Abstract
Gray mold caused by Botrytis cinerea is an important disease in strawberries. This fungus causes significant economic losses since it attacks plants and fruits. In this context, this work aimed to evaluate the effectiveness of Acibenzolar-S-methyl (ASM) and Harpin protein in pre- and post-harvest as inducers of resistance in strawberries to B. cinerea. Strawberry plants (Fragaria x ananassa) from ‘Aromas’ and ‘Camarosa’ cultivars were grown in greenhouse and evaluated in laboratory. Doses of Harpin and ASM in pre- and post-harvest applications were assessed. Yield parameters of strawberry, B. cinerea incidence and injured area in fruit, fruit firmness, CO2 assimilation rate, and phenylalanine ammonia-lyase (PAL) activity were analyzed. Elicitors application in pre- and post-harvest conditions promoted a decrease of B. cinerea incidence and injured area in strawberry fruits. The results suggest that Harpin and ASM treatment show a significant impact on strawberry fruit disease. The control may be associated with the PAL induction, responsible for inducing defense responses. Harpin and ASM represent a promising alternative to synthetic fungicides for B. cinerea control during post-harvest storage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Strawberry (Fragaria x ananassa) is an important crop worldwide in terms of commercial value. According to the Food and Agriculture Organization (FAO), world production exceeded 8 million tons in 2018; in Brazil, it was 3481 tons (FAO 2020). The fruit is a source of many essential nutrients, including potassium, vitamin C, folate, antioxidants (Giampieri et al. 2012, Skrovankova et al. 2015), phenolic compounds, micronutrients, and ascorbic acid (Giampieri et al. 2012; Afrin et al. 2016; Battino et al. 2016). Additionally, strawberry fruits are part of a growing trend that highlights plant-derived antioxidants for their proven health benefits (Nile and Park 2014).
Gray mold caused by Botrytis cinerea is an important disease. This fungus attacks the plants and fruits of strawberries, causing significant economic losses (Dean et al. 2012; Fernández-Ortuño et al. 2012, Tomazeli et al. 2016; Amiri et al. 2019). The problem can be more severe during post-harvest as the pathogen can remain quiescent and damage tissues exclusively when the fruit is ripe (Dean et al. 2012). The most used treatment against gray mold is the extensive use of pesticides from different chemical groups, such as methyl-benzimidazole-carbamate, dicarboximide, carboxamides, and anilinopyrimidines (Zhao et al. 2010). The pesticides residues remain in the fruits and promote risks to human health, besides contamination of the environment (Finiti et al. 2014). Occasionally, the development of pathogen-resistant biotypes to fungicides occurs (Fernández-Ortuño et al. 2012; Leroch et al. 2013; Amiri et al. 2014; Fernández-Ortuño et al. 2014; Rupp et al. 2017). In Florida, for example, the standard spray programs recommend an average of 20 weekly applications (Amiri et al. 2019). However, recent regulations considerably restricted their use.
One substantial challenge of this crop is developing eco-friendly alternatives for control, aiming a more sustainable agriculture. Alternative products such as elicitors are among the potential options for reducing the use of chemical fungicides. These products do not directly inhibit the action of pathogens, biotrophic and necrotrophic, but induce a faster and stronger activation of “Innate Immune Defense Responses” in the host plant (Terry and Joyce, 2004; Tian et al. 2006; Cao et al. 2011; Ren et al. 2012). Upon pathogen attack, pathogen/microbes associated molecular patterns (PAMPs/MAMPs), also called elicitors, activate pattern-recognition receptors (PRRs) in the host, resulting in a downstream signaling cascade, usually through WRKY transcription factors, leading to PAMP-triggered immunity (PTI). Pathogens evolved effectors that suppress PTI, resulting in effector-triggered susceptibility (ETS). In turn, plants have acquired resistance (R) proteins that recognize specific effectors, resulting in a secondary immune response called effector-triggered immunity (ETI) (Miller et al. 2017).
Among the elicitors used in the activation of innate immune defense responses in plants and fruits against diseases, we can mention: (i) Harpin protein; an acidic, glycine-rich, protease-sensitive, and heat stable, encoded by the hrpN gene of the bacterium Erwinia amylovora; in plants are responsible for enhancing plant disease resistance by activating the immunity induced by PAMPs (Wei et al. 1992; Choi et al. 2012, 2013); and (ii) ASM (ASM, benzo(1,2,3) thiadiazole-7-carbothioic acid S-methyl ester, or simply Acibenzolar-S-methyl) derived from benzothiadiazole, a functional analog of salicylic acid (Mandal et al. 2008). These elicitors, when recognized by the corresponding resistance proteins (or R-genes), activate passive and active defense mechanisms in plants (Eitas and Dang 2010), including the phenylpropanoid pathway activation (Liu et al. 2014), accumulation of pathogenesis-related proteins (PRs) and PR gene expression (Cao et al. 2011).
