Abstract
Background
No consensus currently exists regarding the optimal approach for peritoneal dialysis catheter placement. We aimed to compare the outcomes of percutaneous and surgical peritoneal dialysis catheter placement.
Methods
A systematic review of the literature was performed using the MEDLINE, Cochrane Library, and Scopus databases (end-of-search date: August 29th, 2020). We included studies comparing percutaneous (blind, under fluoroscopic/ultrasound guidance, and “half-perc”) and surgical peritoneal dialysis catheter placement (open and laparoscopic) in terms of their infectious complications (peritonitis, tunnel/exit-site infections), mechanical complications (leakage, inflow/outflow obstruction, migration, hemorrhage, hernia, bowel perforation) and long-term outcomes (malfunction, removal, replacement, surgery required, and mortality).
Results
Thirty-four studies were identified, including thirty-two observational studies (twenty-six retrospective and six prospective) and two randomized controlled trials. Percutaneous placement was associated with significantly lower rates of tunnel/exit-site infection [relative risk (RR) 0.72, 95% confidence interval (CI) 0.56–0.91], catheter migration (RR 0.68, 95% CI 0.49, 0.95), and catheter removal (RR 0.73, 95% CI 0.60–0.88). The 2-week and 4-week rates of early tunnel/exit-site infection were also lower in the percutaneous group (RR 0.45, 95% CI 0.22–0.93 and RR 0.41, 95% CI 0.27–0.63, respectively). No statistically significant difference was observed regarding other outcomes, including catheter survival and mechanical complications.
Conclusion
Overall, the quality of published literature on the field of peritoneal dialysis catheter placement is poor, with a small percentage of studies being randomized clinical trials. Percutaneous peritoneal dialysis catheter placement is a safe procedure and may result in fewer complications, such as tunnel/exit-site infections, and catheter migration, compared to surgical placement.
Protocol registration
PROSPERO CRD42020154951.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Peritoneal dialysis (PD) is an important therapeutic option for the management of end-stage renal disease, with comparable short-term and long-term outcomes to hemodialysis [1, 2]. Access to the peritoneal cavity is achieved via the placement of a peritoneal dialysis catheter (PDC). A variety of techniques have been developed to facilitate PDC placement. The classic surgical approaches include open surgery or laparoscopy. The percutaneous approach, on the other hand, allows for a minimally-invasive placement of the catheter in the Interventional Radiology suite using the modified Seldinger technique, often under fluoroscopic/radioscopic guidance [3,4,5]. Patient comorbidities, expertise of the healthcare provider, resource availability and urgency for PD initiation are factors that often influence the choice between the different techniques [3].
Both infectious and mechanical complications directly related to the placement of the PDC are a primary cause of catheter failure and mortality in patients receiving PD [6,7,8]. The rate of complications varies with the placement technique due to inherent strengths and weaknesses associated with each. However, there is currently no consensus regarding the optimal technique for PDC placement. Previous meta-analyses on the subject have failed to provide a definitive answer and were often limited by small sample sizes [9,10,11]. Therefore, we sought to perform an updated systematic review and meta-analysis, aiming to compare the effects of surgical and percutaneous PDC placement techniques on catheter-related complications and catheter survival.
Materials and methods
Study design and inclusion/exclusion criteria
This systematic review and meta-analysis was performed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines (Online Resource 1) [12]. A protocol was developed and agreed upon by all authors prior to the beginning of this study and was made publicly available at the PROSPERO database (registration number CRD42020154951). An institutional review board approval was not required to conduct this study. We applied the PICO (Population/Participants, Intervention, Comparison, and Outcome) framework to define the study selection criteria as follows:
-
P (Participants): Adult patients (age ≥ 18 years) of any sex or race undergoing peritoneal dialysis catheter placement, regardless of indication. Both first-time insertions and re-insertions were considered.
-
I (Interventions): Percutaneous and surgical peritoneal dialysis catheter placement. Percutaneous techniques included catheter placement via the Seldinger technique, with or without fluoroscopic/ultrasound guidance, as well the newer “half-perc” techniques. Surgical techniques included catheter placement via open surgery, mini-laparotomy or laparoscopic surgery.
-
C (Comparison): Studies were deemed eligible only if percutaneous catheter placement was directly compared to surgical catheter placement for the outcomes of interest.
-
O (Outcomes): The primary outcome measures were the rates of 3-month and 1-year catheter survival, overall infectious complications, overall mechanical complications peritonitis, and tunnel/exit-site infection. The secondary outcome measures were the rates of leakage, inflow/outflow obstruction, catheter migration, post-procedural hemorrhage, hernia, bowel perforation, catheter malfunction, surgery required due to catheter-related causes, catheter removal, catheter replacement, post-procedural mortality and overall mortality.
Original clinical studies, including both randomized trials and non-randomized prospective/retrospective comparative studies, published in English, reporting on both surgical and percutaneous PDC placement for the outcomes of interest were deemed eligible for inclusion. The exclusion criteria were defined as follows: (1) articles published in any non-English language, (2) irrelevant articles, (3) animal and in vitro studies, (4) case reports, (5) narrative or systematic reviews and meta-analyses, (6) editorials, letters to the editors, perspectives, comments, errata that did not provide any primary patient data, (7) published abstracts without any published full text, and (8) non-comparative studies (1 study arm only). No search filters were applied.
All eligible studies were closely assessed for any partially or completely overlapping populations according to their author lists, study centers, recruitment dates and data collection dates. Among studies with overlapping populations, we selected those with the largest number of patients and/or data for the outcomes of interest.
Search strategy
Eligible studies were identified by searching through the MEDLINE (via PubMed), Scopus, and Cochrane Library databases (end-of-search date: August 29th, 2020) by two independent reviewers (S.M.E. and G.A.S.). The following search algorithm was used: peritoneal AND dialysis AND catheter AND (insert* OR placement). Any disagreements on article inclusion were resolved by a third independent reviewer (K.P.E.). The reference lists of the included studies were also searched for any missed but otherwise eligible articles, based on the “snowball” methodology [13].
