Abstract
Herbicide use has raised concerns which results in the need to find an alternative approach, using natural methods, of controlling weeds. Phytotoxic substances released from plants have been used to manage weeds biologically. Rumex maritimus, belonging to the Polygonaceae family, is well known to have a wide range of biological properties. However, the phytotoxic properties of R. maritimus is not well documented. Thus, we conducted an investigation into the phytotoxic potential of R. maritimus and attempted to find out its phytotoxic substances. Leaf, stem and root extracts of R. maritimus exhibited a strong inhibitory effects on root and shoot length of Lactuca sativa L., Medicago sativa L., Lolium multiflorum Lam., Phleum pratense L. With increasing extract concentration, inhibition increased. One substance, 2-methoxystypandrone, was purified after a series of chromatography and characterized by spectral data. 2-Methoxystypandrone reduced the seedling length of Lepidium sativum at concentration ≥ 3 μM. The required 50% growth inhibition concentration of Lepidium sativum seedling for 2-methoxystypandrone was ranged 5.8–11.8 μM. Therefore, it is suggested that the phytotoxic effects of R. maritimus on the test plants may be caused due to the presence of 2-methoxystypandrone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
From the initial release of synthetic herbicides in the 40s, their application has been continuously increasing in crop fields in the management of weeds (Powles and Shaner 2001), and overall yearly sales of herbicides are estimated to be 17 billion dollars worldwide (Kraehmer 2012). In agriculture currently, weeds result in approximately 34% of loss of yield, and the management of weeds is mainly reliant on using synthetic herbicides (Jabran et al. 2015; Tanveer et al. 2015). But, synthetic herbicides, the most reliable and used herbicides are now facing some challenges. Environmental concerns, weed population shifts, negative health effects on animals and humans, and herbicide-resistant weeds are major issues of concern regarding the use of herbicides and reduce its efficacy (Marwat et al. 2011; Starling et al. 2014). Additionally, it is not possible for herbicides to be used in fields where crops are being cultivated organically (Jabran et al. 2015). Thus, seeking a natural alternative to synthetic herbicides is required for the management of weeds (Chai et al. 2015; Islam et al. 2017).
In this concerns, phytotoxic substances might be important to be used as nature-friendly alternative to synthetic herbicides (Asaduzzaman et al. 2014). There is a possibility that phytotoxic substances could be used either directly or as lead substances for bioherbicide development to control weeds as they can affect the growth of target plants (Quintana et al. 2008; Gomaa and AbdElgawad 2012). There have been many phytotoxic studies using a number of plants, with medicinal plants garnering much attention in recent years (Islam et al. 2014; Kato-Noguchi et al. 2014; Suwitchayanon et al. 2017).
Rumex maritimus L. (Polygonaceae) is an annual, herbaceous erect plant, up to 1.2 m tall and native to marshy areas extending in the North American (Mexico, USA), European (UK, The Netherlands), and Asian countries (India, Nepal, Bangladesh) (Clapham et al. 1968; Van Der Sman et al. 1992; Chopra et al. 2002). Rumex maritimus has medicinal properties such as antidiarrheal, antimotility (Rouf et al. 2003), antimicrobial (Kuddus et al. 2011), neuropharmacological (Islam et al. 2003). R. maritimus seeds have previously treated back and bloat pain, and they also have aphrodisiac, astringent, carminative, and antimicrobial properties. The same seeds can be used as an aphrodisiac and as a tonic, whilst R. maritimus leaves can be used to treat burns (Kirtikar and Basu 1980). It has been reported that some of the plants that have medicinal values also have phytotoxic properties and phytotoxic substances (Suwitchayanon et al. 2013, 2017). Chemical investigations of R. maritimus have shown that it contains anthraquinone, chromone, and flavone derivatives (Ahmed et al. 1991). Although R. maritimus is well known to have wide range of biological properties, no phytotoxic activity of R. maritimus has yet been found. Thus, an investigation was conducted into the phytotoxic potential of R. maritimus with the objective of phytotoxic substances identification.