These elicitors were promising in activating innate immune defense responses to several diseases in fruits and vegetables. The incidence of Phakopsora zizyphi-vulgaris decreased by up to 87.1% in jujube treated with Harpin concerning the control treatment (Li et al. 2012). The Harpin in B. cinerea treatments reduced the percentage of rotten fruit in cultivars of pepper (Tezcan et al. 2013). The Harpin treatment was effective in controlling infection of B. cinerea and Penicillium expansum in kiwifruit by activating enzymes involved in defense response, such as polyphenol oxidase, peroxidase, and superoxide dismutase (Tang et al. 2015). Harpin and ASM increased peroxidase enzyme activity and, in consequence, reduced the injured area and sporulation caused by Colletotrichum gloeosporioides in apples (Alamino et al. 2013). The elicitors induced resistance to Monilinia fructicola, reducing fungal development (Danner et al. 2008). Seeds treated with ASM or Harpin provided up to 90 and 47% protection against Penicillium digitatum and P. italicum, respectively, in yellow passion fruit plants (Boro et al. 2011). In this context, this work hypothesizes that ASM and Harpin protein sprayed in pre- and post-harvest induce resistance against B. cinerea in strawberries under post-harvest conditions.
2 Material and methods
The experiment was carried out in a greenhouse, with an average temperature of 20 °C, located at the Federal University of Technology - Paraná, Pato Branco campus, PR, Brazil (26°11′50″ S, 52°41′26″ W, and 820 m above sea level).
2.1 Effect of elicitors application in pre-harvest on gray mold
The strawberry plants (Fragaria x ananassa) cv. ‘Aromas’ were purchased from the Maxi Mudas company (Feliz, RS, Brazil). The soil showed the following chemical characteristics: organic matter: 37.53 g dm− 3; phosphorus: 55.16 mg dm− 3; potassium: 1.28 cmolc dm− 3; calcium: 6.92 cmolc dm− 3; magnesium: 2.54 cmolc dm− 3; base saturation: 78.45%; cation exchange capacity: 13.69 cmolc dm− 3; and pH(CaCl2) 5.7. During the growing period, the plants were watered by sprinkling every two days for approximately 15 min. Before applying the elicitors, the fungicide Azoxystrobin [0.3 g active ingredient (a.i.) L− 1] was applied at 10, 25, and 40 d after transplantation to maintain plant health.
Two elicitors: four doses of Harpin [commercial product ProAct™ (0, 100, 200 and 300 mg L− 1, 1% a.i.)] and five doses of ASM [commercial product Bion® (0, 100, 200, 300, and 400 mg L− 1, 50% a.i.)] were evaluated. The planting of seedlings was carried out in seedbeds in a greenhouse (1.0 m wide 60.0 m long × 0.30 m high). The experimental design was a block randomized with nine treatments and three replications (9 × 3). Each block consisted of a seedbed 60 m long; each experimental unit had 7.5 m long and consisted of 25 plants with the spacing of 0.3 m x 0.3 m. The elicitor spraying was carried out at 50, 58, 66, 74, and 82 d after transplanting. The inoculation of the fungus B. cinerea (104 conidia L− 1) was performed 24 h after the first spraying of elicitors (50 d after transplanting),
Evaluation of gas exchange was carried out using a portable infrared gas analyzer (IRGA, model LI-6400xt, LI-COR Inc., Lincoln, NE, USA) 48 h after applying the first treatments. The microclimate conditions in the measuring chamber were 26 °C, photosynthetically active radiation (PAR) of 1300 µmol m− 2 s− 1, relative air humidity of 61%, and 400 µmol CO2 mol− 1. Four fully expanded leaves per plant in three plants per plot were evaluated.
The yield assessments were carried twice a week in a group of 20 central plants per experimental unit. The number and mass of fruits were evaluated to determine the number of marketable fruits per plant (NFP); the mass of marketable fruits per plant (MFP - commercial fruits were those that were free from injuries and deformities caused by diseases); the average mass of marketable fruits (AMF); fruit yield (FY); and gray mold incidence (%) in fruits. Eight yield evaluations were performed (twice a week for four weeks), starting at 68 and finishing 100 d after transplanting.