Data extraction
Data tabulation and extraction was performed by two independent reviewers (S.M.E. and G.A.S) and using a standardized, pre-piloted form. Any disagreements were identified and resolved by reaching a consensus and/or discussion with a third reviewer (K.P.E.). The following data were extracted: (1) study characteristics (first author, year of publication, study design, study center, study period, number of patients, number of procedures, PDC type for each group) (2) patient characteristics (age in years, sex, number of prior abdominal surgeries, underlying comorbidities, primary renal disease, follow up period in months, break-in period in days) (3) primary outcomes (3-month and 1-year catheter survival rate, overall mechanical complication rate, overall infectious complication rate, peritonitis rate, and tunnel/exit-site infection rate), and (4) secondary outcomes (leakage rate, inflow/outflow obstruction rate, catheter migration rate, post-procedural hemorrhage rate, hernia rate, bowel perforation rate, catheter malfunction rate, surgical PDC revision rate, catheter removal rate, catheter replacement rate, post-procedural mortality rate and overall mortality rate). Additional data were recorded regarding early infectious and mechanical complications, occurring up to 2 weeks and 4 weeks following catheter placement.
The overall infectious complication rate was defined as the number of patients affected by at least one episode of peritonitis, tunnel infections and/or exit-site infection. The overall mechanical complication rate was defined as the number of patients affected by at least one episode of any non-infectious complication, including leakage, inflow/outflow obstruction, catheter migration, post-procedural hemorrhage, hernia, and bowel perforation. Catheter survival was defined as the number of catheters remaining in place after a specific time period following placement (3 months and 1 year), taking into account any censoring of patients due to death, transplantation or transfer to hemodialysis as applied by the authors. The catheter removal rate was defined as the number of catheters removed as a result of an infectious or mechanical complication divided by the total number of catheters; removals due to death, transplantation, switching to hemodialysis, and voluntary patient choice were not considered for this outcome. The rest of the outcomes were defined according to the International Society of Peritoneal Dialysis [14].
Quality of evidence assessment
We used the Newcastle–Ottawa scale (NOS) [15] to assess the quality of non-randomized studies; a score of ≥ 6 denoted high study quality. A 3-month duration and a 90% rate were a priori set as the cutoffs for the items assessing whether follow-up length and adequacy, respectively.
We used the Cochrane Collaboration’s tool to assess the quality of randomized controlled trials (RCTs), which evaluates the following types of bias: selection, performance, detection, attrition, and reporting [16].
Statistical analysis
Frequencies and percentages were used to summarize categorical variables, while means and standard deviations (SDs) were used to summarize continuous variables. We applied the method proposed by Hozo et al. to estimate the means and SDs of continuous variables, whenever medians and ranges were provided instead [17]. We estimated all relative rates based on available data for each variable of interest. We handled all available data according to the principles stated in the Cochrane Handbook [18].
Meta-analysis was carried out to compare surgical and percutaneous PDC insertion for all primary and secondary outcomes. A subgroup analysis was performed according to the type of surgical PDC placement (open surgical, laparoscopic or both), as previous meta-analyses have shown differences in the outcomes between open surgical and laparoscopic PDC placement [19, 20]. An additional subgroup analysis was performed regarding patients with no previous abdominal operation history. Selection bias is often present in studies utilizing the percutaneous technique and patients with a previous abdominal surgery are often excluded or moved to the surgical group instead [21]. Thus, a history of previous abdominal operations may act as a confounder when comparing the two placement techniques. Based on extracted data, relative risks (RRs) and 95% confidence intervals (CIs) were calculated by means of 2 × 2 tables for each categorical outcome; RR greater than 1 indicated that the outcome was more frequently present in the percutaneous group. Continuity correction of 0.5 in studies with zero cell frequencies was adopted. Between-study heterogeneity was assessed through Cochran Q statistic and by estimating I2. High heterogeneity was confirmed with a significance level of P < 0.05 and I2 ≥ 50%. We used the random-effects model (DerSimonian-Laird) to calculate the pooled effect estimates for all outcomes, due to the significant between-study heterogeneity [22]. Publication bias was assessed via funnel plots and Egger’s test for each outcome of interest; publication bias was determined to be present for any p value < 0.1 on Egger’s test [23]. Statistical significance was set at 0.05 and all p values were two-tailed. Statistical analyses were performed using STATA IC 16.0 (StataCorp LLC, College Station, Texas).
Results
Study selection and characteristics
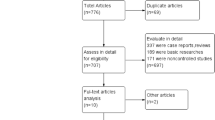
Through our systematic search, 1794 unique articles were retrieved, of which 110 underwent full-text evaluation for eligibility. Ultimately, 34 studies reporting on 6067 PDC placements (2946 percutaneous and 3121 surgical) fulfilled the inclusion criteria and were included in our meta-analysis [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57] (Fig. 1). Detailed study and patient characteristics of the included studies are presented in Tables 1 and 2, respectively. More patients had a history of previous abdominal surgery in the surgical group (26.8%) compared to the percutaneous group (8.4%). The average break-in period was shorter in the percutaneous group (14.7 ± 24.8 days) compared to the surgical group (22.4 ± 31.7). The remaining baseline characteristics were similar among the two groups.
Study quality and publication bias assessment
Of the 34 included studies in this meta-analysis, 32 were non-randomized studies (26 retrospective and 6 prospective), and 2 were randomized controlled trials. The NOS was used to assess the quality of the 24 non-randomized studies, with a mean score of 6.4 ± 1.0 (Online Resource 2A).
Two randomized controlled trials were also included in the meta-analysis and were assessed separately using the Cochrane Collaboration’s Tool. The risk of selection and attrition bias was low in both studies, while the risk of performance and detection bias was high in both cases. The risk of reporting bias was low in Voss et al. and high in Atapour et al., respectively (Online Resource 2B).
Egger’s test revealed no publication bias in the funnel plots of any of the studied outcomes, with the exception of the 2-week tunnel and exit-site infection rate (p = 0.084).
Meta-analyses of primary outcomes
The results of the meta-analyses for all primary outcomes are summarized in Table 3. Forest plots of all primary outcomes are presented in Figs. 2, 3, 4 and 5.
Three-month catheter survival
The 3-month catheter survival rate was reported in 3 studies [24, 35, 42]. No statistically significant difference was found between the percutaneous group (74.9%; n = 250/334) and the surgical group (71.4%; n = 302/423) (RR 1.05, 95% CI 0.95–1.15; p = 0.33). Statistical heterogeneity was low (I2 = 0.0%) (Fig. 2a).
