Abstract
Distal renal tubular acidosis (dRTA) is a tubular disorder with a primary defect of urinary acidification and acid excretion in the collecting duct system. Consequently, patients develop hyperchloremic metabolic acidosis with an inappropriately alkaline urine. Inherited forms of dRTA are due to mutations in at least three distinct genes: SLC4A1, ATP6V1B1, ATP6V0A4. Mutations in SLC4A1-(AE1) are inherited either in an autosomal dominant manner or in a recessive one. ATP6V1B and ATP6V0A4 mutations affect two different subunits of the vacuolar H+-ATPase proton-pump, the B1 and a4 subunits, and are inherited in an autosomal recessive manner. Clinical manifestations of inherited forms of dRTA usually occur during infancy or childhood. However, heterozygous carriers of ATP6V1B1 and ATP6V0A4 mutations may have a higher risk of developing nephrolithiasis and nephrocalcinosis in adulthood, respectively. In full forms of dRTA, patients may present with mild clinical symptoms, such as mild metabolic acidosis and incidental detection of kidney stones, as well as with more severe manifestations such as failure to thrive, severe metabolic acidosis, and nephrocalcinosis. Progressive sensorineural hearing loss develops in the majority of patients with recessive dRTA (ATP6V1B1 and ATP6V0A4 mutations). Some patients with recessive dRTA may also develop abnormal widening of the vestibular aqueduct. This review will discuss our current understanding of the pathophysiology of inherited forms of dRTA, diagnosis and prognosis of patients, and therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Renal acid excretion
Next to the ventilation of CO2 by the lungs, the kidneys play a central role in the long-term control of acid–base homeostasis. The daily excretion of acid and the regeneration of approximately 1 mmol bicarbonate per kg bodyweight (e.g. 70 mmol in an average person of 70 kg body weight per day) are critical tasks. The importance of these processes becomes most evident in syndromes or diseases affecting overall kidney function or more specifically in forms of acquired or inherited renal tubular acidosis.
Maintenance and control of systemic acid–base balance by the kidney is achieved through three major processes: (1) the reabsorption of filtered bicarbonate, (2) the excretion of acid mostly in the form of ammonium and titratable acidity, and (3) the de novo synthesis of bicarbonate to replenish bicarbonate lost in metabolism [1].
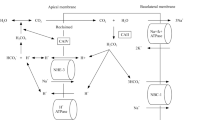
The kidneys filter daily about 180 l of primary urine containing a total of approximately 4500 mEq bicarbonate which in a healthy person is entirely reabsorbed along the nephron. About 80% of the filtered bicarbonate is reclaimed in the proximal tubule via secretion of protons by NHE sodium-proton exchangers (mostly the NHE3/SLC9A3 isoform) and proton pumps (H+-ATPases) and, as suggested recently, the sodium-dependent bicarbonate cotransporter (NBCn2) [2]. Because of the luminal activity of carbonic anhydrases [carbonic anhydrase type IV, (CAIV)] the formation of CO2 and H2O from HCO3 − and H+ is facilitated. CO2 and H2O then diffuse into proximal tubule cells where the process is reversed by the cytosolic carbonic anhydrase type II (CAII). The resulting HCO3 − is released into blood by the basolateral sodium-bicarbonate cotransporter (NBCe1/SLC4A4) whereas protons are recycled into urine across the luminal membrane (Fig. 1). A fraction of bicarbonate is also reabsorbed through the paracellular pathway in the proximal tubule driven by the luminal accumulation of chloride and the lumen-negative potential.
Scheme of mechanisms underlying bicarbonate reabsorption in the proximal tubule. NBCe1 electrogenic sodium-bicarbonate-cotransporter 1, NBCn2 electroneutral sodium-bicarbonate-cotransporter 2, NHE3 sodium-proton-exchanger 3, V-ATPase vacuolar-type H+-ATPase, CAII carbonic anhydrase type II, CAIV carbonic anhydrase type IV
The remaining bicarbonate (approx. 20% of the filtered load) is then reabsorbed along the thick ascending limb of the loop of Henle by transcellular mechanisms similar to those in the proximal tubule.
Metabolism consumes bicarbonate (i.e. in the urea cycle) and produces acids that require buffering by bicarbonate. The kidney replenishes bicarbonate by de novo generation of bicarbonate from ammoniagenesis in the proximal tubule and by hydration of CO2 in acid-secretory type A intercalated cells. In the proximal tubule, glutamine is taken up mostly from blood and fueled into ammoniagenesis and gluconeogenesis releasing ammonia and bicarbonate ions. Ammoniagenesis is stimulated during acidosis (by enhanced glutamine uptake and higher enzymatic fluxes) and contributes to the renal adaptation. Renal ammonium excretion is a process involving several steps. First ammonium is secreted into urine in the proximal tubule (a fraction is also released back into circulation), mostly reabsorbed by the Na/K/2Cl-cotransporter NKCC2 in the thick ascending limb of the loop of Henle, accumulated in the interstitium and finally secreted by the cells lining the collecting duct system into urine in the form of ammonia (see below).
Final urinary acidification and fine-tuning of renal acid-excretion occurs in the collecting system consisting of the connecting tubule, and the cortical and medullary parts of the collecting duct [3]. The first intercalated cells appear already in the late distal convoluted tubule.
Acid-secretory type A intercalated cells not only mediate ammonia excretion into urine but are also responsible for urinary acidification coupled to de novo synthesis of bicarbonate (Fig. 2). CO2 is hydrated with the help of the cytosolic CAII forming protons and bicarbonate. Bicarbonate is released into blood via the basolateral chloride-bicarbonate exchanger AE1 (anion exchanger 1, SLC4A1) whereas protons are pumped into urine by vacuolar-type H+-ATPases located in the luminal membrane [3, 4]. As discussed below, rare genetic mutations in SLC4A1 or two different subunits of the multimeric H+-ATPase (consisting of more than 14 subunits with multiple isoforms) cause inherited forms of distal renal tubular acidosis (dRTA) [5,6,7]; secretion of protons into urine acidifies urine to a maximal pH of around 4.5–4.0. Further acidification of urine is impossible as proton pumps must work against a steep proton gradient (intracellular pH 7.2, luminal pH 4.5). However, 1 l of urine of pH 4.5 contains only 30 µmol of protons, a minute amount compared to the requirement to excrete 70 mmol of acid. Urinary buffers, so-called titratable acidity (the term refers to the method to measure titratable acidity by back-titrating acidified urine), help to buffer protons and thereby to increase the amount of excreted acid. The main “titratable acid” is phosphate, but creatinine and urate also contribute to variable extents. Proton secretion is also tightly coupled to ammonia secretion where luminal ammonia (NH3) captures free protons and is trapped in urine in the form of ammonium (NH4 +). Ammonia secretion by intercalated cells (and also by neighboring principal cells) is mediated by two related gas channels belonging to the family of the rhesus blood group proteins, namely RhBG and RhCG (Fig. 2) [8,9,10].
Type-A acid-secretory intercalated cells in the collecting system and structure of the V-type H+-ATPase (insert). The red/orange parts of the pump belong to the V1-part, the blue subunits to the V0-sector; the B- and a-subunits, mutated in inherited dRTA, are indicated and occur in different isoforms. The B1 or a4 isoforms, respectively, can be mutated in patients with dRTA and nephrocalcinosis. AE1 anion exchanger 1, RhCG rhesus blood group family type C glycoprotein, RhBG rhesus blood group family type B glycoprotein, CAII carbonic anhydrase type II, V-ATPase vacuolar-type H+-ATPase. (Color figure online)
The sum of urinary ammonium plus titratable acidity minus bicarbonate is called net acid secretion. For the sake of simplicity, urinary phosphate can be taken as an approximation for titratable acidity, and urinary bicarbonate can be neglected when urine pH is below pH 6.5 [8].