2 Material and methods
2.1 Collection of plant material
Leaves, stems and roots of Rumex maritimus L. were collected at the vegetative stage from the crop field of the Agronomy Field Laboratory, Bangladesh Agricultural University and from Sutiakhali village, Mymensingh, Bangladesh during August–December 2014. Tap water was used to clean particles of dirt, left to air dry in room temperature to avoid direct sunlight, and then ground into powder. The powder was subsequently packed and refrigerated (2 °C) for further extraction.
2.2 Bioassay test species
Five plant species’ seeds were used as testing plants for the bioassay: Lepidium sativum L., Phleum pratense L., Lolium multiflorum Lam., Medicago sativa L., and Lactuca sativa L. Lepidium sativum, Phleum pratense, Medicago sativa, and Lactuca sativa were selected for their characteristics of seedling growth, and Lolium multiflorum was selected as, in crop fields, it is a predominant weed (Kato-Noguchi et al. 2016).
2.3 Extraction
Rumex maritimus powder (leaves, stems and roots, 100 g) was extracted with 850 mL of 70% aqueous methanol for two days. Then it was filtrated through using a layer filter paper (Number 2, 125 mm, Toyo Roshi Kaisha, Japan). The residue was extracted again with methanol (850 mL) for one day and then put through filtration. Two filtrates were combined and then evaporated at 40 °C until complete dryness. After evaporation, the obtained crude extracts of 100 g plant was dissolved in 400 mL of methanol.
2.4 Growth bioassay experiment
Islam and Kato-Noguchi (2016) described a method of growth bioassay, which was used here. The concentrations used for bioassay were equivalent to the extracts obtained from 0.01, 0.03, 0.1, and 0.3 g DW R. maritimus mL−1. Tested concentrations of corresponded extracts were then dropped on to a sheet of filter paper inside Petri dishes. Seeds (n = 10) of Medicago sativa, Lactuca sativa, Lolium multiflorum and Phleum pratense were then placed inside the Petri dishes. Before placement of seeds, the methanolic extracts in the petri dishes were evaporated inside a draft chamber.
Phleum pratense and Lolium multiflorum seeds were pre-sprouted (sprouting condition: soaked in water and kept for 90 and 68 h at 25 °C in darkness, respectively). An experiment without treatment extracts served as control. The Petri dishes were then moved to a plant growth chamber (25 °C) in darkness for two days’ incubation. By comparing with the control seedlings, the % length of the seedlings was calculated.
2.5 Isolation and purification of the active substance
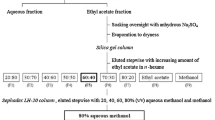
As stated above, Rumex maritimus powder (1025 g) was extracted and evaporated to yield aqueous residue. The pH of the residue was then fixed to 7.0 by using NaOH (1 N). The residue was then partitioned (7 times) against ethyl acetate (150 mL each time). Using the growth bioassay by Lepidium sativum as narrated above, biological activity of both fractions (ethyl acetate and aqueous) was checked. After overnight soaking in anhydrous Na2SO4, ethyl acetate extract was dried and chromatographed through the silica gel column (silica gel 60, mesh 70–230, spherical, 60 g; Nacalai Inc., Kyoto, Japan). A mixture of n-hexane and ethyl acetate was used as an elution solvent for silica gel chromatography. The amount of ethyl acetate (150 mL step−1) was increased step by step (10% in each step, v/v), and 300 mL methanol was used for final elution. Two fractions [60 (fraction 5) and 70% (fraction 6) ethyl acetate in n-hexane] in this chromatography were active as obtained from growth bioassay of Lepidium sativum. After combing of these two fractions of silica gel, the purification was proceeded by using Sephadex LH-20 chromatography (GE Healthcare, 50 g, Uppsala, Sweden). In this separation step, aqueous methanol was used as elution solvent (100 mL in each step), and increased step by step (20% in each step, v/v). Final elution was made with 200 mL of methanol. Elution made with 60 and 80% aqueous methanol showed inhibitory activity. After evaporation of active fractions, the residues were then chromatographed using C18 cartridge (YMC Co., Ltd., Kyoto). The elution solvent in C18 cartridge was the same as Sephadex LH-20 column (15 mL step−1, 30 mL for methanol, 20% increase in every step, v/v). The most growth inhibitory fraction [60% aqueous methanol (fraction 3)] of C18 cartridge was then dried for final purification by HPLC (using a column size of 500 mm × 10 mm ID, S-5 µ, ODS AQ-325; YMC Ltd., Kyoto, Japan), eluted at a flow rate of 1.5 mL per min with 60% (v/v) aqueous methanol. The wavelength for detection was 220 nm. One active peak fraction was found eluted in 53–58 min (Figs. 1S, 2S). The substance was characterized using HRESIMS, 1H-NMR (spectra were recorded at 400 MHz in a spectrometer with CDCl3 and CD3OD), and 13C NMR (at 100 MHz with CD3OD).