One-year catheter survival
The 1-year catheter survival rate was reported in 5 studies [24, 26, 42, 50, 51]. No statistically significant difference was found between the percutaneous group (71.2%; n = 332/466) and the surgical group (62.8%; n= 446/710) (RR 1.02, 95% CI 0.97–1.07; p = 0.49). Statistical heterogeneity was low (I2 = 0.0%) (Fig. 2b).
Overall infectious complications
The overall infectious complication rate was reported in 5 studies [29, 30, 39, 43, 50]. No statistically significant difference was found between the percutaneous group (23.6%; n = 183/777) and the surgical group (28.5%; n = 163/573) (RR 0.82, 95% CI 0.60–1.11; p = 0.20). Statistical heterogeneity was low (I2 = 47.9%) (Fig. 3a).
Overall mechanical complications
The overall mechanical complication rate was reported in 6 studies [28,29,30, 39, 43, 50]. No statistically significant difference was found between the percutaneous group (16.8%; n = 139/827) and the surgical group (16.7%; n = 126/753) (RR 1.00, 95% CI 0.72–1.38; p = 0.98). Statistical heterogeneity was low (I2 = 45.7%) (Fig. 3b).
Peritonitis rate
The peritonitis rate was reported in 24 studies [24, 26,27,28,29,30, 32, 33, 35,36,37,38, 42, 44, 46,47,48,49,50,51, 54, 55, 57]. No statistically significant difference was found between the percutaneous group (15.7%; n = 321/2042) compared to the surgical group (15.2%; n = 334/2205) (RR 0.94, 95% CI 0.75–1.18; p = 0.60). Statistical heterogeneity was low (I2 = 49.8%) (Fig. 4). No statistically significant difference was observed in the subgroup analysis between the percutaneous and the laparoscopic group (RR 1.10, 95% CI 0.78–1.56) or the open surgical group (RR 0.98, 95% CI 0.74–1.29).
Tunnel and exit-site infection rate
The tunnel and exit site infection rate was reported in 24 studies [24, 26,27,28,29,30, 32, 33, 36,37,38, 40, 43, 44, 46,47,48,49,50,51, 53,54,55, 57], and was significantly lower in the percutaneous group (10.2%; n = 191/1882) compared to the surgical group (12.8%; n = 263/2054) (RR 0.72, 95% CI 0.56–0.91; p = 0.01). Statistical heterogeneity was low (I2 = 24.5%) (Fig. 5). No statistically significant difference was observed in the subgroup analysis between the percutaneous and the laparoscopic group (RR 0.95, 95% CI 0.76–1.19), while the tunnel and exit-site infection rate was significantly lower in the percutaneous group compared to the open surgical group (RR 0.68, 95% CI 0.48–0.95).
Meta-analyses of secondary outcomes
The results of the meta-analyses for all secondary outcomes are summarized in Table 3. Forest plots of all secondary outcomes are presented in Online Resource 3.
Leakage
The leakage rate was reported in 28 studies [24,25,26,27,28,29,30, 32,33,34, 36,37,38, 40, 42,43,44,45,46,47,48,49,50,51, 53,54,55, 57]. No statistically significant difference was found between the percutaneous group (7.4%; n = 159/2148) and the surgical group (5.9%; n = 136/2321) (RR 1.29, 95% CI 0.95–1.76; p = 0.11). Statistical heterogeneity was low (I2 = 32.8%) (Online Resource 3A). No statistically significant difference was observed in the subgroup analysis between the percutaneous and the laparoscopic group (RR 1.05, 95% CI 0.63–1.76), while the leakage rate was significantly higher in the percutaneous group compared to the open surgical group (RR 1.67, 95% CI 1.15–2.44).
Inflow/outflow obstruction
The inflow/outflow obstruction rate was reported in 11 studies [28,29,30, 36, 38, 43, 44, 46, 47, 54, 57]. No statistically significant difference was found between the percutaneous group (8.4%; n = 88/1043) and the surgical group (8.5%; n = 76/891) (RR 0.86, 95% CI 0.64–1.15; p = 0.51). Statistical heterogeneity was low (I2 = 0.0%) (Online Resource 3B). No statistically significant difference was observed in the subgroup analysis between the percutaneous and the laparoscopic group (RR 0.70, 95% CI 0.18–2.71) or the open surgical group (RR 0.85, 95% CI 0.48–1.52).
Catheter migration rate
The catheter migration rate was reported in 14 studies [25, 29,30,31,32,33, 36, 40, 43, 45, 46, 49, 51, 54], and was significantly lower in the percutaneous group (6.1%; n = 64/1058) compared to the surgical group (8.7%; n = 81/927) (RR 0.68, 95% CI 0.49–0.95; p = 0.02). Statistical heterogeneity was low (I2 = 0.0%) (Online Resource 3C). No statistically significant difference was observed in the subgroup analysis between the percutaneous and the laparoscopic group (RR 1.36, 95% CI 0.36–5.17), while the migration rate was significantly lower in the percutaneous group compared to the open surgical group (RR 0.65, 95% CI 0.46–0.93).
Post-procedural hemorrhage rate
The post-procedural hemorrhage rate was reported in 21 studies [24, 26, 28,29,30, 32, 33, 36, 38, 40, 42, 43, 45,46,47, 49,50,51, 53, 54, 57]. No statistically significant difference was found between the percutaneous group (2.5%; n = 45/1785) and the surgical group (2.6%; n = 48/1864) (RR 1.09, 95% CI 0.72–1.66; p = 0.68). Statistical heterogeneity was low (I2 = 0.0%) (Online Resource 3D). No statistically significant difference was observed in the subgroup analysis between the percutaneous and the laparoscopic group (RR 0.65, 95% CI 0.09–4.56) or the open surgical group (RR 1.14, 95% CI 0.72–1.80).