2.2 Effect of elicitors application in pre-harvest on gray mold in post-harvest
Twenty-four hours after the fourth treatment application (75 days after transplanting), fruits from the same experiment described above were harvested at the point of maturation to perform the analyses. The harvested fruits were disinfested by immersion in sodium hypochlorite (NaClO) 5% (v/v) of active chlorine for 30 s, and rinsed in distilled water. After disinfection, the fruits were set in plastic boxes with capacity for six fruits each; the plastic boxes were covered with a moistened paper towel. Then, the fruits were inoculated with a conidia solution of B. cinerea with 105 conidia mL− 1 with a spray bottle. The boxes remained in the laboratory at room temperature (average of 22 °C). The fruits used for this experiment were harvested from the plants of the experiment mentioned above. The nine treatments carried out in the field were maintained with three replications (9 × 3) with six fruits in each experimental unit.
The fruits were evaluated 96 h after inoculation. The diameter values of each lesion in four mutually perpendicular directions were recorded. The means of diameters were used to calculate the circumference area of the lesion, using the equation: A = πr2. The pulp firmness was measured with a TR penetrometer (Model Fruit Test 327) with an 8 mm tip; the results were expressed in Newton (N).
2.3 Effect of elicitors application in post-harvest on the incidence and injured area of gray mold and PAL activity in strawberry
“Camarosa’ fruits were obtained from an organic producer in Palmas, PR, Brazil (26°29’3” S and 48°59’26” W; 1115 m above sea level), and used for the measurements. The fruits were harvested when they reached physiological maturity and selected based on size, firmness, color, and absence of injuries or diseases. The fruits were disinfected according to the methodology previously mentioned. The disinfected fruits were separated into groups of 12 fruits and placed in plastic boxes. The fruits were divided into two groups of six fruits inside boxes. A mechanical injury with 3 mm depth was performed in one group in the two equatorial portions of the fruit.
The experiment was carried out in a 2 × 4 factorial scheme. Two inoculation ways (wounded and unwounded fruits) and three treatments with elicitors, two commercial products containing Harpin [Messenger® (3% of a.i.) and Harpin αβ ProAct™ (1% of a.i.) at the dose of 80 mg (a.i.) L− 1], and a commercial product containing ASM [ActiGard® 50 WG (50% of a.i.) at the dose of 5 mg (a.i.) mL− 1] were evaluated. Sterile water was used as the control treatment.
The treatments were applied with a spray bottle, and the boxes with the fruits remained in the laboratory at room temperature (average of 22 °C). The fruits were inoculated with a conidial suspension of B. cinerea with 105 conidia mL− 1 12 h after application. The incidence and injured area of B. cinerea and pulp firmness as previously described were evaluated 48 h after inoculation. Tissue samples were collected from areas adjacent to the lesions, and they were stored in liquid nitrogen (−180 °C) for the determination of phenylalanine ammonia-lyase (PAL) activity according to the methodology described by Rodrigues et al. (2006).
2.4 Preparation of spore solution for fruit inoculation
The inoculum was isolated from diseased fruits and cultivated in a culture medium of potato-dextrose agar and then kept for 15 d in a BOD incubator at 25 °C, with a photoperiod of 12 h. The concentration of spores was set in suspension using a hemocytometer. The inoculum used for both experiments was grown using this methodology.
2.5 Data analysis
The data were tested for the normality and homogeneity assumptions. When meeting the premises, the data were submitted to analysis of variance, and the means were compared by the Tukey test (p ≤ 0.05). All the statistical analyses were carried out with the R software, version 3.5.0. Data referring to the injured area was transformed to arcsine (\(\surd\)X /100). The graphs were built using the ggplot2 package (Wickham 2016).
3 Results
3.1 Elicitors application in pre-harvest
The application of Harpin and ASM in the ‘Aromas’ strawberry cultivar did not affect the number of marketable fruits per plant (Fig. 1a), the mass of marketable fruits plant (Fig. 1b), the average mass of marketable fruits (Fig. 1c), net photosynthesis 24 hours before (Fig. 1d) and after (Fig. 1e) and fruit yield (Fig. 1f). However, the application of elicitors reduced the incidence and injured area of fruits (Figs. 2 and 3). The incidence data were adjusted to the second-degree polynomial function and the dose of 0.2 g (a.i.) L− 1 of Harpin and ASM had the highest control of gray mold. For both elicitors, the greatest reduction occurred up to the dose of 0.2 g (a.i.) L− 1, followed by a increase in Harpin at higher doses (Fig. 2).