2.6 Bioassay of the identified growth inhibitory substance
Identified active substance was dissolved in 1 mL of methanol to prepare treatment concentrations for bioassay and transferred to filter papers (qualitative, number 2; Toyo) in Petri dishes (28 mm). After evaporation of methanol in a laminar flow, the filter paper was moistened with Tween 20 (0.05%, 600 µL). Seeds of Lepidium sativum (n = 10) were then placed on the filter paper inside the Petri dish. The shoot and root length of the seedlings were measured as stated above after 48 h of incubation in darkness at 25 °C.
2.7 Statistical analysis
All bioassays followed a completely randomised block and had three replications and 10 seedlings. The whole experiment was repeated two times. Mean results are expressed as mean ± standard error. ANOVA was conducted using the SPSS statistical package, USA (Version 16) for Windows. Data for significant differences between control and treatment was tested by Tukey’s HSD at the 0.05 probability level. The I 50 values were determined using GraphPad Prism software package, USA (Version 6).
3 Results
3.1 Phytotoxic potential of R. maritimus extracts
R. maritimus extracts showed significant inhibitory effects on tested plants (Fig. 1), and the effects were varied in response to the extract concentration. The growth of Lactuca sativa and Medicago sativa seedlings were completely inhibeted by the extract obtained from 0.1 g DW of Rumex maritimus mL−1. The extract, at identical concentration, inhibited root and shoot lengths of Lolium multiflorum and Phleum pratense to 29.8, 13.6% and 27.2, 27.5% of control length, respectively. At 0.3 g DW equivalent extract mL−1, the root length of Phleum pratense and seedling of Lolium multiflorum experienced complete inhibition, whereas the shoot growth of Phleum pratense inhibited by 8.9% of control length. A significant inhibition level (19–76% of the control length) was observed on tested plants at concentration of 0.01 g DW equivalent extract mL−1. Different levels of inhibition resulted when the plants were exposed to the other concentrations of extracts (Fig. 1). The I 50 values on seedling growth of all tested plants ranged between 0.1 and 42.9 mg DW equivalent extract mL−1 (Table 1).