Hernia rate
The hernia rate was reported in 13 studies [24, 27, 29, 31, 32, 36, 38, 42, 43, 46, 47, 50, 53]. No statistically significant difference was found between the percutaneous group (3.6%; n = 41/1128) and the surgical group (6.2%; n = 70/1133) (RR 0.71, 95% CI 0.49–1.02; p = 0.13). Statistical heterogeneity was low (I2 = 0.0%) (Online Resource 3E). No statistically significant difference was observed in the subgroup analysis between the percutaneous and the laparoscopic group (RR 0.83, 95% CI 0.48–1.41) or the open surgical group (RR 0.61, 95% CI 0.36–1.04).
Bowel perforation rate
The bowel perforation rate was reported in 18 studies [24, 27,28,29,30,31, 33, 34, 36, 40, 43, 44, 47, 49,50,51, 53, 56]. No statistically significant difference was found between the percutaneous group (0.4%; n = 5/1323) and the surgical group (0.1%; n = 1/1534) (RR 1.45, 95% CI 0.62–3.38; p = 0.40). Statistical heterogeneity was low (I2 = 0.0%) (Online Resource 3F). No statistically significant difference was observed in the subgroup analysis between the percutaneous and the laparoscopic group (RR 1.55, 95% CI 0.34–7.16) or the open surgical group (RR 1.47, 95% CI 0.51–4.25).
Catheter malfunction rate
The catheter malfunction rate was reported in 11 studies [24, 27, 29, 32, 40, 48,49,50,51, 53, 56]. No statistically significant difference was found between the percutaneous group (12.5%; n = 107/854) and the surgical group (17.2%; n = 180/1047) (RR 0.86, 95% CI 0.67–1.09; p = 0.20). Statistical heterogeneity was low (I2 = 0.0%) (Online Resource 3G). No statistically significant difference was observed in the subgroup analysis between the percutaneous and the laparoscopic group (RR 0.91, 95% CI 0.67–1.23) or the open surgical group (RR 0.81, 95% CI 0.50–1.33).
Catheter-related surgery required rate
The catheter-related surgery required rate was reported in 5 studies [30, 36, 42, 49, 56]. No statistically significant difference was found between the percutaneous group (13.1%; n = 40/395) and the surgical group (9.9%; n = 46/463) (RR 1.03, 95% CI 0.68–1.55; p = 0.90). Statistical heterogeneity was low (I2 = 0.0%) (Online Resource 3H). No statistically significant difference was observed in the subgroup analysis between the percutaneous and the laparoscopic group (RR 1.08, 95% CI 0.55–2.12) or the open surgical group (RR 0.98, 95% CI 0.54–1.80).
Catheter removal rate
The catheter removal rate was reported in 12 studies [27, 29, 32, 33, 38,39,40, 43, 44, 46, 47, 56], and was lower in the percutaneous group (21.8%; n = 309/1478) compared to the surgical group (25.8%; n = 309/1418) (RR 0.73, 95% CI 0.60–0.88; p = < 0.001). Statistical heterogeneity was low (I2 = 23.6%) (Online Resource 3I). In the subgroup analysis, the catheter removal rate was lower in the percutaneous group, when compared against the laparoscopic group (RR 0.66, 95% CI 0.45–0.99) and the open surgical group (RR 0.71, 95% CI 0.53–0.94).
Catheter replacement rate
The catheter replacement rate was reported in 6 studies [28, 29, 32, 40, 42, 43]. No statistically significant difference was found between the percutaneous group (8.6%; n = 47/546) and the surgical group (13.1%; n = 74/566) (RR 0.78, 95% CI 0.55–1.11; p = 0.17). Statistical heterogeneity was low (I2 = 0.0%) (Online Resource 3J).
Post-procedural mortality rate
The post-procedural mortality rate was reported in 6 studies [30, 32, 47, 48, 51, 57]. No statistically significant difference was found between the percutaneous group (0.0%; n = 0/597) and the surgical group (1.4%; n = 6/440) (RR 0.42, 95% CI 0.10–1.87; p = 0.26). Statistical heterogeneity was low (I2 = 0.0%) (Online Resource 3K). No statistically significant difference was observed in the subgroup analysis between the percutaneous and the laparoscopic group (RR 0.94, 95% CI 0.06–14.51 or the open surgical group (RR 0.98, 95% CI 0.01–2.00).
Overall mortality rate
The overall mortality rate was reported in 12 studies [29, 30, 33, 40, 42, 43, 46, 48, 51, 52, 56]. No statistically significant difference was found between the percutaneous group (12.7%; n = 140/1105) and the surgical group (13.3%; n = 171/1287) (RR 1.03, 95% CI 0.73–1.45; p = 0.86). Statistical heterogeneity was high (I2 = 51.2%) (Online Resource 3L). No statistically significant difference was observed in the subgroup analysis between the percutaneous and the laparoscopic group (RR 2.17, 95% CI 0.20–23.71), or the open surgical group (RR 0.92, 95% CI 0.66–1.29).
Early outcomes
An additional analysis was performed on the rates of peritonitis, tunnel and exit-site infections, and leakage, specifically occurring at 2 weeks and 4 weeks following catheter placement. The forest plots of these meta-analyses are presented in Online Resource 3M-R and their results are summarized in Table 3.
Subgroup analysis
An additional subgroup analysis was performed for patients with no previous abdominal operation history. No statistically significant difference was detected regarding the rates of peritonitis, tunnel and exit-site infection, leakage, inflow/outflow obstruction, migration, post-procedural hemorrhage, hernia, bowel perforation, and catheter removal (Online Resource 4).
Discussion
The results of this systematic review and meta-analysis show that percutaneous PDC placement is an overall safe technique and, compared to surgical placement, may lead to lower rates of tunnel/exit-site infection, catheter migration, and catheter removal. With the exception of peritonitis rate in Boujelbane et al., previous meta-analyses have failed to detect any significant difference in the outcomes of the two methods [9,10,11]. In Tullavardhana et al. and Boujelbane et al., the lack of statistically significant results can be attributed to several methodological flaws; these include limiting their search strategy to one database, omitting important outcomes such as catheter migration, mixing count data and dichotomous data in the meta-analyses of binary outcomes, adding the rates of individual non-mutually exclusive events to obtain cumulative rates, and extracting survival data from Kaplan–Meier curves, without taking into account censored data for death or other causes [18]. Our systematic search yielded 24 additional studies and 18 additional studies compared to Tullavardhana et al. and Boujelbane et al., respectively. The inclusion criteria of our study were almost identical with those of Tullavardhana et al. and narrower compared to those of Boujelbane et al., as the latter also included pediatric patients in their sample. The anatomic differences of children may predispose them to certain complications, such as catheter migration, and thus we decided to exclude them from our analysis [58]. The large difference in the number of included studies cannot be solely explained by our more recent search date and is rather the result of a more rigorous search strategy. Indeed, just 11 of the 34 included studies were published after the last search date of Tullavardhana et al. and Boujelbane et al. [24, 25, 35, 37, 40, 43, 46, 49,50,51, 56]. On the other hand, Htay et al. only included RCTs [10]. Despite the higher quality of evidence provided, this strategy may compromise the statistical power of the meta-analysis, when available RCTs on the subject are limited.