The activity of type A intercalated cells and hence net acid secretion is enhanced during acidosis and decreased during alkalosis.
Next to type A intercalated cells, a second type of intercalated cell, type B intercalated cells, is expressed in the distal convoluted tubule, connecting the tubule and cortical collecting duct. These cells harbor the chloride/bicarbonate exchanger pendrin (SLC26A4) on their luminal membrane and play an important role in the secretion of bicarbonate during alkalosis and the reabsorption of chloride [11, 12]. The latter may be important for the control of NaCl homeostasis and blood pressure control [13,14,15,16,17,18].
Case report
A 35-year-old male patient with recurrent urolithiasis and nephrocalcinosis was referred to our stone clinic for metabolic evaluation. He had been diagnosed with dRTA when he presented at the age of 6 with severe metabolic acidosis (pH 6.98, bicarbonate 3.3 mmol/l) and alkaline urine pH of 7.0. Ultrasound of the kidneys demonstrated bilateral medullary nephrocalcinosis. After careful analysis of his pedigree, an autosomal recessive inheritance was suspected. Consequently, alkali treatment with potassium citrate and sodium bicarbonate was initiated. However, due to non-adherence he suffered from repeated episodes of nephrolithiasis during adolescence and young adulthood. Stone analysis revealed 100% calcium phosphate. In addition, as a consequence of the repeated pain therapy with opioids he became opioid dependent. At presentation in our stone clinic he had developed chronic kidney disease (CKD) Kidney Disease Improving Global Outcomes (KDIGO) stage G 3a–b and sensorineural hearing loss. Ultrasound of his kidneys demonstrated bilateral nephrocalcinosis (Fig. 3). Adherence to therapy was still problematic since hypokalemia (serum potassium 3.1 mmol/l) and metabolic acidosis (bicarbonate 15 mmol/l) were still present. Bone densitometry indicated osteopenia with normal levels of calcium, parathyroid hormone, 25-OH- and 1,25-(OH)2-vitamin D3. Serum phosphate was low (0.67 mmol/l). Genetic analysis in the Department of Genetics at the European Georges Pompidou Hospital in Paris revealed a homozygous mutation (p.Gln753*) in the ATP6V0A4 gene encoding for the a4 subunit of the vacuolar H+-ATPase. Despite ongoing repetitive kidney stone episodes, his kidney function had remained stable over the last 3 years.
Mechanisms of inherited forms of distal renal tubular acidosis
Inherited forms of renal acid–base disturbances are rare and caused by mutations in transport proteins and enzymes located in acid-secretory intercalated cells in the collecting duct system, mutations of components of the angiotensin II—aldosterone system regulating renal acid excretion, or by mutations leading to malformations of the kidney [1]. The various types of renal tubular acidosis affect mostly specific transport pathways localized in distinct nephron segments which provide the basis for the nomenclature of these acid–base disturbances. In the following, we will focus on defects underlying type I renal tubular acidosis (dRTA I, classic distal renal tubular acidosis).
Classic dRTA is characterized by the inability to acidify urine below pH 5.3 in the presence of metabolic acidosis. Consecutively, the excretion of ammonium and titratable acids is also reduced leading to an overall reduction in urinary acid excretion [1]. Patients develop hyperchloremic metabolic acidosis usually with a normal anion gap often associated with hypokalemia. During childhood and adolescence, failure to thrive, growth retardation, rickets, and nephrolithiasis or nephrocalcinosis may occur and lead to the initial diagnosis. Patients may also develop polyuria which may be triggered by the reduced capacity to concentrate urine due to hypercalciuria, hypokalemia or nephrocalcinosis [19,20,21].
Incomplete dRTA presents also with inadequate urinary acidification but patients usually have normal blood gases, i.e. normal blood pH and bicarbonate. The defect can be revealed with the various types of acid challenge tests (ammonium chloride or fludrocortisone–furosemide test, see below) where urine pH does not acidify below 5.3 [22].
To date, mutations in genes encoding for three distinct transport proteins have been identified as causing classic dRTA: in the chloride-bicarbonate exchanger AE1/SLC4A1 or in the B1/ATP6V1B1 and a4/ATP6V0A4 subunits of the vacuolar-type H+-ATPase [5, 6, 23, 24]. However, not all cases of inborn dRTA can be explained by mutations in these genes suggesting that mutations in additional genes may contribute to inherited dRTA. Candidate genes may include the K+/Cl−-cotransporter KCC4 (SLC12A7) [25], the Forkhead transcription factor Foxi1 [26], the Cl−/HCO3 −-exchanger SLC26A7 [27], the ammonia channel RhCG (SLC42A3) [8], the hensin (DMBT1)-CXCL12 signal complex [28, 29], or other H+-ATPase subunits [30]. In Europe, mutations in ATP6V1B1 and ATP6V0A4 appear to be more prevalent whereas in other regions, the relative occurrence of mutations may be different.
Mutations in SLC4A1 can be inherited in both an autosomal dominant (heterozygous mutations) and autosomal recessive (homozygous mutations) manner. In contrast, mutations in the ATP6V1B1 and ATP6V0A4 genes follow an autosomal recessive pattern but the significance of heterozygous mutations (i.e. only one mutated allele detectable) has recently been discussed (see below) [31].
The proton pump consists of a protein complex of two major domains, the cytosolic catalytic V1 domain hydrolyzing ATP (with eight subunits A–H) and the membrane-bound V0 domain mediating the proton transfer with the a, c, c″, d, and e subunits [32]. The B1 subunit is part of the V1 domain whereas the a4 subunit belongs to the V0 domain (Fig. 2). The B1 subunit is found only in a few organs including kidney, inner ear, epididymis and lung. In kidney, the B1 subunit is highly enriched in all types of intercalated cells but is also detected at lower levels in the thick ascending limb of Henle. The a4 subunit is also enriched in all types of intercalated cells but is also highly abundant in the proximal tubule and in the thick ascending limb of the loop of Henle [33]. The subunit is also expressed in epididymis and the stria vascularis of the inner ear [24, 34]. The expression of both subunits, B1 and a4, in the inner ear may explain the occurrence of sensorineural deafness in patients with mutations in these subunits. Nevertheless, the progression of sensorineural deafness is variable in patients and does not respond to alkali therapy [35, 36]. Some patients may also develop dizziness possibly because of an enlarged vestibular aqueduct (EVA) observed in some but not all patients [36]. Whether alterations in the function of proton pumps in the epididymis occur and affect male fertility in these patients is still unknown.
Based on experiments in yeast and cell culture models it appears that most of the mutations identified in the B1 subunit cause either dysfunction or impaired assembly of the protein complex [37, 38]. Accordingly, mice lacking the B1 subunit have a reduced capacity to acidify urine and develop more severe metabolic acidosis when acid-loaded. When crossed with hypercalciuric mice, B1 deficient mice develop severe nephrocalcinosis with hydronephrosis [19, 39, 40].