Effect of Rumex maritimus extracts on the shoot and root lengths of Lactuca sativa, Medicago sativa, Lolium multiflorum, and Phleum pratense seedlings exposed to different concentrations of Rumex maritimus extracts mL−1. Values are presented as mean ± SE for every treatment. Bars and similar letters indicate standard deviation from the mean and no significant difference among means within group, respectively (Tukey’s HSD, at 0.05 probability level)
3.2 Purification of growth inhibitory substance in aqueous methanolic extract
At 0.9 g DW equivalent extract mL−1, fractions 5 and 6 in silica gel chromatography completely (100%) inhibited the seedling growth of Lepidium sativum (Fig. 2). After a series of chromatography, one active substance was identified through spectroscopic analysis. The substance molecular formula was estimated to be C14H13O5 by HRESIMS at m/z 261.0776 [M+H]+ (calcd for C14H13O5, 261.0763, Δ = + 1.3 mmu). The 1H NMR (400 MHz, CDCl3) spectrum showed δH 7.52 (s, 1 H, H-8), 6.11 (s, 1 H, H-3), 3.93 (s, 3 H, H-14), 2.59 (s, 3 H, H-12), 2.35 (s, 3 H, H-13), and 1H NMR (400 MHz, CDCl3) showed δH 7.51 (s, 1 H, H-8), 6.27 (s, 1 H, H-3), 3.93 (s, 3 H, H-14), 2.56 (s, 3 H, H-12), 2.33 (s, 3 H, H-13). The 13C NMR (100 MHz, CD3OD) showed δ C 204.8 (C-11), 162.7 (C-2), 144.2 (C-7), 137.3 (C-6), 121.8 (C-8), 110.1 (C-3), 57.1 (C-14), 31.6 (C-12), 19.6 (C-13). The compound was identified as 2-methoxystypandrone (Fig. 3) after comparing its spectral data with a literature (Rauwald and Meithing 1983).
Effect of fractions eluted in silica gel column on Lepidium sativum seedling length by R. maritimus extract. Values are presented as mean ± SE for every treatment. Bars and similar letters indicate standard deviation from the mean and no significant difference among means within group, respectively (Tukey’s HSD, at 0.05 probability level)
3.3 Biological activity of 2-methoxystypandrone
2-Methoxystypandrone reduced the seedling length of Lepidium sativum in a concentration-dependent manner. A significant inhibition started from ≥ 3 μM concentration on plants tested with 2-methoxystypandrone (Figs. 4, 5). The length of Lepidium sativum root and shoot were decreased by 32.5, 47.0, 66.0, 81.3, 88.5, 97.3, 99.8% and 29.9, 65.8, 75.5, 79.9, 93.0, 99.6, 99.9% as compared to control length at concentration of 3, 10, 30, 100, 300, 1000 and 3000 µM, respectively. The I 50 values of 2-methoxystypandrone were 5.8 μM on shoot and 11.8 μM on root growth of Lepidium sativum (Table 1).
Effect of 2-methoxystypandrone on shoot and root length of Lepidium sativum. Values are presented as mean ± SE for every treatment. Bars and similar letters indicate standard deviation from the mean and no significant difference among means within group, respectively (Tukey’s HSD, at 0.05 probability level)
4 Discussion
Rumex maritimus extracts showed significant inhibition of seedling length of Lactuca sativa, Lolium multiflorum, Medicago sativa, and Phleum pratense. This inhibition was extract concentration-dependent. The I 50 values of the tested plants were different, which indicates that the inhibition was also species-dependent. The concentration- and species-dependent inhibition on tested plants were also found in Lilium sp. (Chai et al. 2015), Aglaia odorata (Kato-Noguchi et al. 2016), Mangifera indica (Suzuki et al. 2016), Marsilea crenata (Islam et al. 2017). The growth inhibitory effects of R. maritimus extracts suggests that the extracts may possess phytotoxic substances.