Infectious complications are common in the setting of peritoneal dialysis. According to our pooled data, the overall rate of infectious complications was 23.6% in the percutaneous group and 28.5% in surgical group. However, only a limited number of studies reported data on this specific outcome. Most included studies reported data on the incidence of peritonitis and tunnel and exit-site infections individually. Infectious complications were also a major [27, 33, 40] or even the most common [28, 29, 31, 38, 39, 41] cause of catheter removal in many of the included studies. Percutaneous PDC placement resulted in significantly lower tunnel/exit-site infection rates. The lower incidence of this complication may account for the lower complication-related catheter removal rate seen in the percutaneous group. Surgical placement may predispose patients to infectious complications due to its highly invasive nature, providing a larger port of entry for microorganisms [32]. Specifically, early tunnel and exit-site infections, occurring at 2 weeks and 4 weeks following catheter placement, were also lower in the percutaneous group. The close temporal association of these early infections to the PDC placement implicates that the advantage of the percutaneous group may stem from the technique itself rather than other risk factors unrelated to catheter placement. On the other hand, both early and overall peritonitis rates were similar between the two groups. Early utilization of the PDC early after placement is a known advantage of the percutaneous technique over its surgical counterpart, but it also is a risk factor for early peritonitis and thus may initially offset any potential advantage of percutaneous placement [24, 59].
Mechanical complications also affect a significant proportion of patients undergoing PDC placement. Overall, 16.8% of patients in the surgical group and 16.7% in the percutaneous group developed a mechanical complication. Just as with infectious complications, mechanical complications were also a major cause of catheter removal in many of the included studies [27,28,29, 31, 39]. Multiple concerns have been raised over the years regarding the occurrence of mechanical complications with percutaneous PDC placement. Leakage, particularly occurring during the first weeks following the placement, may occur more frequently because of earlier catheter utilization compared to surgically inserted catheters [28]. The lack of direct visualization may predispose patients to bowel perforation, which can be a life-threatening complication. However, the risk varies substantially with operator experience and history of previous abdominal operations [24, 60]. Higher post-procedural hemorrhage rate may be more common with percutaneous placement, as bleeding control can be achieved intraoperatively with surgical placement [47]. On the other hand, hernias may occur more frequently with surgical placements due to the larger incision sites [28]. Surgical placement is also thought to promote mechanical complications through increased fibrous tissue formation [32]. Despite their theoretical basis, none of these associations were corroborated by our meta-analyses. Instead, percutaneous and surgical PDC placement were shown to be overall equally safe in terms of mechanical complications.
Migration is a complication that often leads to malfunction, eventually requiring revision or removal of the catheter [29, 56]. Depending on the severity, restoring function can be achieved via non-surgical or surgical manipulation [61]. In our study, migration was less common with percutaneous placement. However, the rates of catheter malfunction and catheter-related surgery were not significantly different between the two groups, as would be expected. Many of the studies reporting on catheter migration did not report on either of these two additional outcomes, which may explain this inconsistency. Catheter migration may also be influenced by the catheter type [61] or certain interventions during placement, such as the addition of a subcutaneous sling to prevent catheter movement [40]. Sivaramakrishnan et al. hypothesized that the latter may have significantly contributed to the lower incidence of catheter migration in the percutaneous group, rather than the technique itself [40].
Both techniques proved to be very safe, as post-procedural mortality was minimal and none of the deaths was directly related to the procedure [30]. Overall mortality varied significantly across studies, presumably due to the differences in the follow-up duration. Most studies did not provide a detailed breakdown of the causes leading to death. Perakis et al. reported that more than 60% of all patient deaths were caused by underlying cardiovascular disease, but also more than 20% were attributed to infectious complications [31]. Medani et al. concluded that all deaths were unrelated to peritoneal dialysis itself, with the exception of a fungal peritonitis case [42]. Due to the heterogeneity in patient characteristics across studies, including underlying comorbidities, it is hard to extrapolate a definitive conclusion about the long-term patient outcomes. As previously said, percutaneous catheter placement was associated with lower risk for infectious complications, such as tunnel and exit-site infections. Taking into consideration that a small but significant minority of patient deaths are attributed to infectious complications, percutaneous catheter placement might therefore indirectly decrease overall mortality, when all other risk factors for death (e.g., underlying comorbidities) are accounted for. However, further studies are needed, ideally in the form of randomized controlled trials, to confirm this statement.
In terms of catheter-specific long-term outcomes, both techniques displayed similar results. Catheter survival did not significantly differ between the methods for both 3 months and 1 year following placement. Most studies reported data on catheter survival in the form of Kaplan–Meier curves, censoring patients for catheter removal due to death, transplantation, or transfer to hemodialysis, thus preventing us from extracting accurate dichotomous data. The results from individual studies in this case were inconclusive; some studies favored percutaneous placement [29, 37, 40], others favored surgical placement [28, 35], while many reported comparable catheter survival rates between the two methods [24, 26, 30, 31, 33, 42, 50]. These findings suggest that neither of the two methods seems to have a clear advantage over the other in terms of catheter survival. Nonetheless, more data are needed before definitive conclusions can be made.