Lack of the a4 subunit in mice causes severe dRTA with hypokalemia, nephrocalcinosis, and reduced bone mineral density [41, 42]. The mice develop also a massive hearing loss and a reduced sense of smell. The absence of the a4 subunit from the proximal tubule is associated with low molecular weight proteinuria suggesting an important role of this subunit in receptor-mediated endocytosis [42]. In at least one series of patients with mutations in either ATP6V1B1 or ATP6V0A4, mutations in the latter were associated with a more severe clinical presentation and reduced kidney function [42].
The chloride-bicarbonate exchanger AE1 (SLC4A1) is expressed both in acid-secretory type A intercalated cells and red blood cells. Mutations in SLC4A1 cause either dRTA or red blood cell abnormalities including spherocytosis or South-East Asian ovalocytosis (SAO). Importantly, most mutations cause either dRTA or hematological abnormalities but only a few mutations affect both systems. The mode of inheritance is usually autosomal dominant but few autosomal recessive mutations have been described. The most frequent recessive mutation, G701D, causes dRTA that can be associated with hemolytic anemia. Interactions of AE1 with the chaperone glycophorin have been identified to underlie the separation of renal and red blood cell mutations as this molecule is only expressed in red blood cells and is able to rescue “renal” mutations bringing them to the red blood cell membrane [43]. A series of additional mutations has been identified that are more common in South-East Asia and are mostly associated with a red blood cell phenotype. It has been speculated that some of these mutations may confer resistance to malaria [44]. In contrast to the recessive mutations, patients with a Caucasian background harbor more frequently dominant mutations, R589H being the most common one that, rather, causes dRTA [45, 46]. Several types of AE1 mutations have been described that may cause either intracellular retention of mutant proteins or even mistargeting to the luminal membrane of type A intercalated cell models [47,48,49]. In mice, complete absence of AE1 causes severe metabolic acidosis and reduced renal excretion [50]. Introduction of the R589H mutation in mice (in mice this mutation corresponds to R607H) causes dysfunction of intercalated cells with reduced expression of proton pumps [45].
Inherited distal renal tubular acidosis as an underlying cause of nephrocalcinosis or kidney stones in adults
Nephrocalcinosis is caused by various disorders with different pathophysiologies including primary hyperoxaluria, sarcoidosis, medullary sponge kidney, primary hyperparathyroidism, distal RTA and others. Depending on the underlying cause patients may develop CKD with progression to end-stage kidney disease requiring renal replacement therapy. Thus, correct and timely diagnosis is of prime importance. Clinical manifestation of inherited dRTA can vary among patients depending on the underlying gene mutation. Hereditary recessive distal RTA due to B1 or a4 subunit mutations of the H+-ATPase typically manifests during infancy or childhood and presents with severe symptoms such as vomiting, failure to thrive, diarrhea or constipation, polyuria, nephrocalcinosis or rickets/osteomalacia. However, some cases may present with milder symptoms including a mild metabolic acidosis, hypocitraturia, incidental detection of kidney stones or renal calcification. In particular, patients with autosomal dominant dRTA due to mutations in the SLC4A1 gene may present first clinical symptoms only during adulthood.
As a consequence of metabolic acidosis skeletal buffers such as carbonate and phosphate in combination with calcium are removed from the bones resulting in bone demineralization and subsequently hypercalciuria. Additionally, expression of renal calcium transport proteins is decreased in metabolic acidosis further promoting calcium excretion and thus development of nephrocalcinosis and kidney stones (Fig. 3) [19, 51].
Consequently, in patients with nephrocalcinosis or repetitive episodes of kidney stones dRTA is an important differential diagnosis and should be considered, particularly if stone analysis detects calcium phosphate-containing stones in the presence of metabolic acidosis or if there is evidence of impaired hearing or deafness.
Diagnosis of inherited dRTA
Distal RTA results from a defective urinary acidification and is characterized by an inappropriate alkaline urine pH in the context of a hyperchloremic, normal anion gap metabolic acidosis with preserved glomerular filtration rate (GFR). The mutated genes, namely B1 (ATP6V1B1) and a4 (ATP6V0A4) subunit as well as AE1 (SLC4A1), are also expressed in extrarenal tissues, including the epididymis and cells of the stria vascularis of the inner ear (B1 and a4), and erythrocytes (AE1), respectively. The diagnosis is primarily based on typical clinical and laboratory abnormalities and confirmed by genetic analysis. The phenotype includes renal and extrarenal clinical symptoms. Specialized tests to test for urinary acidification capacity are mentioned below and are mainly required for diagnosis of incomplete forms of dRTA.
Short ammonium chloride loading test
Diagnosis of the renal defect is established by the short ammonium chloride loading test (= the short test of urinary acidification), which was refined and validated by Wrong and Davies in a seminal study several decades ago [22]. The principle of the short ammonium chloride loading test is based on the principal mechanism of hydrogen ion or acid secretion by the “healthy” kidney, namely excretion of all hydrogen ions combined with ammonia (NH3) as ammonium (NH4 +). Meanwhile, several animal studies have confirmed the crucial role of ammoniagenesis and ammonium excretion in renal acid excretion [52,53,54,55].
The original protocol of the test is explained briefly. After emptying the bladder, urine is collected hourly under paraffin oil and thymol or toluene for 10 h. After two hourly collections of urine, ammonium chloride capsules are given orally at a dose of 0.1 g (≈ 1.9 meq) per kg body weight over an hour. Blood gas analysis is performed before and after 2–4 h after ingestion of ammonium chloride. Urinary pH is measured hourly using a pH electrode. In this test, urinary pH below 5.3 excludes a urinary acidification defect and the test is terminated. Wrong and Davies investigated a total of 68 subjects, 10 healthy controls and 58 patients with different forms of renal diseases, including general renal failure without evidence of tubular abnormality, complete or incomplete renal tubular acidosis, and prolonged hypercalcemia and others. By using the ammonium chloride test, the authors demonstrated that the test is a reliable method to evaluate the ability of the kidney to excrete acid. Recently the test has been applied in several human studies [56,57,58]. Mostly, it has been used to selectively screen for complete or incomplete forms of dRTA in recurrent kidney stone formers [57, 58]. However, although the ammonium chloride loading test is still the ‘gold standard’ to test for defective urinary acidification, many patients suffer from unpleasant gastrointestinal side effects of ammonium chloride such as nausea and vomiting and also are not pleased about the duration of the test for a maximum of 8 h. Thus, Walsh et al. developed a simpler but effective and well-tolerated alternative test that will be discussed in detail in the next paragraph.
The simultaneous furosemide and fludrocortisone test as an alternative to ammonium chloride
The simultaneous furosemide plus fludrocortisone test (f + f test) is based on previous studies describing a stimulation of H+ secretion in response to oral furosemide application [59]. The f + f test has been tested in complete and incomplete dRTA and it is less specific than the gold standard ammonium chloride test. The test is thought to be based on the stimulation of electrogenic sodium reabsorption by the epithelial sodium channel ENaC in the collecting duct system due to enhanced delivery of sodium after blockade of Na+-reabsorption by the loop diuretic furosemide in the thick ascending limb (TAL) [60]. Higher activity of ENaC would cause a more lumen-negative potential in the collecting duct system and thereby increase the driving force for proton secretion. The mineralocorticoid fludrocortisone would stimulate ENaC activity, but also direct effects of aldosterone on H+-ATPase activity have been described [61, 62]. More recently, an alternative explanation has been provided whereby furosemide would stimulate NHE3-dependent proton secretion in the TAL and thereby increase urinary acidification [63]. Why TAL proton secretion would be reduced in dRTA patients is unclear, but could be related to the expression of the ATP6V1B1 and ATP6V0A4 transcripts in the TAL [33]. However, a more recent study conducted in healthy human volunteers provides support for the initial hypothesis that furosemide-induced urinary acidification requires ENaC-activity as the furosemide-induced drop in urinary pH was blunted when the ENaC-inhibitor amiloride was coadministered [64].