Number of marketable fruits per plant (MFP) (a), mass of marketable fruits per plant (MMFP) (b), average mass of marketable fruits (AMF) (c), net photosynthesis (AN) 24 h before (d) and after (e) the treatment (cv. Aromas), and fruit yield (FY) (f) of strawberry plants according to the application of Acibenzolar-S-Methyl (ASM) and Harpin αβ. The black bars represent the standard deviation
Gray mold incidence in post-harvest of strawberry fruits (cv. Aromas) according to the treatment with acibenzolar-s-methyl (ASM) (blue) and Harpin αβ (black) in pre-harvest. The regression is significant by F-test at (p ≤ 0.05). Each point corresponds to one of the three replications. (Color figure online)
Gray mold injured area (cm2) in post-harvest of strawberry fruits (cv. Aromas) according to the treatment with acibenzolar-s-methyl (ASM) (blue) and Harpin αβ (black) in pre-harvest. The regression is significant by F-test at (p ≤ 0.05). Each point corresponds to one of the three replications. (Color figure online)
At post-harvest, the Harpin and ASM doses applied in pre-harvest influenced the size of the lesion area of gray mold (Fig. 3). Both products exhibited a significant decrease of the B. cinerea injured area at all tested doses, and no other microorganisms causing post-harvest rot were observed. We observed a reduction in the injured area of 84.9 and 82.4% with 0.3 g (a.i.) L− 1 of Harpin and ASM, respectively, and 89.9% for ASM dose of 0.4 g (a.i.) L− 1 (Fig. 3). As with gray mold incidence, the greatest reduction in the injured area occurred up to the dose of 0.2 g (a.i.) L− 1, from which there was a tendency towards stabilization with a slight tendency to increase the injured area at higher doses.
We found a significant increase in the pulp firmness with the elicitor application (Fig. 4). The rise in pulp firmness occurred up to the dose of 0.2 g (a.i.) L− 1; from there, an inverse effect occurred; the firmness of the fruit was reduced.
Pulp firmness in post-harvest of strawberry fruits (cv. Aromas) according to the treatment with acibenzolar-s-methyl (ASM) (blue) and Harpin αβ (black) in pre-harvest The regression is significant by F-test at (p ≤ 0.05). Each point corresponds to one of the three replications. (Color figure online)
3.2 Effect of post-harvest elicitors application on the incidence and injured area of gray mold
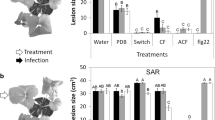
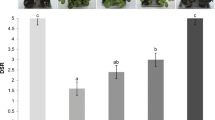
The incidence of gray mold was 97.2% in the control treatment and 51.4% in the fruits treated with Harpin protein and 63.9% in the treatments with Harpin αβ protein, and ASM, following the same pattern for the injured area, showing significantly greater damage in the control treatment, and less damage in treatments with elicitors (Table 1). The fruits with a mechanical injury were not significantly different concerning the healthy fruits for the variable injured area (Table 1) and PAL activity (Table 2). However, the unwounded fruits had higher pulp firmness when compared to the wounded fruits (Table 2; Fig. 5).
4 Discussion
Multiple pre-harvest Harpin and ASM applications efficiently reduced the incidence and injured area of B. cinerea in strawberry fruits in post-harvest. A similar result was observed with ASM applied during the winter growing period, which caused a delay of gray mold development on harvested strawberry fruit (Terry and Joyce 2000). The ASM and Harpin applied on ‘Camarosa’ strawberries cultivar induced innate immunity or resistance in the plants while reducing leaf blight and gray mold (Tomazeli et al. 2016). The Harpin treatment effectively controlled the infection of B. cinerea and Penicillium expansum in kiwifruit (Tang et al. 2015). In tomato, Harpin treatment had a positive effect against B. cinerea and Alternaria alternata (Zhu and Zhang 2016). Zhang et al. (2011) observed that multiple ASM applications in pre-harvest reduced latent infections caused by Alternaria alternata and Fusarium spp. in muskmelon (Cucumis melo L. cv. Yindi) fruit. The same authors observed that after ten days of storage, the fruit quality had an improvement when a greater number of ASM applications were performed. This fact suggests that plant cells can be stimulated repeatedly by ASM during fruit development and, consequently, be induced to develop greater resistance (Zhang et al. 2011).