When examining the laparoscopic and open surgical procedures separately, percutaneous placement resulted in lower rates of tunnel/exit-site infection and catheter migration compared to the open surgical but not the laparoscopic group. Two systematic reviews in the past have shown an advantage of laparoscopy, especially in regard to mechanical complications [19, 20], while another study showed a long-term cost benefit over the open approach [62]. Therefore, laparoscopy might be the preferred option as the standard surgical approach, provided an operator experienced with this technique is available. In the second subgroup analysis, patients with a history of previous abdominal operations were excluded from both groups. Despite the lower absolute complication rates of the percutaneous group for most outcomes, none of these differences were statistically significant. However, no certain conclusions can be made, as the statistical power of the subgroup analyses was compromised by the smaller samples, compared to the cumulative analyses.
The results of this systematic review and meta-analysis should be interpreted with caution due to its inherent limitations. We only included articles in the English language which may have introduced some language bias in our search results. Most of the included articles (94.1%; n = 32/34) were observational non-randomized studies, which are characterized by a lower quality of evidence compared to RCTs. Selection bias is common among studies reporting on percutaneous PDC placement [21]. Some studies entirely excluded patients with a history of an abdominal operation [29, 32, 34, 36, 38, 46], while others allocated patients to the surgical group if technical difficulties were expected or contraindications to percutaneous placement were present [27, 30, 31, 35, 41, 49, 54]. The latter scenario included severe obesity and previous abdominal operations. To address this issue, we decided to perform subgroup analyses that were not included in the original protocol, which could also introduce a certain degree of bias. The definition of many complications varied widely across studies, which can directly affect the reported incidence of certain complications, as in the case of leakage in Voss et al. [38]. There was also significant heterogeneity in the prophylactic antibiotic protocols used. Many studies did not report on the operator’s experience with either placement method or were less experienced with the percutaneous method [24, 54]. This is important, as the operator’s experience can directly affect the outcomes of the procedure [64]. A considerable amount of studies did not have adequate follow-up length and rates, which might have resulted in under-reporting of complication rates. The statistical power of funnel plots and Egger’s test to detect publication bias is limited when less than 10 studies report data on an outcome of interest, as was the case in four primary (3-month catheter survival, 1-year catheter survival, overall infectious complications, and overall mechanical complications) and three secondary outcomes (catheter-related surgery required, catheter replacement, and post-procedural mortality) in our main analysis. Therefore, they should be interpreted with caution [18]. Finally, the amount of data for many outcomes was limited. This was caused either due to selective reporting or lack of data extractability, such as death/transplant-censored Kaplan Meier curves for survival data. As a result, the statistical power for some outcomes was low.
In conclusion, percutaneous PDC placement is an overall safe procedure with comparable outcomes to surgical placement, and may potentially lead to fewer infectious complications, such as peritonitis and tunnel/exit-site infections, catheter migrations, and removals. Therefore, it might be safe to say that the percutaneous method can be initially attempted for all low-risk patients, such as those with normal BMI and no history of previous abdominal operation. Taking into account other advantages of the percutaneous technique, such as the lower cost and faster PD initiation, it may become the preferred approach for low-risk patients [24, 63]. In contrast, the surgical method can be reserved for cases of failed percutaneous placement or high-risk patients, as it is more invasive and delays PDC utilization without leading to better outcomes. Choosing between the open surgical and the laparoscopic approach in this scenario will mainly depend on the surgeon’s experience and availability. Nonetheless, the quality of the published literature on the topic remains poor. Well-designed RCTs comparing the outcomes between the percutaneous and surgical technique are still needed to inform decisions on optimal PDC placement.
Availability of data and material
The data are available upon request.
References
Mehrotra R, Devuyst O, Davies SJ, Johnson DW (2016) The current state of peritoneal dialysis. J Am Soc Nephrol 27:3238–3252
Wong B, Ravani P, Oliver MJ et al (2018) Comparison of patient survival between hemodialysis and peritoneal dialysis among patients eligible for both modalities. Am J Kidney Dis 71:344–351. https://doi.org/10.1053/j.ajkd.2017.08.028
Crabtree JH, Chow KM (2017) Peritoneal dialysis catheter insertion. Semin Nephrol 37:17–29. https://doi.org/10.1016/j.semnephrol.2016.10.004
Peppelenbosch A, Van Kuijk WHM, Bouvy ND et al (2008) Peritoneal dialysis catheter placement technique and complications. NDT Plus. https://doi.org/10.1093/ndtplus/sfn120
Shahbazi N, McCormick BB (2011) Peritoneal dialysis catheter insertion strategies and maintenance of catheter function. Semin Nephrol 31:138–151. https://doi.org/10.1016/j.semnephrol.2011.01.003
Woodrow G, Turney JH, Brownjohn AM (1997) Technique failure in peritoneal dialysis and its impact on patient survival. Perit Dial Int 17:360–364. https://doi.org/10.1177/089686089701700411
Piraino B, Bernardini J, Sorkin M (1986) The influence of peritoneal catheter exit-site infections on peritonitis, tunnel infections, and catheter loss in patients on continuous ambulatory peritoneal dialysis. Am J Kidney Dis 8:436–440. https://doi.org/10.1016/S0272-6386(86)80171-8