In a first study with the f + f test, Walsh et al. investigated eight patients with previously diagnosed dRTA and a control group of 11 healthy probands [56]. All participants were subjected to a short ammonium chloride test followed by the f + f test. Briefly, a baseline urine sample was collected from all participants followed by oral administration of 40 mg furosemide and 1 mg fludrocortisone. Urine collection was performed hourly and urine pH was measured using an electrode pH meter for 6 h after the baseline sample. Notably, there were no adverse effects with the f + f test. All healthy probands were able to acidify their urine below pH 5.3 with the f + f test or the ammonium chloride test while urine pH of dRTA patients remained above pH 5.3 indicating defective urinary acidification. In a follow-up study, the f + f test was further used in a preselected cohort of kidney stone formers [57]. In this study, the authors confirmed a distinct sensitivity and excellent negative predictive value of this test to exclude incomplete dRTA in patients with kidney stones or nephrocalcinosis or both. However, this study was retrospectively performed and only patients with a clinical suspicion for an acidification defect were tested. Consequently, the reliability of the f + f test, especially in the diagnosis of incomplete dRTA, remains to be further validated by ideally a prospective blinded study in a cohort of unselected patients. In addition, due to limited specificity, patients tested negative with f + f test may require confirmation by the ammonium chloride loading test. This finding has also been confirmed in another study by Dhayat et al. who prospectively subjected an unselected cohort of 170 stone formers to sequential ammonium chloride and f + f testing [65]. Furthermore, the authors also tested for non-provocative laboratory parameters to predict incomplete dRTA and demonstrated by using a morning fasting urinary pH at a threshold of < 5.3 with a plasma potassium threshold of > 3.8 mmol/l that incomplete dRTA can reliably be excluded. Thus, future studies are required to verify the value and impact of the f + f test in diagnosing incomplete dRTA.
Hearing test
The ATP6V1B1 and ATP6V0A4 forms are also expressed in extrarenal tissues such as in the stria vascularis of the inner ear. Thus, the majority of patients with the recessive forms of dRTA develop progressive bilateral sensorineural hearing loss which is interestingly more common in patients with B1 mutations than in those with a4 mutations [6, 35, 41, 42, 46]. Some patients also present with other abnormalities of the auricular system such as abnormal widening of the vestibular aqueduct (enlarged vestibular aqueduct, EVA) which is usually bilaterally present (Fig. 4) [36]. However, this type of abnormality is not specific for hereditary dRTA since it may also be present in patients with Pendred or branchio-oto-renal syndrome.
Enlarged vestibular aqueduct in a patient with inherited dRTA. MRI images of the temporal bone and labyrinth with bilateral enlarged endolymphatic duct (arrow) (a). b Three-dimensional reconstruction of the labyrinth showing the enlarged endolymphatic duct and sac (arrow) and bulbous dysplasia of the apical turn of the cochlea (short arrow). Right (c) and left (d) temporal bone with enlargement of the bony vestibular aqueducts (long arrow) in comparison to the diameter of the posterior semicircular canal (short arrow). Taken from [36]
To test for sensorineural hearing abnormalities a standard audiogram has to be performed investigating masked and unmasked bone and air conduction at different frequencies. To detect other auricular abnormalities, both, magnetic resonance imaging (MRI) or computed tomography (CT) can be used for the diagnosis of enlarged vestibular aqueducts (Fig. 4) [36].
Patients with inherited dRTA due to mutations in SLC4A1 may present concomitantly with Southeast Asian ovalocytosis (SAO), mainly in the Malay archipelago, the Philippines, Indonesia and southern Thailand. SAO is a hematologic disease that is clinically characterized by hemolytic anemia, oval-shape erythrocytes in the peripheral blood smear, and the presence of the hemizygous deletion of amino acids 400–408 (also known as SAO mutation) [66].
Long-term perspectives
Clinical outcome/Progression to CKD
To date, very few data exist on the long-term clinical outcome of inherited dRTA patients. Most studies have primarily investigated the genotype–phenotype characteristics of these patients at diagnosis. The most recent report has investigated one of the largest cohorts of patients with dRTA to date [67]. Among 89 patients clinically diagnosed with inherited dRTA, mutations in ATP6V1B1, ATP6V0A4, and SLC4A1 were found in 71.9% of all subjects. There was no significant difference regarding male and female distribution for all genes. Mean age of onset was around 5.5 years, although patients with SLC4A1 mutations typically presented at an older age (12–13 years old) compared to those with ATP6V1B1 and ATP6V0A4 mutations. As expected, sensorineural hearing loss was present in the majority of cases with ATP6V1B1 (92%) and ATP6V0A4 (56.7%) mutations, with a significantly earlier onset in patients carrying the ATP6V1B1 mutation. Another common finding was nephrocalcinosis that was detected in 93.6% of all mutated patients without differences among the different types of mutated genes. In this cohort, hypokalemia was more frequent and severe in patients with H+-ATPase mutations compared to those with SLC4A1 mutations. Notably, a significant proportion of subjects with pathogenic mutations suffered from CKD (31.3%), defined according to the KDIGO criteria (eGFR < 90 ml/min/1.73 m2) during the long-term follow-up, presenting after pubertal growth spurt. These findings are novel and of particular importance since inherited dRTA was always considered as a “benign” disease with regard to kidney function [46]. However, the pathophysiology of CKD is unclear and it has been debated to be caused by tubulointerstitial damage due to nephrocalcinosis and persistent hypokalemia. In addition, repeated pre-renal hits with acute kidney injury may also result in chronic kidney damage. Further studies are required to confirm these findings in larger cohorts. A previous smaller study including 19 children with genetically confirmed inherited dRTA also reported earlier age of onset in patients with ATP6V1B1 and ATP6V0A4 mutations compared to those with SLC4A1 mutations [46]. Metabolic acidosis was more profound in children with ATP6V0A4 mutations. Interestingly, in this cohort a substantial number of patients presented with partial proximal tubular dysfunction (partial Fanconi syndrome) that resolved after alkali treatment. However, the underlying mechanisms are unclear—it has been suggested that they may be associated with the role of the proton pump in receptor-mediated endocytosis, and the co-expression of the a4 subunit together with the chloride transporter CLC-5 in the proximal tubule cells and α-intercalated cells of the collecting ducts [68]. As described by Palazzo et al., nephrocalcinosis was very common and detected in all but one patient and reported to present with different degrees (mild to moderate or marked). In addition, three patients developed kidney stones, while there was no correlation between the severity of nephrocalcinosis and development of kidney stones. Also in this cohort, a significant number of the children presented with CKD (KDIGO G2, eGFR 60–90 ml/min/1.73 m2) at the last follow-up (at the age of up to 15 years). There was no significant correlation between the genetic diagnosis and CKD, however, there was a trend towards ATP6V0A4 mutations being more common in patients with CKD at the last follow-up. This observation is supported by animal data from Atp6v0a4-deficient mice that demonstrated impaired proximal tubule function [42]. Furthermore, in this study data analysis from a total of 99 patients with ATP6V1B1 and ATP6V0A4 mutations demonstrated a more severe phenotype in patients with ATP6V0A4 mutations compared to patients carrying the ATP6V1B1 mutation.