One substance was isolated and characterized as 2-methoxystypandrone (Fig. 3), a naphthoquinone, which is one of the most potent substances in the quinone series (Liu et al. 2012). 2-Methoxystypandrone has also been found in Rhamnus fallax B. Z. (Rauwald and Meithing 1983), Rumex japonicus (Nishina et al. 1993), and Polygonum cuspidatum (Chiou et al. 2010; Chern et al. 2014; Kuang et al. 2014), and it is synthesized from trimethoxybenzaldehyde (Hughes and Sargent 1989). However, although 2-methoxystypandrone has been found in many other plants, this is the first report of 2-methoxystypandrone in R. maritimus. In the study, the seedling growth of Lepidium sativum was inhibited by 2-methoxystypandrone in a concentration-dependent manner. Singh et al. (2001) and Liu et al. (2012) also reported similar concentration-dependent biological activities of 2-methoxystypandrone. 2-Methoxystypandrone has also been documented having a large range of biological properties, for example anti-cancer (Liu et al. 2012), antitumor (Kuang et al. 2014), anti-resorption (Chiou et al. 2010), antimicrobial (Nishina et al. 1993), ischemic stroke activity (Chern et al. 2014), and HRV 3C-protease inhibitory activity (Singh et al. 2001). The I 50 values of 2-methoxystypandrone for the seedling growth of Lepidium sativum ranged from 5.5 to 12.0 μM. The findings also agree with the findings of Liu et al. (2012), who stated I 50 values for 2-methoxystypandrone of less than 10 μM for different biological activities. The methoxy group at the C-2 position in 2-methoxystypandrone may be a reason for its inhibitory activities. A significant reduction in the activity (15-fold lower activity) of 2-methoxystypandrone was observed when the methoxy group at C-2 was transposed to the C-3 position (Singh et al. 2001). In addition, 2-methoxystypandrone lost its activity completely when the methoxy group at the C-2 position was replaced with a hydroxyl group (Singh et al. 2001). Carbonyl groups may be another reason for the activity of 2-methoxystypandrone because it has three α, β-unsaturated carbonyl groups (Kuang et al. 2014). The phytotoxic substances released from the plants are thought to suppress the normal growth and development of adjacent plants by affecting their physiological processes (Quintana et al. 2008). The phytotoxic substances can also be a nature-friendly potent source of bio-herbicides (Suwitchayanon et al. 2017). Therefore, the medicinal plant R. maritimus could potentially be used in biological weed management because of the phytotoxic effects of 2-methoxystypandrone.
In conclusion, concentration- and species-dependent inhibitory activities were found using the aqueous methanolic extracts of R. maritimus on tested plants. An active phytotoxic substance was purified from R. maritimus extracts using column chromatography, and its chemical structure was identified as 2-methoxystypandrone by means of HRESIMS, and 1H- and 13C nuclear magnetic resonance spectroscopic analyses. 2-Methoxystypandrone showed significant growth inhibitory effects on Lepidium sativum seedling length in a concentration-dependent manner. These findings indicate that 2-methoxystypandrone may have contribution to the phytotoxic effects of R. maritimus.
References
Ahmed M, Datta BK, Rouf ASS (1991) Anthraquinone, chromone and flavone derivatives from Rumex maritimus. Pharmazie 46:548–549
Asaduzzaman M, An M, Pratley JE, Luckett DJ, Lemerle D (2014) Canola (Brassica napus) germplasm shows variable allelopathic effects against annual ryegrass (Lolium rigidum). Plant Soil 380:47–56. https://doi.org/10.1007/s11104-014-2054-4