Singh N, Davidson I, Minhajuddin A et al (2010) Risk factors associated with peritoneal dialysis catheter survival: a 9-year single-center study in 315 patients. J Vasc Access 11:316–322. https://doi.org/10.5301/JVA.2010.5774
Tullavardhana T, Akranurakkul P, Ungkitphaiboon W, Songtish D (2016) Surgical versus percutaneous techniques for peritoneal dialysis catheter placement: a meta-analysis of the outcomes. Ann Med Surg 10:11–18. https://doi.org/10.1016/j.amsu.2016.07.007
Htay H, Johnson DW (2019) Catheter type, placement, and insertion techniques for preventing catheter-related infections in maintenance peritoneal dialysis patients: summary of a cochrane review. Am J Kidney Dis 74:703–705. https://doi.org/10.1053/j.ajkd.2019.07.005
Boujelbane L, Fu N, Chapla K et al (2015) Percutaneous versus surgical insertion of PD catheters in dialysis patients: a meta-analysis. J Vasc Access 16:498–505. https://doi.org/10.5301/jva.5000439
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700
Wohlin C (2014) Guidelines for snowballing in systematic literature studies and a replication in software engineering. In: Proceedings of the 18th international conference on evaluation and assessment in software engineering. ACM, New York, NY, USA, pp 38:1–38:10
Gokal R, Alexander S, Ash S et al (1998) Peritoneal catheters and exit-site practices toward optimum peritoneal access: 1998 update. Perit Dial Int 18:11–33. https://doi.org/10.1177/089686089801800102
Stang A (2010) Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605. https://doi.org/10.1007/s10654-010-9491-z
Higgins JPT, Altman DG, Gøtzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:1–9. https://doi.org/10.1136/bmj.d5928
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13. https://doi.org/10.1186/1471-2288-5-13
Higgins J, Green S (eds) (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration
Hagen SM, Lafranca JA, Steyerberg EW et al (2013) Laparoscopic versus open peritoneal dialysis catheter insertion: a meta-analysis. PLoS ONE. https://doi.org/10.1371/journal.pone.0056351
Shrestha BM, Shrestha D, Kumar A et al (2018) Advanced laparoscopic peritoneal dialysis catheter insertion: systematic review and meta-analysis. Perit Dial Int 38:163–171
Crabtree JH (2008) Fluoroscopic placement of peritoneal dialysis catheters: a harvest of the low-hanging fruits. Perit Dial Int 28:134–137. https://doi.org/10.1177/089686080802800207
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188. https://doi.org/10.1016/0197-2456(86)90046-2
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Abdel Aal AK, Guest SS, Moawad S et al (2018) Outcomes of fluoroscopic and ultrasound-guided placement versus laparoscopic placement of peritoneal dialysis catheters. Clin Kidney J 11:549–554. https://doi.org/10.1093/ckj/sfx132
Alkatheeri AMA, Blake PG, Gray D, Jain AK (2016) Success of urgent-start peritoneal dialysis in a large Canadian renal program. Perit Dial Int 36:171–176. https://doi.org/10.3747/pdi.2014.00148
Khositrangsikun K, Chujohn W, Kanokkantapong C, Kanjanabuch T (2011) Comparison of the Seldinger technique and surgical technique in Tenckhoff catheter insertion in CAPD patients: a single center experience. J Med Assoc Thai 94(Suppl 4):77–80
Maher E, Wolley MJ, Abbas SA et al (2014) Fluoroscopic versus laparoscopic implantation of peritoneal dialysis catheters: a retrospective cohort study. J Vasc Interv Radiol 25:895–903. https://doi.org/10.1016/j.jvir.2014.01.023
Mellotte GJ, Ho CA, Morgan SH et al (1993) Peritoneal dialysis catheters: a comparison between percutaneous and conventional surgical placement techniques. Nephrol Dial Transplant 8:626–630. https://doi.org/10.1093/oxfordjournals.ndt.a092552
Özener Ç, Bihorac A, Akoglu E (2001) Technical survival of CAPD catheters: comparison between percutaneous and conventional surgical placement techniques. Nephrol Dial Transplant 16:1893–1899. https://doi.org/10.1093/ndt/16.9.1893
Park YS, Il MS, Kim DK et al (2014) The outcomes of percutaneous versus open placement of peritoneal dialysis catheters. World J Surg 38:1058–1064. https://doi.org/10.1007/s00268-013-2346-5
Perakis KE, Stylianou KG, Kyriazis JP et al (2009) Long-term complication rates and survival of peritoneal dialysis catheters: the role of percutaneous versus surgical placement. Semin Dial 22:569–575. https://doi.org/10.1111/j.1525-139X.2009.00621.x
Rosenthal MA, Yang PS, Liu ILA et al (2008) Comparison of outcomes of peritoneal dialysis catheters placed by the fluoroscopically guided percutaneous method versus directly visualized surgical method. J Vasc Interv Radiol 19:1202–1207. https://doi.org/10.1016/j.jvir.2008.05.006
Roueff S, Pagniez D, Moranne O et al (2002) Simplified percutaneous placement of peritoneal dialysis catheters: comparison with surgical placement. Perit Dial Int J Int Soc Perit Dial 22:267–269. https://doi.org/10.1177/089686080202200216
Sampathkumar K, Mahaldar A, Sooraj Y et al (2008) Percutaneous CAPD catheter insertion by a nephrologist versus surgical placement: a comparative study. Indian J Nephrol 18:5. https://doi.org/10.4103/0971-4065.41280
Stonelake S, Baharani J, Thomas M et al (2019) Outcomes of the weighted peritoneal dialysis catheter in patients at risk of percutaneous catheter failure. Perit Dial Int 39:142–146. https://doi.org/10.3747/pdi.2017.00233
Atapour A, Asadabadi HR, Karimi S et al (2011) Comparing the outcomes of open surgical procedure and percutaneously peritoneal dialysis catheter (PDC) insertion using laparoscopic needle: a two month follow-up study. J Res Med Sci 16:463–468
Sun TYT, Voss D, Beechey D, Lam-Po-Tang M (2016) Comparison of peritoneal dialysis catheter insertion techniques: peritoneoscopic, radiological and laparoscopic: a single-centre study. Nephrology 21:416–422. https://doi.org/10.1111/nep.12621
Voss D, Hawkins S, Poole G, Marshall M (2012) Radiological versus surgical implantation of first catheter for peritoneal dialysis: a randomized non-inferiority trial. Nephrol Dial Transplant 27:4196–4204. https://doi.org/10.1093/ndt/gfs305
Nicholas J, Thomas M, Adkins R et al (2014) Percutaneous and surgical peritoneal dialysis catheter placements have comparable outcomes in the modern era. Perit Dial Int 34:552–556. https://doi.org/10.3747/pdi.2013.00125