In summary, clinical outcome of inherited dRTA patients seems to be good, particularly if diagnosis is established early with subsequent initiation of alkali treatment. In contrast to frequent presence of nephrocalcinosis and kidney stones in this population, some patients may develop CKD. The underlying mechanisms of CKD have not been fully identified yet and seem to be associated with the respective gene. Further studies are required with larger patient cohorts and longer follow-ups, especially from the time period after transition to adult care, to evaluate the risk to progress to end-stage renal disease.
Pregnancy
Female CKD patients are at increased risk for complications during pregnancy and therefore intensive monitoring and interdisciplinary care is highly recommended in this population [69,70,71]. However, patients with inherited dRTA usually have a normal kidney function with preserved eGFR and therefore are often not perceived as CKD patients. Nevertheless, several case reports have described severe complications during pregnancy in female patients with different types of RTA [72,73,74,75]. We recently reported a series of three pregnant women with inherited dRTA with exacerbated acid–base disturbances during pregnancy [76]. All three patients presented with profound hypokalemia and worsening of metabolic acidosis during pregnancy. In addition to a potentially higher requirement for alkali therapy and potassium supplementation during pregnancy, physicians have to pay particular attention to hyperemesis gravidarum that might be a cause for stopping intake of alkali therapy and subsequent deterioration of acid–base and electrolyte status. Other complications such as recurrent urinary tract infections and obstruction should also be considered because of pre-existing nephrocalcinosis and/or kidney stones. Consequently, in pregnant women with inherited dRTA interdisciplinary management including the obstetrician and the nephrologist is recommended. Furthermore, in addition to regular monitoring of creatinine and proteinuria, acid–base and electrolyte status should also be tested regularly to prevent life-threatening hypokalemia and decompensation of metabolic acidosis.
Stone risk in heterozygous carriers?
A recent study of Dhayat et al. investigated the in vivo impact of a single-nucleotide polymorphism (SNP) in the coding region of the B1 subunit causing a change in the amino acid sequence (c.481G.A; p.E161K) of the H+-ATPase that causes greatly diminished pump function in vitro, and on urinary acidification in recurrent kidney stone formers [58]. Among 555 patients with stone disease, 5.8% were heterozygous for the respective SNP and demonstrated a trend to higher urinary pH values. Of the patients with p.E161K SNP, 52.4% were even identified with incomplete dRTA using the short NH4Cl loading test to confirm a urinary acidification defect in these patients (= urine pH > 5.3). As expected, there was a higher prevalence of calcium phosphate stones in p.E161K carriers when compared to wild-type subjects. As mentioned above, the simultaneous furosemide and fludrocortisone test is a valid alternative to the ammonium chloride test. Thus, Shavit et al. compared the results of both f + f and short NH4Cl test in recurrent stone formers who were screened for dRTA [57]. Urinary acidification defect as a result of incomplete or complete dRTA was present in 50% of the 34 patients who underwent to both tests. The comparison of both tests revealed a sensitivity of 100% but a specificity of only 24% for the f + f test. Therefore, in patients with an abnormal f + f test who are clinically not suspicious of defective urinary acidification, confirmation by NH4Cl test should be performed.
Therapy of inherited dRTA
To date, therapy of inherited dRTA consists of alkali treatment to correct metabolic acidosis and avoid complications such as failure to thrive, growth retardation, and rickets [46]. Physicians have to consider that in contrast to adults, who usually require stable and low doses of bicarbonate such as 0.5–1 mEq/kg/day, growing children and infants may need substantially higher doses, especially if genetic diagnosis includes mutations in the B1 or a4-subunit of the H+-ATPase compared to patients with SLC4A1 mutations. Unfortunately, there is no amelioration of the progressive hearing loss and progressive nephrocalcinosis with alkali therapy. Potassium-containing formulations such as potassium citrate should be preferred since patients usually present with a hypokalemic metabolic acidosis. However, potassium citrate may be unpleasant for some patients because of gastrointestinal side effects. Hence, also sodium bicarbonate or other alkali formulations can be used or added to therapy. Pediatricians may also use Shohl’s solution containing sodium citrate that can be more easily dose-adjusted in children. In the presence of severe hypercalciuria, thiazides can be administered to reduce renal calcium excretion. However, they should be used carefully since the risk of hypokalemia and polyuria may increase. If polyuria is severe indomethacin can be added to therapy.
Because of progressive and irreversible hearing loss, hearing devices and language teaching are inevitable and thus of prime importance to ensure normal intellectual development and social integration of these patients.
Summary and conclusion
dRTA is a rare inherited tubular disorder impairing the kidney’s ability to acidify urine and excrete acid. The clinical manifestations depend on the gene mutated. In severe cases, patients may present after birth with failure to thrive, vomiting, dehydration, and profound disturbances of acid–base balance and electrolytes. In milder cases, nephrocalcinosis or -lithiasis may be the first clinical presentations. Next to treatment of metabolic acidosis, the progressive loss of hearing should be treated with hearing aids to ensure a normal intellectual development of children. Early genetic diagnosis and counseling of parents is important. During pregnancy, women with dRTA may suffer from exacerbations of their metabolic acidosis and experience severe electrolyte disturbances requiring a close monitoring of these parameters.