Chai M, Zhu X, Cui H, Jiang C, Zhang J, Shi L (2015) Lily cultivars have allelopathic potential in controlling Orobanche aegyptiaca Persoon. PLoS ONE 10:e0142811. https://doi.org/10.1371/journal.pone.0142811
Chern CM, Wange YH, Liou KT, Hou YC, Chen CC, Shen YC (2014) 2-Methoxystypandrone ameliorates brain function through preserving BBB integrity and promoting neurogenesis in mice with acute ischemic stroke. Biochem Pharmacol 87:502–514. https://doi.org/10.1016/j.bcp.2013.11.018
Chiou WF, Liao JF, Huang CY, Chen CC (2010) 2-Methoxystypandrone represses RANKL-mediated osteoclastogenesis by down-regulating formation of TRAF6–TAK1 signalling complexes. Br J Pharmacol 161:321–335. https://doi.org/10.1111/j.1476-5381.2010.00823.x
Chopra RN, Nayar SL, Chopra IC (2002) Glossary of Indian medicinal plants. National Institute of Science Communication and Information Resources, New Delhi
Clapham AR, Tutin TG, Warburg EF (1968) Excursion flora of the British Isles. Cambridge University Press, Cambridge
Gomaa NH, AbdElgawad HR (2012) Phytotoxic effects of Echinochloa colona (L.) Link. (Poaceae) extracts on the germination and seedling growth of weeds. Span J Agric Res 10:492–501
Hughes AB, Sargent MV (1989) Synthesis of 2-methoxystypandrone: comments on the structure of ventilaginone. J Chem Soc Perkin Trans 1:449–452. https://doi.org/10.1039/P19890000449
Islam MS, Kato-Noguchi H (2016) Allelopathic potential of the weed Fimbristylis dichotoma (L.) on four dicotyledonous and four monocotyledonous test plant species. Res Crops 17:388–394. https://doi.org/10.5958/2348-7542.2016.00064.4
Islam MS, Rahman MT, Rouf ASS, Rahman F (2003) Evaluation of neuropharmacological effects of Rumex maritimus Linn. (Polygonaceae) root extracts. Pharmazie 58:738–741
Islam AKMM, Ohno O, Suenaga K, Kato-Noguchi H (2014) Two novel phytotoxic substances from Leucas aspera. J Plant Physiol 171:877–883. https://doi.org/10.1016/j.jplph.2014.03.003
Islam MS, Iwasaki A, Suenaga K, Kato-Noguchi H (2017) Isolation and identification of two potential phytotoxic substances from the aquatic fern Marsilea crenata. J Plant Biol 60:75–81. https://doi.org/10.1007/s12374-016-0408-6
Jabran K, Mahajan G, Sardana V, Chauhan BS (2015) Allelopathy for weed control in agricultural systems. Crop Prot 72:57–65. https://doi.org/10.1016/j.cropro.2015.03.004
Kato-Noguchi H, Pukclai P, Ohno O, Suenaga K (2014) Isolation and identification of a plant growth inhibitor from Tinospora tuberculata Beumee. Acta Physiol Plant 36:1621–1626. https://doi.org/10.1007/s11738-014-1537-5
Kato-Noguchi H, Suzuki M, Noguchi K, Ohno O, Suenaga K, Laosinwattana C (2016) A potent phytotoxic substance in Aglaia odorata Lour. Chem Biodivers 13:549–554. https://doi.org/10.1002/cbdv.201500175
Kirtikar KR, Basu BD (1980) Indian medicinal plants. International Book Distributors, Dehradun
Kraehmer H (2012) Innovation: changing trends in herbicide discovery. Outlooks Pest Manag 23:115–118. https://doi.org/10.1564/23jun06
Kuang S, Qi C, Liu J, Sun X, Zhang Q, Sima Z, Liu J, Li W, Yu Q (2014) 2-Methoxystypandrone inhibits signal transducer and activator of transcription 3 and nuclear factor-κB signaling by inhibiting Janus kinase 2 and IκB kinase. Cancer Sci 105:473–480. https://doi.org/10.1111/cas.12359
Kuddus MR, Ali MB, Rumi F, Aktar F, Rashid MA (2011) Evaluation of polyphenols content and cytotoxic, membrane stabilizing and antimicrobial activities of seed of Rumex maritimus Linn. Bangladesh Pharm J 14:67–71
Liu J, Zhang Q, Chen K, Liu J, Kuang S, Chen W, Yu Q (2012) Small-molecule STAT3 signalling pathway modulators from Polygonum cuspidatum. Planta Med 78:1568–1570. https://doi.org/10.1055/s-0032-1315121