The gray mold incidence and injured area results evidenced that post-harvest ASM and Harpin treatments efficiently reduced the incidence and injured area of B. cinerea on strawberry fruits of the ‘Aromas’ cultivar. Other studies also had demonstrated control of plant and fruit diseases with the use of ASM. The ASM treatment effectively controlled pink rot caused by Trichothecium roseum on muskmelon fruit during storage at room temperature (Ren et al. 2012; Ge et al. 2015). ASM dipping treatment significantly decreased injury diameter on the pear fruit inoculated with Penicillium expansum (Ge et al. 2017).
The reduction of incidence and injured area of gray mold in fruits after treatment with ASM can be attributed to several factors: signal transduction pathways for SAR after treatment followed by defense responses (Tripathi et al. 2010); the increased of gene expression and activities of peroxidase and polyphenol oxidase (Lin et al. 2011); the increased of the production of reactive oxygen species, by increasing the activities of NADPH oxidase, superoxide dismutase, and enzymes in the AsA–GSH cycle (Ge et al. 2017). The Harpin spray induced the transcript expression of defense-related genes, including chitinase, β-1,3-glucanase, and PAL based on RT-qPCR analysis, increased the activity of these enzymes, and the content of total phenolic compounds and lignin (Zhu and Zhang 2016).
The PAL activity in strawberry fruit was affected by post-harvest ASM and Harpin treatment, probably causing the accumulation of metabolites to create physical barriers to suppress pathogen infection. An increase in PAL activity is associated with the biosynthesis of active metabolites such as phytoalexins, phenols, salicylic acid, and lignins in plant defense pathways (Lorens et al. 2017; Wang et al. 2017; Yang et al. 2018). The increase in PAL activity also increases the amount of lignin in fruits; consequently, a pulp firmness increase (Yang et al. 2018). In this work, the pulp firmness increased when utilized Harpin and ASM treatment. Similar results were observed in other fruits. For muskmelon, the firmness values were significantly enhanced by the post-harvest ASM treatment (Zhang et al. 2011).
The response of plants to the elicitor depends on several factors related to the plant and the doses, the type of elicitor, and even stresses. Based on this, the lesser effect caused by the higher doses used on the incidence of the disease, on the area injured by B. cinerea, and on the firmness of the fruits occurred because the higher doses activate different pathways on the plant, and this may promote negative interactions, resulting in lower defense response. During the elicitation process, the response of plants is more dependent on the genetic of plants than the nature of elicitors (Vallejo et al. 2003; Baenas et al. 2014). Combined to this, the concentration of elicitors and the interval between treatment and harvest can also induce different responses that are dependent on the characteristics of each species, being necessary to find the effective dose and the appropriate application time for each species (Vasconsuelo et al. 2007). Studies report an additive or synergistic response after treatment with elicitors, as different signal transduction pathways seem to exist in response to environmental stresses and the elicitor used. These pathways can antagonize or harmonize with each other, leading to negative or additive interactions, respectively (Zhang et al. 2003; Cho et al. 2008; Cevallos-Casals and Cisneros-Zevallos, 2010).
The elicitor application in pre and post-harvest promoted decreased B. cinerea incidence and injured area on strawberry fruits, presenting a potential use to increase post-harvest storage. This result suggests that Harpin and ASM treatment have a significant impact on strawberry fruit disease. This control was probably associated with the PAL activity, which was responsible for inducing defense responses, which increased the pulp firmness due to lignin deposition. Thus, these elicitors represent a promising alternative to synthetic fungicides for B. cinerea control during post-harvest storage.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
Code availability
Not applicable.