References
Wagner CA, Devuyst O, Bourgeois S, Mohebbi N (2009) Regulated acid–base transport in the collecting duct. Pflugers Arch 458(1):137–156. doi:10.1007/s00424-009-0657-z
Guo YM, Liu Y, Liu M, Wang JL, Xie ZD, Chen KJ, Wang DK, Occhipinti R, Boron WF, Chen LM (2017) Na+/HCO3− cotransporter NBCn2 mediates HCO3− reclamation in the apical membrane of renal proximal tubules. J Am Soc Nephrol. doi:10.1681/ASN.2016080930
Gottschalk CW, Lassiter WE, Mylle M (1960) Localization of urine acidification in the mammalian kidney. Am J Physiol 198:581–585
Alper SL, Natale J, Gluck S, Lodish HF, Brown D (1989) Subtypes of intercalated cells in rat kidney collecting duct defined by antibodies against erythroid band 3 and renal vacuolar H+-ATPase. Proc Natl Acad Sci USA 86(14):5429–5433
Karet FE, Gainza FJ, Gyory AZ, Unwin RJ, Wrong O, Tanner MJ, Nayir A, Alpay H, Santos F, Hulton SA, Bakkaloglu A, Ozen S, Cunningham MJ, di Pietro A, Walker WG, Lifton RP (1998) Mutations in the chloride-bicarbonate exchanger gene AE1 cause autosomal dominant but not autosomal recessive distal renal tubular acidosis. Proc Natl Acad Sci USA 95(11):6337–6342
Karet FE, Finberg KE, Nelson RD, Nayir A, Mocan H, Sanjad SA, Rodriguez-Soriano J, Santos F, Cremers CW, Di Pietro A, Hoffbrand BI, Winiarski J, Bakkaloglu A, Ozen S, Dusunsel R, Goodyer P, Hulton SA, Wu DK, Skvorak AB, Morton CC, Cunningham MJ, Jha V, Lifton RP (1999) Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nat Genet 21(1):84–90
Karet FE, Finberg KE, Nayir A, Bakkaloglu A, Ozen S, Hulton SA, Sanjad SA, Al-Sabban EA, Medina JF, Lifton RP (1999) Localization of a gene for autosomal recessive distal renal tubular acidosis with normal hearing (rdRTA2) to 7q33-34. Am J Hum Genet 65(6):1656–1665
Biver S, Belge H, Bourgeois S, Van Vooren P, Nowik M, Scohy S, Houillier P, Szpirer J, Szpirer C, Wagner CA, Devuyst O, Marini AM (2008) A role for Rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature 456(7220):339–343
Bourgeois S, Bounoure L, Christensen EI, Ramakrishnan SK, Houillier P, Devuyst O, Wagner CA (2013) Haploinsufficiency of the ammonia transporter Rhcg predisposes to chronic acidosis: Rhcg is critical for apical and basolateral ammonia transport in the mouse collecting duct. J Biol Chem 288(8):5518–5529. doi:10.1074/jbc.M112.441782
Bounoure L, Ruffoni D, Muller R, Kuhn GA, Bourgeois S, Devuyst O, Wagner CA (2014) The role of the renal ammonia transporter Rhcg in metabolic responses to dietary protein. J Am Soc Nephrol 25(9):2040–2052. doi:10.1681/ASN.2013050466
Wagner CA, Mohebbi N, Capasso G, Geibel JP (2011) The anion exchanger pendrin (SLC26A4) and renal acid–base homeostasis. Cell Physiol Biochem 28(3):497–504. doi:10.1159/000335111
Wagner CA, Finberg KE, Stehberger PA, Lifton RP, Giebisch GH, Aronson PS, Geibel JP (2002) Regulation of the expression of the Cl–/anion exchanger pendrin in mouse kidney by acid–base status. Kidney Int 62(6):2109–2117
Kim YH, Kwon TH, Frische S, Kim J, Tisher CC, Madsen KM, Nielsen S (2002) Immunocytochemical localization of pendrin in intercalated cell subtypes in rat and mouse kidney. Am J Physiol Renal Physiol 283(4):F744–F754
Hafner P, Grimaldi R, Capuano P, Capasso G, Wagner CA (2008) Pendrin in the mouse kidney is primarily regulated by Cl- excretion but also by systemic metabolic acidosis. Am J Physiol Cell Physiol 295(6):C1658–C1667
Frische S, Kwon TH, Frokiaer J, Madsen KM, Nielsen S (2003) Regulated expression of pendrin in rat kidney in response to chronic NH4Cl or NaHCO3 loading. Am J Physiol Renal Physiol 284(3):F584–F593
Pech V, Kim YH, Weinstein AM, Everett LA, Pham TD, Wall SM (2006) Angiotensin II increases chloride absorption in the cortical collecting duct in mice through a pendrin-dependent mechanism. Am J Physiol Renal Physiol 292(3):F914–F20
Verlander JW, Hassell KA, Royaux IE, Glapion DM, Wang ME, Everett LA, Green ED, Wall SM (2003) Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: role of pendrin in mineralocorticoid-induced hypertension. Hypertension 42(3):356–362
Wagner CA (2016) Pendrin-A new target for diuretic therapy? J Am Soc Nephrol 27(12):3499–3501. doi:10.1681/ASN.2016070720
Renkema KY, Velic A, Dijkman HB, Verkaart S, van der Kemp AW, Nowik M, Timmermans K, Doucet A, Wagner CA, Bindels RJ, Hoenderop JG (2009) The calcium-sensing receptor promotes urinary acidification to prevent nephrolithiasis. J Am Soc Nephrol 20(8):1705–1713. doi:10.1681/ASN.2008111195
Gueutin V, Vallet M, Jayat M, Peti-Peterdi J, Corniere N, Leviel F, Sohet F, Wagner CA, Eladari D, Chambrey R (2013) Renal beta-intercalated cells maintain body fluid and electrolyte balance. J Clin Investig 123(10):4219–4231. doi:10.1172/JCI63492
Sebastian A, McSherry E, Morris RC Jr (1976) Impaired renal conservation of sodium and chloride during sustained correction of systemic acidosis in patients with type 1, classic renal tubular acidosis. J Clin Investig 58(2):454–469
Wrong O, Davies HE (1959) The excretion of acid in renal disease. Q J Med 28(110):259–313
Bruce LJ, Cope DL, Jones GK, Schofield AE, Burley M, Povey S, Unwin RJ, Wrong O, Tanner MJ (1997) Familial distal renal tubular acidosis is associated with mutations in the red cell anion exchanger (Band 3, AE1) gene. J Clin Investig 100(7):1693–1707
Smith AN, Skaug J, Choate KA, Nayir A, Bakkaloglu A, Ozen S, Hulton SA, Sanjad SA, Al-Sabban EA, Lifton RP, Scherer SW, Karet FE (2000) Mutations in ATP6N1B, encoding a new kidney vacuolar proton pump 116-kD subunit, cause recessive distal renal tubular acidosis with preserved hearing. Nat Genet 26(1):71–75
Boettger T, Hubner CA, Maier H, Rust MB, Beck FX, Jentsch TJ (2002) Deafness and renal tubular acidosis in mice lacking the K–Cl co-transporter Kcc4. Nature 416(6883):874–878
Blomqvist SR, Vidarsson H, Fitzgerald S, Johansson BR, Ollerstam A, Brown R, Persson AE, Bergstrom GG, Enerback S (2004) Distal renal tubular acidosis in mice that lack the forkhead transcription factor Foxi1. J Clin Investig 113(11):1560–1570
Xu J, Song P, Nakamura S, Miller M, Barone S, Alper SL, Riederer B, Bonhagen J, Arend LJ, Amlal H, Seidler U, Soleimani M (2009) Deletion of the chloride transporter slc26a7 causes distal renal tubular acidosis and impairs gastric acid secretion. J Biol Chem 284(43):29470–29479
Gao X, Eladari D, Leviel F, Tew BY, Miro-Julia C, Cheema F, Miller L, Nelson R, Paunescu TG, McKee M, Brown D, Al-Awqati Q (2010) Deletion of hensin/DMBT1 blocks conversion of {beta}- to {alpha}-intercalated cells and induces distal renal tubular acidosis. Proc Natl Acad Sci USA. doi:10.1073/pnas.1010364107