Sivaramakrishnan R, Gupta S, Agarwal SK et al (2016) Comparison of outcomes between surgically placed and percutaneously placed peritoneal dialysis catheters: a retrospective study. Indian J Nephrol 26:268–274. https://doi.org/10.4103/0971-4065.163425
Liberek T, Chmielewski M, Lichodziejewska-Niemierko M et al (2003) Survival and function of Tenckhoff peritoneal dialysis catheter after surgical or percutaneous placement: one centre experience. Int J Artif Organs 26:174–175. https://doi.org/10.1177/039139880302600213
Medani S, Shantier M, Hussein W et al (2012) A comparative analysis of percutaneous and open surgical techniques for peritoneal catheter placement. Perit Dial Int 32:628–635. https://doi.org/10.3747/pdi.2011.00187
Kim JH, Kim MJ, Ye B-M et al (2020) Percutaneous peritoneal dialysis catheter implantation with no break-in period: a viable option for patients requiring unplanned urgent-start peritoneal dialysis. Kidney Res Clin Pract. https://doi.org/10.23876/j.krcp.20.006
Rana TA, Cramp H, Akoh JA (2011) Evaluation of medical insertion of peritoneal dialysis catheters. Nephrourol Mon 3:46–53
Picó-Vicent L, Cases JM, Picó-Mira L, Contreras JP (2000) Post-implantation events of swan-neck catheters for PD. EDTNA-ERCA J 26:17–18. https://doi.org/10.1111/j.1755-6686.2000.tb00112.x
Kang SH, Park JW, Cho KH, Do JY (2020) Comparison of peritoneal dialysis catheter insertion techniques by nephrologists: surgical vs blind methods. Semin Dial. https://doi.org/10.1111/sdi.12904
Borazan A, Comert M, Ucan BH et al (2006) The comparison in terms of early complications of a new technique and percutaneous method for the placement of CAPD catheters. Ren Fail 28:37–42. https://doi.org/10.1080/08860220500461237
Dinç B, Dinçkan A, Çiyiltepe H et al (2012) Comparison of laparoscopic and conventional methods for continuous ambulatory peritoneal dialysis catheter insertion in terms of complications. Turk Nephrol Dial Transplant J 21:156–160. https://doi.org/10.5262/tndt.2012.1002.09
Demiriz B, Bulut M, Gök Oʇuz E et al (2014) A comparison of laparoscopic and conventional methods in peritoneal dialysis catheter placement in patients with end-stage renal disease: single center experience. Turk Nephrol Dial Transplant J 23:181–184. https://doi.org/10.5262/tndt.2014.1003.02
Xie D, Zhou J, Cao X et al (2020) Percutaneous insertion of peritoneal dialysis catheter is a safe and effective technique irrespective of BMI. BMC Nephrol 21:1–10. https://doi.org/10.1186/s12882-020-01850-5
Zhang D, Peng Y, Zheng T et al (2020) An analysis of the “half-Perc” versus open surgical placement method for a peritoneal dialysis catheter: a non-inferiority cohort study. BMC Nephrol 21:1–9. https://doi.org/10.1186/s12882-020-01936-0
Brunier G, Hiller JA, Drayton S et al (2010) A change to radiological peritoneal dialysis catheter insertion: three-month outcomes. Perit Dial Int 30:528–533. https://doi.org/10.3747/pdi.2009.00114
Chula DC, Campos RP, de Alcântara MT et al (2014) Percutaneous and surgical insertion of peritoneal catheter in patients starting in chronic dialysis therapy: a comparative study. Semin Dial. https://doi.org/10.1111/sdi.12147
Dequidt C, Vijt D, Veys N, Van Biesen W (2003) Bed-side blind insertion of peritoneal dialysis catheters. EDTNA-ERCA J 29:137–139. https://doi.org/10.1111/j.1755-6686.2003.tb00294.x
Gajjar AH, Rhoden DH, Kathuria P et al (2007) Peritoneal dialysis catheters: laparoscopic versus traditional placement techniques and outcomes. Am J Surg 194:872–876. https://doi.org/10.1016/j.amjsurg.2007.08.038
Glavinovic T, Kashani M, Al-Sahlawi M et al (2019) A peritoneal dialysis access quality improvement initiative: a single-center experience. Perit Dial Int 39:437–446. https://doi.org/10.3747/pdi.2018.00233
Henderson S, Brown E, Levy J (2009) Safety and efficacy of percutaneous insertion of peritoneal dialysis catheters under sedation and local anaesthetic. Nephrol Dial Transplant 24:3499–3504. https://doi.org/10.1093/ndt/gfp312
LaPlant MB, Saltzman DA, Segura BJ et al (2018) Peritoneal dialysis catheter placement, outcomes and complications. Pediatr Surg Int 34:1239–1244. https://doi.org/10.1007/s00383-018-4342-1
Keswani M, Redpath Mahon AC, Richardson T et al (2019) Risk factors for early onset peritonitis: the SCOPE collaborative. Pediatr Nephrol 34:1387–1394. https://doi.org/10.1007/s00467-019-04248-0
Jacobs IG, Gray RR, Elliott DS, Grosman H (1992) Radiologic placement of peritoneal dialysis catheters: preliminary experience. Radiology 182:251–255. https://doi.org/10.1148/radiology.182.1.1727292
Al-Hwiesh AK (2016) A modified peritoneal dialysis catheter with a new technique: farewell to catheter migration. Saudi J Kidney Dis Transpl 27:281–289. https://doi.org/10.4103/1319-2442.178261
Davis WT, Dageforde LA, Moore DE (2014) Laparoscopic versus open peritoneal dialysis catheter insertion cost analysis. J Surg Res 187:182–188. https://doi.org/10.1016/j.jss.2013.09.041
Medani S, Hussein W, Shantier M et al (2015) Comparison of percutaneous and open surgical techniques for first-time peritoneal dialysis catheter placement in the unbreached peritoneum. Perit Dial Int 35:576–585. https://doi.org/10.3747/pdi.2013.00003
Dombros N, Dratwa M, Feriani M et al (2005) European best practice guidelines for peritoneal dialysis. 3 peritoneal access. Nephrol Dial Transplant 20:ix8–ix12. https://doi.org/10.1093/ndt/gfi1117
Acknowledgements
We want to thank Dimitrios Kosmidis for his assistance in designing the tables and supplementary material of this manuscript.
Funding
The authors declare no funding source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Since this study only used published data, no Institutional Review Board approval was necessary.
Consent to participate
Since this study only used published data, no patient written consent was necessary.
Consent for publication
Since this study only used published data, no consent for publication was necessary.
Code availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Esagian, S.M., Sideris, G.A., Bishawi, M. et al. Surgical versus percutaneous catheter placement for peritoneal dialysis: an updated systematic review and meta-analysis. J Nephrol 34, 1681–1696 (2021). https://doi.org/10.1007/s40620-020-00896-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-020-00896-w