References
Afrin S, Gasparrini M, Forbes-Hernandez TY, Reboredo-Rodriguez P, Mezzetti B et al (2016) Promising health benefits of the strawberry: a focus on clinical studies. J Agric Food Chem. https://doi.org/10.1021/acs.jafc.6b00857
Alamino DA, Cabral B, Danner MA, Marchese JA (2013) Induction of resistance to bitter rot in apples by the use of elicitors in the postharvest. Pesqui Agropecu Brasil. https://doi.org/10.1590/S0100-204X2013000300002
Amiri A, Heath SM, Peres NA (2014) Resistance to fluopyram, fluxapyroxad, and penthiopyrad in Botrytis cinerea from strawberry. Plant Dis. https://doi.org/10.1094/PDIS-07-13-0753-RE
Amiri A, Zuniga AI, Cordova LG, Peres NA (2019) The Importance of Selecting Appropriate Rotation and Tank-Mix Partners for Novel SDHIs to Enhance Botrytis Fruit Rot Control in Strawberry. Plant Dis. https://doi.org/10.1094/PDIS-07-18-1276-RE
Baenas N, García-Viguera C, Moreno DA (2014) Elicitation: a tool for enriching the bioactive composition of foods. Molecules. https://doi.org/10.3390/molecules190913541
Battino M, Forbes-Hernandez T, Gasparrini M, Afrin S, Mezzetti B et al (2016) The effects of strawberry bioactive compounds on human health. Acta Hortic. https://doi.org/10.17660/ActaHortic.2017.1156.54
Boro MC, Beriam LOS, Guzzo SD (2011) Induced resistance against Xanthomonas axonopodis pv. passiflorae in passion fruit plants. Trop Plant Pathol. https://doi.org/10.1590/S1982-56762011000200002
Cao S, Hu Z, Zheng Y, Yang Z, Lu B (2011) Effect of BTH on antioxidant enzymes radical-scavenging activity and decay in strawberry fruit. Food Chem. https://doi.org/10.1016/j.foodchem.2010.08.051
Cevallos-Casals BA, Cisneros-Zevallos L (2010) Impact of germination on phenolic content and antioxidant activity of 13 edible seed species. Food Chem. https://doi.org/10.1016/j.foodchem.2009.09.030
Chester KS (1933) The problem of acquired physiological immunity in plants. Q. Rev. Biol. https://www.jstor.org/stable/2808427
Cho H-Y, Son SY, Rhee HS, Yoon S-YH, Lee-Parsons CWT, Park JM (2008) Synergistic effects of sequential treatment with methyl jasmonate, salicylic acid and yeast extract on benzophenanthridine alkaloid accumulation and protein expression in Eschscholtzia californica suspension cultures. J Biotechnol. https://doi.org/10.1016/j.jbiotec.2008.02.020
Choi MS, Heu S, Paek NC, Koh HJ, Lee JS et al (2012) Expression of hpa1 gene encoding a bacterial Harpin protein in Xanthomonas oryzae pv oryzae enhances disease resistance to both fungal and bacterial pathogens in rice and Arabidopsis. Plant Pathol J. 28(4):364–372. https://doi.org/10.5423/PPJ.OA.09.2012.0136
Choi MS, Kim W, Lee C, Oh CS (2013) Harpins multifunctional proteins secreted by gram-negative plant-pathogenic bacteria. Mol Plant Microbe Interact. https://doi.org/10.1094/MPMI-02-13-0050-CR
CSC – CALIFORNIA STRAWBERRY COMMISSION; THE CALIFORNIA MINOR CROPS COUNCIL. A pest management strategic plant for strawberry in California 2013. https://www.ipmcenters.org/pmsp/pdf/CASTRAWBERRYPDF
Danner MA, Sasso SAZ, Medeiros JGS, Marchese J, Mazaro JM (2008) Indução de resistência à podridão-parda em pêssegos pelo uso de elicitores em pós-colheita. Pesqui Agropecu Brasil. https://doi.org/10.1590/S0100-204X2008000700002
Dean R, Van Kan J, Pretorius ZA, Hammond-Kosack KE, Di Pietro A et al (2012) The top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol. https://doi.org/10.1111/j.1364-3703.2011.00783.x
Ebel J, Cosio EG (1994) Elicitors of plant defense responses. Inter Rev Cytol doi. https://doi.org/10.1016/S0074-7696(08)62404-3
Eitas TK, Dangl JL (2010) NB-LRR proteins: pairs pieces perception partners and pathways. Cur Opin Plant Biol. https://doi.org/10.1016/j.pbi.2010.04.007
FAO (2020) Food and Agriculture Organization of the United Nations. FAOSTAT. Available from: http://www.fao.org/faostat/en/#data/QC (accessed March 2021)
Fernández-Ortuño D, Chen F, Schnabel G (2012) Resistance to pyraclostrobin and boscalid in Botrytis cinerea isolates from strawberry fields in the Carolinas. Plant Dis. https://doi.org/10.1094/PDIS-12-11-1049-RE