Schwartz GJ, Gao X, Tsuruoka S, Purkerson JM, Peng H, D’Agati V, Picard N, Eladari D, Al-Awqati Q (2015) SDF1 induction by acidosis from principal cells regulates intercalated cell subtype distribution. J Clin Investig 125(12):4365–4374. doi:10.1172/JCI80225
Smith AN, Borthwick KJ, Karet FE (2002) Molecular cloning and characterization of novel tissue-specific isoforms of the human vacuolar H+-ATPase C, G and d subunits, and their evaluation in autosomal recessive distal renal tubular acidosis. Gene 297(1–2):169–177
Zhang J, Fuster DG, Cameron MA, Quinones H, Griffith C, Xie XS, Moe OW (2014) Incomplete distal renal tubular acidosis from a heterozygous mutation of the V-ATPase B1 subunit. Am J Physiol Renal Physiol 307(9):F1063–F1071. doi:10.1152/ajprenal.00408.2014
Wagner CA, Finberg KE, Breton S, Marshansky V, Brown D, Geibel JP (2004) Renal vacuolar H+-ATPase. Physiol Rev 84:1263–1314
Stehberger P, Schulz N, Finberg KE, Karet FE, Giebisch G, Lifton RP, Geibel JP, Wagner CA (2003) Localization and regulation of the ATP6V0A4 (a4) vacuolar H+-ATPase subunit defective in an inherited form of distal renal tubular acidosis. J Am Soc Nephrol 14:3027–3038
Smith AN, Finberg KE, Wagner CA, Lifton RP, Devonald MA, Su Y, Karet FE (2001) Molecular cloning and characterization of Atp6n1b: a novel fourth murine vacuolar H+-ATPase a-subunit gene. J Biol Chem 276(45):42382–42388
Stover EH, Borthwick KJ, Bavalia C, Eady N, Fritz DM, Rungroj N, Giersch AB, Morton CC, Axon PR, Akil I, Al-Sabban EA, Baguley DM, Bianca S, Bakkaloglu A, Bircan Z, Chauveau D, Clermont MJ, Guala A, Hulton SA, Kroes H, Li, Volti G, Mir S, Mocan H, Nayir A, Ozen S, Rodriguez Soriano J, Sanjad SA, Tasic V, Taylor CM, Topaloglu R, Smith AN, Karet FE (2002) Novel ATP6V1B1 and ATP6V0A4 mutations in autosomal recessive distal renal tubular acidosis with new evidence for hearing loss. J Med Genet 39(11):796–803
Mohebbi N, Vargas-Poussou R, Hegemann SC, Schuknecht B, Kistler AD, Wuthrich RP, Wagner CA (2013) Homozygous and compound heterozygous mutations in the ATP6V1B1 gene in patients with renal tubular acidosis and sensorineural hearing loss. Clin Genet 83(3):274–278. doi:10.1111/j.1399-0004.2012.01891.x
Yang Q, Li G, Singh SK, Alexander EA, Schwartz JH (2006) Vacuolar H+-ATPase B1 subunit mutations that cause inherited distal renal tubular acidosis affect proton pump assembly and trafficking in inner medullary collecting duct cells. J Am Soc Nephrol 17(7):1858–1866
Fuster DG, Zhang J, Xie XS, Moe OW (2008) The vacuolar-ATPase B1 subunit in distal tubular acidosis: novel mutations and mechanisms for dysfunction. Kidney Int 73(10):1151–1158
Finberg KE, Wagner CA, Bailey MA, Paunescu TG, Breton S, Brown D, Giebisch G, Geibel JP, Lifton RP (2005) The B1 subunit of the H+ATPase is required for maximal urinary acidification. Proc Nat Acad Sci USA 102(38):13616–13621
Rothenberger F, Velic A, Stehberger PA, Kovacikova J, Wagner CA (2007) Angiotensin II stimulates vacuolar H+-ATPase activity in renal acid-secretory intercalated cells from the outer medullary collecting duct. J Am Soc Nephrol 18(7):2085–2093
Lorente-Canovas B, Ingham N, Norgett EE, Golder ZJ, Karet Frankl FE, Steel KP (2013) Mice deficient in H+-ATPase a4 subunit have severe hearing impairment associated with enlarged endolymphatic compartments within the inner ear. Dis Model Mech 6(2):434–442. doi:10.1242/dmm.010645
Hennings JC, Picard N, Huebner AK, Stauber T, Maier H, Brown D, Jentsch TJ, Vargas-Poussou R, Eladari D, Hubner CA (2012) A mouse model for distal renal tubular acidosis reveals a previously unrecognized role of the V-ATPase a4 subunit in the proximal tubule. EMBO Mol Med 4(10):1057–1071. doi:10.1002/emmm.201201527
Tanphaichitr VS, Sumboonnanonda A, Ideguchi H, Shayakul C, Brugnara C, Takao M, Veerakul G, Alper SL (1998) Novel AE1 mutations in recessive distal renal tubular acidosis. Loss-of-function is rescued by glycophorin A. J Clin Investig 102(12):2173–2179
Khositseth S, Bruce LJ, Walsh SB, Bawazir WM, Ogle GD, Unwin RJ, Thong MK, Sinha R, Choo KE, Chartapisak W, Kingwatanakul P, Sumboonnanonda A, Vasuvattakul S, Yenchitsomanus P, Wrong O (2012) Tropical distal renal tubular acidosis: clinical and epidemiological studies in 78 patients. QJM 105(9):861–877. doi:10.1093/qjmed/hcs139
Mumtaz R, Trepiccione F, Hennings JC, Huebner AK, Serbin B, Picard N, Ullah A, Paunescu TG, Capen DE, Lashhab RM, Mouro-Chanteloup I, Alper SL, Wagner CA, Cordat E, Brown D, Eladari D, Hubner CA (2017) Intercalated cell depletion and vacuolar H+-ATPase mistargeting in an Ae1 R607H Knockin model. J Am Soc Nephrol 28(5):1507–1520. doi:10.1681/ASN.2016020169
Besouw MTP, Bienias M, Walsh P, Kleta R, Van’t Hoff WG, Ashton E, Jenkins L, Bockenhauer D (2017) Clinical and molecular aspects of distal renal tubular acidosis in children. Pediatr Nephrol 32(6):987–996. doi:10.1007/s00467-016-3573-4
Devonald MA, Smith AN, Poon JP, Ihrke G, Karet FE (2003) Non-polarized targeting of AE1 causes autosomal dominant distal renal tubular acidosis. Nat Genet 33(2):125–127
Kittanakom S, Cordat E, Akkarapatumwong V, Yenchitsomanus PT, Reithmeier RA (2004) Trafficking defects of a novel autosomal recessive distal renal tubular acidosis mutant (S773P) of the human kidney anion exchanger (kAE1). J Biol Chem 279(39):40960–40971
Walsh S, Turner CM, Toye A, Wagner C, Jaeger P, Laing C, Unwin R (2007) Immunohistochemical comparison of a case of inherited distal renal tubular acidosis (with a unique AE1 mutation) with an acquired case secondary to autoimmune disease. Nephrol Dial Transplant 22(3):807–812
Stehberger PA, Shmukler BE, Stuart-Tilley AK, Peters LL, Alper SL, Wagner CA (2007) Distal renal tubular acidosis in mice lacking the AE1 (band3) Cl–/HCO3 – exchanger (slc4a1). J Am Soc Nephrol 18(5):1408–1418
Lemann J Jr, Gray RW, Maierhofer WJ, Cheung HS (1986) The importance of renal net acid excretion as a determinant of fasting urinary calcium excretion. Kidney Int 29(3):743–746 (S0085-2538(15)33643-7)
Chan K, Busque SM, Sailer M, Stoeger C, Broer S, Daniel H, Rubio-Aliaga I, Wagner CA (2016) Loss of function mutation of the Slc38a3 glutamine transporter reveals its critical role for amino acid metabolism in the liver, brain, and kidney. Pflugers Arch 468(2):213–227. doi:10.1007/s00424-015-1742-0
