Huss-Marp J, Darsow U, Brockow K, Pfab F, Weichenmeier I, Schober W, Petersson CJ, Borres MP, Ring J, Behrendt H. Can immunoglobulin E-measurement replace challenge tests in allergic rhinoconjunctivits to grass pollen? Clin Exp Allergy 2011 Aug;41:1116–24
Ferrer M, Sanz ML, Sastre J, Bartra J, del Cuvillo A, Montoro J, Jauregui I, Davila I, Mullol J, Valero A. Molecular diagnosis in allergology: application of the microarray technique. J Investig Allergol Clin Immunol 2009;19 Suppl 1:19–24
Renault NK, Mirotti L, Alcocer MJ. Biotechnologies in new high-throughput food allergy tests: why we need them. Biotechnol Lett 2007 Mar;29:333–9
Sanz ML, Blazquez AB, Garcia BE. Microarray of allergenic component-based diagnosis in food allergy. Curr Opin Allergy Clin Immunol 2011 Jun;11:204–9
Ahlgrim C, Gutermuth J, Ænell A, Borres MP, Schäffner I, Darsow U et. al Comparison of molecular multiplex and singleplex analysis of IgE to grass pollen allergens in untreated German grass pollen allergic patients. J Invest All Clin Immunol 2015; Vol 25 (in press)
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions (as proposed by International Committee of Medical Journal Editors [ICMJE])
J. Huss-Marp: design of the study, data collection, statistical analysis, preparation and revision of the manuscript; J. Gutermuth: data collection and revision of the manuscript; I. Schäffner: preparation and revision of the manuscript; U. Darsow: data collection and revision of the manuscript; F. Pfab: data collection and revision of the manuscript; K. Brockow: data collection and revision of the manuscript; J. Ring: design of the study and revision of the manuscript; H. Behrendt: design of the study and revision of the manuscript; T. Jakob: data collection and revision of the manuscript; C. Ahlgrim: statistical analysis, preparation and revision of the manuscript
Cite this as: Huss-Marp J, Gutermuth J, Schäffner I, Darsow U, Pfab F, Brockow K, Ring J, Behrendt H, Jakob T, Ahlgrim C. Comparison of molecular and extract-based allergy diagnostics with multiplex and singleplex analysis. Allergo J Int 2015;24:46–53 DOI: 10.1007/s40629-015-0045-5
Funding
This work was supported by a research grant from Thermo Fisher Scientific, Phadia GmbH, Freiburg
Conflict of interest
Schäffner I and Huss-Marp J were employees of Thermo Fisher Scientific. Jakob T has received honoraria for consulting and for serving on the speakers buro of Thermo Fisher Scientific and has received research funding from Thermo Fisher Sceintific. The other authors state that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Huss-Marp, J., Gutermuth, J., Schäffner, I. et al. Comparison of molecular and extract-based allergy diagnostics with multiplex and singleplex analysis. Allergo J Int 24, 46–53 (2015). https://doi.org/10.1007/s40629-015-0046-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40629-015-0046-4