Fernández-Ortuño D, Grabke A, Bryson PK, Amiri A, Peres NA et al (2014) Fungicide resistance profiles in Botrytis cinerea from strawberry fields of seven southern US states. Plant Dis. https://doi.org/10.1094/PDIS-09-13-0970-RE
Finiti I, Leyva M, de la O, Vicedo B, Gomez-Pastor R, Lopez-Cruz J et al (2014) Hexanoic acid protects tomato plants against Botrytis cinerea by priming defence responses and reducing oxidative stress. Mol Plant Pathol. https://doi.org/10.1111/mpp.12112
Ge Y, Deng H, Bi Y, Li C, Liu Y, Dong B (2015) Postharvest ASM dipping and DPI pre-treatment regulated reactive oxygen species metabolism in muskmelon (Cucumis melo L.) fruit. Postharvest Biol Tec. https://doi.org/10.1016/j.postharvbio.2014.09.001
Ge Y, Wei M, Li C, Chen Y, Lv J, Li J (2017) Effect of acibenzolar-S-methyl on energy metabolism and blue mould of Nanguo pear fruit. Sci Hortic. https://doi.org/10.1016/j.scienta.2017.07.012
Giampieri F, Tulipani S, Alvarez-Suarez JM, Quiles JL, Mezzetti B et al (2012) The strawberry: composition, nutritional quality, and impact on human health. Nutrition. https://doi.org/10.1016/j.nut.2011.08.009
Leroch M, Plesken C, Weber RW, Kauff F, Scalliet G et al (2013) Gray mold populations in German strawberry fields are resistant to multiple fungicides and dominated by a novel clade closely related to Botrytis cinerea. Appl Environ Microbiol. https://doi.org/10.1128/AEM.02655-12
Li M, Yu ML, Zhang ZQ, Wu YC, Liu ZG et al (2012) Harpin induces rust disease (Phakopsora zizyphi-vulgaris) resistance on winter jujube (Ziziphus jujuba Mill cv Dongzao). Biol Agric Hortic. https://doi.org/10.1080/01448765.2012.727613
Lin J, Gong D, Zhu S, Zhang Li, Zhang Lu (2011) Expression of PPO and POD genes and contents of polyphenolic compounds in harvested mango fruits in relation to Benzothiadiazole-induced defense against anthracnose. Sci Hortic 130(1):85–89. https://doi.org/10.1016/j.scienta.2011.06.014
Liu YY, Ge YH, Bi Y, Li CY, Deng HW et al (2014) Effect of postharvest acibenzolar-S-methyl dipping on phenylpropanoid pathway metabolism in muskmelon (Cucumis melo L.) fruits. Sci Hortic. https://doi.org/10.1016/j.scienta.2014.01.030
Lorens E, García-Agustín P, Lapeña L (2017) Advances in induced resistance by natural compounds: towards new options for woody crop protection. Sci Agric. https://doi.org/10.1590/1678-992x-2016-0012
Mandal B, Mandal S, Csinos AS, Martinez N, Culbreath AK et al (2008) Biological and Molecular Analyses of the Acibenzolar-S-Methyl-Induced Systemic Acquired Resistance in Flue-Cured Tobacco Against Tomato spotted wilt virus. Phytopathol. https://doi.org/10.1094/PHYTO-98-2-0196
Miller RNG, Alves GSC, Sluys MV (2017) Plant immunity: unravelling the complexity of plant responses to biotic stresses. Ann Bot. https://doi.org/10.1093/aob/mcw284
Nile SH, Park SW (2014) Edible berries: Bioactive components and their effect on human health. Nutrition doi. https://doi.org/10.1016/j.nut.2013.04.007
Ren Y, Wang Y, Bi Y, Ge Y, Wang Y, Fan C et al (2012) Postharvest BTH treatment induced disease resistance and enhanced reactive oxygen species metabolism inmuskmelon (Cucumis melo L.) fruit. Eur Food Res Technol. https://doi.org/10.1007/2Fs00217-012-1715-x
Rodrigues AAC, Bezerra Neto E, Coelho RSB (2006) Induced resistance to Fusarium oxysporum f. sp. tracheiphilum in cowpea plants: effectiveness of abiotic inducers and elicited enzymatic activity. Fitopatol Brasil. https://doi.org/10.1590/S0100-41582006000500009
Rupp S, Weber RW, Rieger D, Detzel P, Hahn M (2017) Spread of Botrytis cinerea strains with multiple fungicide resistance in German horticulture. Front Microbiol. https://doi.org/10.3389/fmicb.2016.02075
Skrovankova S, Sumczynski D, Mlcek J, Jurikova T, Sochor J (2015) Bioactive compounds and antioxidant activity in different types of berries. Int J Mol Sci. https://doi.org/10.3390/ijms161024673
Tang J, Liu Y, Li H, Wang L, Huang K, Chen Z (2015) Combining an antagonistic yeast with harpin treatment to control postharvest decay of kiwifruit. Biol Control. https://doi.org/10.1016/j.biocontrol.2015.04.025
Terry LA, Joyce DC (2000) Suppression of grey mould on strawberry fruit with the chemical plant activator acibenzolar. Pest Management Science.