Marwat KB, Khan MA, Hashim S, Nawab K, Khattak AM (2011) Integrated weed management in wheat. Pak J Bot 43:625–633
Nishina A, Kubota K, Osawa T (1993) Antimicrobial components, trachrysone and 2-methoxystypandrone, in Rumex japonicus Houtt. J Agric Food Chem 41:1772–1775. https://doi.org/10.1021/jf00034a047
Powles SB, Shaner DL (2001) Herbicide resistance and world grains. CRC Press, Boca Raton
Quintana N, Weir TL, Du J, Broeckling CD, Rieder JP, Stermitz FR, Paschke MW, Vivanco JM (2008) Phytotoxic polyacetylenes from roots of Russian knapweed (Acroptilon repens (L.) DC.). Phytochemistry 69:2572–2578. https://doi.org/10.1016/j.phytochem.2008.07.015
Rauwald HW, Meithing H (1983) 2-Methoxystypandrone, a new naphthoquinone from Rhamnus fallax. Z Naturforsch 38:17–20
Rouf AS, Islam MS, Rahman MT (2003) Evaluation of antidiarrhoeal activity Rumex maritimus root. J Ethnopharmacol 84:307–310
Singh SB, Graham PL, Reamer RA, Cordingley MG (2001) Discovery, total synthesis, HRV 3C-protease inhibitory activity, and structure–activity relationships of 2-methoxystypandrone and its analogues. Bioorg Med Chem Lett 11:3143–3146. https://doi.org/10.1016/S0960-894X(01)00648-5
Starling AP, Umbach DM, Kamel F, Long S, Sandler DP, Hoppin JA (2014) Pesticide use and incident diabetes among wives of farmers in the agricultural health study. Occup Environ Med 71:629–635. https://doi.org/10.1136/oemed-2013-101659
Suwitchayanon P, Pukclai P, Kato-Noguchi H (2013) Allelopathic activity of Cymbopogon nardus (Poaceae): a preliminary study. J Plant Stud 2:1–6. https://doi.org/10.5539/jps.v2v2p1
Suwitchayanon P, Ohno O, Suenaga K, Kato-noguchi H (2017) N-Octanoyl tyramine, a phytotoxic compound in the roots of Cymbopogon nardus. Acta Physiol Plant 39:123. https://doi.org/10.1007/s11738-017-2419-4
Suzuki M, Khan MSI, Iwasaki A, Suenaga K, Kato-Noguchi H (2016) Allelopathic potential and an allelopathic substance in mango leaves. Acta Agric Scand B 67:37–42. https://doi.org/10.1080/09064710.2016.1215517
Tanveer A, Safdar ME, Suleman M, Tahir M, Zamir SI, Nadeem MA (2015) Assessing the potential of the water soluble allelopaths of Marsilea minuta in rice and wheat. Planta Daninha 33:231–239. https://doi.org/10.1590/0100-83582015000200008
Van Der Sman AJM, Blom CWPM, Van de Steeg HM (1992) Phenology and seed production in Chenopodium rubrum, Rumex maritimus, and Rumex palustris as related to photoperiod in river forelands. Can J Bot 70:392–400. https://doi.org/10.1139/b92-053
Acknowledgements
We thank Dennis Murphy, Professor, Ehime University for his critical English editing on the manuscript. The first author also acknowledges the Government of Japan for providing a scholarship to carry out the research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
40626_2017_95_MOESM1_ESM.tif
Fig.1S HPLC chromatogram of the methanolic extracts of Rumex maritimus. Arrow indicates the active peak fraction in which the inhibitory activity was detected. Supplementary material 1 (TIFF 184 kb)
40626_2017_95_MOESM2_ESM.tif
Fig.2S Effects of the fractions obtained with HPLC. Lepidium sativum was exposed to the concentration equivalent to the extracts obtained from 6.0 g DW of Rumex maritimus mL−1. Values given are means ± SE from two independent experiments with 10 seedlings for each treatment. Supplementary material 2 (TIFF 125 kb)
Rights and permissions
About this article
Cite this article
Islam, M.S., Iwasaki, A., Suenaga, K. et al. 2-Methoxystypandrone, a potent phytotoxic substance in Rumex maritimus L.. Theor. Exp. Plant Physiol. 29, 195–202 (2017). https://doi.org/10.1007/s40626-017-0095-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40626-017-0095-9