Burki R, Mohebbi N, Bettoni C, Wang X, Serra AL, Wagner CA (2015) Impaired expression of key molecules of ammoniagenesis underlies renal acidosis in a rat model of chronic kidney disease. Nephrol Dial Transplant 30(5):770–781. doi:10.1093/ndt/gfu384
Wagner CA, Devuyst O, Belge H, Bourgeois S, Houillier P (2011) The rhesus protein RhCG: a new perspective in ammonium transport and distal urinary acidification. Kidney Int 79(2):154–161. doi:10.1038/ki.2010.386
Weiner ID, Verlander JW (2017) Ammonia transporters and their role in acid–base balance. Physiol Rev 97(2):465–494. doi:10.1152/physrev.00011.2016
Walsh SB, Shirley DG, Wrong OM, Unwin RJ (2007) Urinary acidification assessed by simultaneous furosemide and fludrocortisone treatment: an alternative to ammonium chloride. Kidney Int 71(12):1310–1316
Shavit L, Chen L, Ahmed F, Ferraro PM, Moochhala S, Walsh SB, Unwin R (2016) Selective screening for distal renal tubular acidosis in recurrent kidney stone formers: initial experience and comparison of the simultaneous furosemide and fludrocortisone test with the short ammonium chloride test. Nephrol Dial Transplant 31(11):1870–1876. doi:10.1093/ndt/gfv423
Dhayat NA, Schaller A, Albano G, Poindexter J, Griffith C, Pasch A, Gallati S, Vogt B, Moe OW, Fuster DG (2016) The vacuolar H+-ATPase B1 subunit polymorphism p.E161K associates with impaired urinary acidification in recurrent stone formers. J Am Soc Nephrol 27(5):1544–1554. doi:10.1681/ASN.2015040367
Batlle DC (1986) Segmental characterization of defects in collecting tubule acidification. Kidney Int 30(4):546–554
Kovacikova J, Winter C, Loffing-Cueni D, Loffing J, Finberg KE, Lifton RP, Hummler E, Rossier B, Wagner CA (2006) The connecting tubule is the main site of the furosemide-induced urinary acidification by the vacuolar H+-ATPase. Kidney Int 70(10):1706–1716
Winter C, Schulz N, Giebisch G, Geibel JP, Wagner CA (2004) Nongenomic stimulation of vacuolar H+-ATPases in intercalated renal tubule cells by aldosterone. Proc Nat Acad Sci USA 101(8):2636–2641
Winter C, Kampik NB, Vedovelli L, Rothenberger F, Paunescu TG, Stehberger PA, Brown D, John H, Wagner CA (2011) Aldosterone stimulates vacuolar H(+)-ATPase activity in renal acid-secretory intercalated cells mainly via a protein kinase C-dependent pathway. Am J Physiol Cell Physiol 301(5):C1251–C1261. doi:10.1152/ajpcell.00076.2011
de Bruijn PI, Larsen CK, Frische S, Himmerkus N, Praetorius HA, Bleich M, Leipziger J (2015) Furosemide-induced urinary acidification is caused by pronounced H+ secretion in the thick ascending limb. Am J Physiol Renal Physiol:ajprenal 00154:02015. doi:10.1152/ajprenal.00154.2015
Bech AP, Wetzels JFM, Nijenhuis T (2017) Use of the furosemide fludrocortisone test to clinically assess distal tubular acidification. Am J Kidney Dis. doi:10.1053/j.ajkd.2017.05.009
Dhayat NA, Gradwell MW, Pathare G, Anderegg M, Schneider L, Luethi D, Mattmann C, Moe OW, Vogt B, Fuster DG (2017) Furosemide/fludrocortisone test and clinical parameters to diagnose incomplete distal renal tubular acidosis in kidney stone formers. Clin J Am Soc Nephrol. doi:10.2215/CJN.01320217
Wrong O, Bruce LJ, Unwin RJ, Toye AM, Tanner MJ (2002) Band 3 mutations, distal renal tubular acidosis, and Southeast Asian ovalocytosis. Kidney Int 62(1):10–19. doi:10.1046/j.1523-1755.2002.00417.x
Palazzo V, Provenzano A, Becherucci F, Sansavini G, Mazzinghi B, Orlandini V, Giunti L, Roperto RM, Pantaleo M, Artuso R, Andreucci E, Bargiacchi S, Traficante G, Stagi S, Murer L, Benetti E, Emma F, Giordano M, Rivieri F, Colussi G, Penco S, Manfredini E, Caruso MR, Garavelli L, Andrulli S, Vergine G, Miglietti N, Mancini E, Malaventura C, Percesepe A, Grosso E, Materassi M, Romagnani P, Giglio S (2017) The genetic and clinical spectrum of a large cohort of patients with distal renal tubular acidosis. Kidney Int 91(5):1243–1255. doi:10.1016/j.kint.2016.12.017
Gunther W, Luchow A, Cluzeaud F, Vandewalle A, Jentsch TJ (1998) ClC-5, the chloride channel mutated in Dent’s disease, colocalizes with the proton pump in endocytotically active kidney cells. Proc Natl Acad Sci USA 95(14):8075–8080
Cabiddu G, Castellino S, Gernone G, Santoro D, Moroni G, Giannattasio M, Gregorini G, Giacchino F, Attini R, Loi V, Limardo M, Gammaro L, Todros T, Piccoli GB (2016) A best practice position statement on pregnancy in chronic kidney disease: the Italian Study Group on Kidney and Pregnancy. J Nephrol 29(3):277–303. doi:10.1007/s40620-016-0285-6
Alsuwaida A, Mousa D, Al-Harbi A, Alghonaim M, Ghareeb S, Alrukhaimi MN (2011) Impact of early chronic kidney disease on maternal and fetal outcomes of pregnancy. J Matern Fetal Neonatal Med 24(12):1432–1436. doi:10.3109/14767058.2011.575483
Piccoli GB, Cabiddu G, Attini R, Vigotti FN, Maxia S, Lepori N, Tuveri M, Massidda M, Marchi C, Mura S, Coscia A, Biolcati M, Gaglioti P, Nichelatti M, Pibiri L, Chessa G, Pani A, Todros T (2015) Risk of adverse pregnancy outcomes in women with CKD. J Am Soc Nephrol 26(8):2011–2022. doi:10.1681/ASN.2014050459
Firmin CJ, Kruger TF, Davids R (2007) Proximal renal tubular acidosis in pregnancy. A case report and literature review. Gynecol Obstet Investig 63(1):39–44. doi:10.1159/000094942
Hardardottir H, Lahiri T, Egan JF (1997) Renal tubular acidosis in pregnancy: case report and literature review. J Matern Fetal Med 6(1):16–20. doi:10.1002/(SICI)1520-6661(199701/02)6:1<16::AID-MFM3>3.0.CO;2-V
Srisuttayasathien M (2015) Hypokalemia-induced rhabdomyolysis as a result of distal renal tubular acidosis in a pregnant woman: a case report and literature review. Case Rep Obstet Gynecol 2015:947617. doi:10.1155/2015/947617
Rowe TF, Magee K, Cunningham FG (1999) Pregnancy and renal tubular acidosis. Am J Perinatol 16(4):189–191. doi:10.1055/s-2007-993856
Seeger H, Salfeld P, Eisel R, Wagner CA, Mohebbi N (2017) Complicated pregnancies in inherited distal renal tubular acidosis: importance of acid–base balance. J Nephrol 30(3):455–460. doi:10.1007/s40620-016-0370-x
Acknowledgements
Work of the authors has been supported by the Swiss National Science Foundation and the 6th and 7th EU Frame work projects Eunefron and Eurenomics.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical statement
This article does not contain any studies with human participants performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mohebbi, N., Wagner, C.A. Pathophysiology, diagnosis and treatment of inherited distal renal tubular acidosis. J Nephrol 31, 511–522 (2018). https://doi.org/10.1007/s40620-017-0447-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-017-0447-1