Terry LA, Joyce DC (2004) Elicitors of induced disease resistance in postharvest horticultural crops: a brief review. Postharvest Biol Technol. https://doi.org/10.1016/j.postharvbio.2003.09.016
Tezcan H, Akbudak N, Akbudak B (2013) The effect of harpin on shelf life of peppers inoculated with Botrytis cinerea. J Food Sci Technol. https://doi.org/10.1007/s13197-011-0432-y
Tian S, Wan Y, Qin G, Xu Y (2006) Induction of defense responses against Alternaria rot by different elicitors in harvested pear fruit. Applied Microbiol Biotechnol doi. https://doi.org/10.1007/s00253-005-0125-4
Tomazeli VN, Marchese JA, Danner MA, Perboni AT, Finatto T et al (2016) Improved resistance to disease and mites in strawberry through the use of acibenzolar-S-methyl and harpin to enhance photosynthesis and phenolic metabolism. Theor Exp Plant Physiol. https://doi.org/10.1007/s40626-016-0068-4
Tripathi D, Jiang YL, Kumar D (2010) SABP2 a methyl salicylate esterase is required for the systemic acquired resistance induced by acibenzolar-S‐methyl in plants. FEBS letters. https://doi.org/10.1016/j.febslet.2010.06.046
Vallejo F, García-Viguera C, Tomás-Barberán FA (2003) Changes in broccoli (Brassica oleracea L. var. italica) health-promoting compounds with inflorescence development. J Agric Food Chem. https://doi.org/10.1021/jf0212338
Vasconsuelo A, Boland R (2007) Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Sci. https://doi.org/10.1016/j.plantsci.2007.01.006
Wang M, Gao L, Dong S, Sun Y, Shen Q et al (2017) Role of silicon on plant–pathogen interactions. Front Plant Sci. https://doi.org/10.3389/fpls.2017.00701
Wei ZM, Laby RJ, Zumoff CH, Bauer DW, He SY et al (1992) Harpin elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science. https://doi.org/10.1126/science.1621099
Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York. https://ggplot2.tidyverse.org. Accessed 26 June 2021
Yang C, Liang Y, Qiu D, Zeng H, Yuan J et al (2018) Lignin metabolism involves Botrytis cinerea BcGs1-induced defense response in tomato. BMC plant boil. https://doi.org/10.1186/s12870-018-1319-0
Zhang C, Wu J (2003) Ethylene inhibitors enhance elicitor-induced paclitaxel production in suspension cultures Taxus spp. cells. Enzyme Microb Technol. https://doi.org/10.1016/S0141-0229(02)00266-1
Zhang Z, Bi Y, Ge Y, Wang J, Deng J et al (2011) Multiple pre-harvest treatments with acibenzolar-S-methyl reduce latent infection and induce resistance in muskmelon fruit. Sci Horticul. https://doi.org/10.1016/j.scienta.2011.06.024
Zhao H, Kim YK, Huang L, Xiao CL (2010) Resistance to thiabendazole and baseline sensitivity to fludioxonil and pyrimethanil in Botrytis cinerea populations from apple and pear in Washington State. Postharvest Biol Technol. https://doi.org/10.1016/j.postharvbio.2009.11.013
Zhu Z, Zhang X (2016) Effect of harpin on control of postharvest decay and resistant responses of tomato fruit. Postharvest Biol Technol. https://doi.org/10.1016/j.postharvbio.2015.09.007
Funding
S. Scariotto acknowledges Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) for a postdoctoral fellowship (PNPD - Postdoctoral National Program), Grant # 88887.352204/2019-00. V.N. Tomazeli1, M.V. Paladini, and C.O. Bolina also acknowledge CAPES for a MSc. fellowship, finance code # 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Ethical approval
This article does not contain any studies with human participants or animals performed by any authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Scariotto, S., Tomazeli, V.N., Paladini, M.V. et al. Plant innate immunity in strawberry induced by pathogen-associated molecular pattern harpin and acibenzolar-S-methyl. Theor. Exp. Plant Physiol. 33, 357–367 (2021). https://doi.org/10.1007/s40626-021-00218-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40626-021-00218-w