Abstract
Background
Acute kidney injury (Dasta et al., Nephrol Dial Transplant 23(6):1970–1974, 2008) following cardiac surgery is associated with higher perioperative morbidity and mortality, but its impact on long term development of chronic kidney disease (CKD) is uncertain.
Methods
A total of 350 patients submitted to elective cardiac surgery were evaluated for AKI, defined as an increase in serum creatinine (SCr) ≥ 0.3 mg/dL over baseline value. Univariate and multivariate analysis were used to study pre, intra and postoperative parameters associated with occurrence CKD after 12 months of follow-up.
Results
AKI incidence was 41 % (n = 88). The 12-month prevelence of CKD was 9 % (n = 19) in non-AKI patients versus 25 % (n = 54, p < 0.0001) in the AKI group. The factors identified as independent risk factors for long-term CKD development in the multivariate logistic regression model were age >60 years, hospitalization serum creatinine >0.8 mg/dL, peripheral artery disease, hemorrhage and AKI duration > 3 days.
Conclusion
Patients developing AKI after cardiac surgery presented high prevalence of long-term incident CKD. The duration of AKI was a strong independent risk factor for this late CKD development. Recognition of predictive factors for CKD development following cardiac surgery-associated AKI may help to develop strategies to prevent or halt CKD progression in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute kidney injury (AKI) is a serious complication after cardiac surgery, which is associated to high mortality, increase in hospitalization time and hospital costs. AKI occurs in up to 30 % of patients undergoing cardiac surgery and even minor postoperative changes of serum creatinine are associated with increased hospital costs and mortality [1, 2].
Recently, numerous studies demonstrated a strong association between the severity of AKI and latter progression to chronic kidney disease (CKD). Even mild increases in serum creatinine levels after cardiac surgery are associated in a graded manner with a subsequent increase in the risk of incident CKD and CKD progression [3–6].
Most of the studies on CKD development after AKI were focused on the magnitude of serum creatinine increase and their impact on kidney disease progression [7–10], ignoring AKI duration as a potentially important aspect of the severity of AKI. Not only the duration of AKI after cardiac surgery is directly proportional to long-term mortality, but the duration of serum creatinine rise has also been suggested as a better end-point for AKI trials in general [11, 12]. However, the relationship between duration of AKI and kidney disease progression has not been studied yet.
Thus, we sought to determine the association between the duration of the AKI episode and the long-term CKD development in cardiac surgery patients-associated AKI.
Patients and Methods
The Ethical Institutional Review Board approved the study and waived informed consent due to the purely observational character of the study.
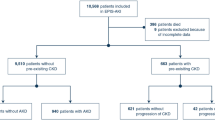
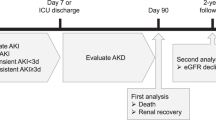
From July/2003 to July/2005 and from July/2006 to July/2007 all 819 patients undergoing elective coronary artery-bypass grafting (CABG), valve replacement, or both, at the Heart Institute, University of São Paulo—Brazil, were prospectively assessed. From the initial sample, 718 patients were discharged alive from the hospital and 348 patients were available for long-term kidney function follow-up, evaluated with serum creatinine measurement (SCr) and creatinine clearance (CrCl) calculation by the Modification of Diet in Renal Disease (MDRD) formula after 12 months from hospital discharge. Patients were evaluated by a single observer 24h prior to surgery to assess pre-operatory variables and then, followed from ICU admission until hospital discharge. Inclusion criteria were patients 18 to 90 years and undergoing elective CABG, valve replacement or both. Exclusion criteria were emergency surgeries, congenital heart disease repair, aortic aneurysm and baseline CKD ≥ stage 3. Chronic kidney disease (CKD) stage 3 was defined as two or more estimated glomerular filtration rate (eGFR) <60 mL/min, calculated by the Modification of Diet in Renal Disease (MDRD) formula, in ≥3 months period. AKI was defined as an increase in serum creatinine ≥0.3 mg/dL over reference value in 48 h, according to AKIN criteria [13]. Duration of AKI was defined by the number of days in which AKI was present.
The variables below were analyzed as possible predictors for CKD development.
Pre-operative variables
Gender, age, race, body mass index (BMI), heart failure (defined as NYHA functional Class II or greater), hypertension, previous myocardial infarction, diabetes mellitus (use of oral glucose-lowering agents or insulin), stroke, previous heart surgery, peripheral vascular disease, left ventricular ejection fraction (LVEF), capillary glucose (mg/dL), serum creatinine (mg/dL) and estimated baseline creatinine clearance (MDRD formula) at hospitalization.
Intra-operative variables
Cardiopulmonary bypass (CPB) time, intraoperative fluid balance and hemorrhage (defined as output of mediastinal or pericardial drains over 1L in 24 h or return to the surgery room due to bleeding within the first 24 h post-surgery or transfusion of 3 or more units of packed red cells in the surgery room or within the first post-operative 48 h).
Early post-operative variables
Central venous pressure (CVP), hemodynamic instability (defined as the need of at least three vasoactive drugs in order to maintain mean blood pressure >65 mm Hg), intra-aortic balloon pump (IABP) use and serum arterial bicarbonate.
Statistical analysis
Data are presented as mean ± standard deviation (SD) or percent and were analyzed using the Statistical Package for Social Sciences (SPSS) for Windows, version 17 (SPSS Inc., Chicago, IL, USA). The Kolmogorov–Smirnov test was used to verify the normality of distribution of continuous variables. Univariate analysis was carried out for baseline characteristics (including AKI diagnosis) and to evaluate the influence of baseline characteristics and AKI on CKD development, a multiple logistic regression analysis was performed. Variables were included in the multivariate analysis if found to be significant at univariate analysis with a P value < 0.1. Continuous variables were expressed as mean ± s.d. and analyzed by unpaired Student’s t-test, using Welch’s adjustment, whenever required. Non-parametric variables were expressed as median and analyzed by Mann–Whitney’s test. Categorical variables were expressed as absolute (n) and relative (%) frequency, and were analyzed by Pearson’s or Fisher’s exact test, whenever appropriate. In the multivariate analysis, results were expressed by the odds ratio and the 95 % confidence interval. Hosmer–Lemeshow goodness-of-fit test was applied to evaluate the calibration of logistic regression model and to check the importance of the discrepancy between observed and expected CKD. Forward stepwise selection procedure was used to select the variables significantly related to CKD development, as assessed by the likelihood ratio test. Odds ratios with 95 % confidence intervals were calculated for statistically significant variables. A p value < 0.05 was considered as statistically significant.
Results
Three hundred and forty eight patients undergoing elective coronary artery bypass graft surgery (CABG), valve surgery or both were initially evaluated for CKD development 12 months after hospital discharge. One hundred thirty-three patients were excluded from study because of baseline CKD ≥ stage 3 and 215 patients remained for final analysis.
Patients’ age was 60 ± 13 years and mean body mass index was 26.2 ± 4.5. There was a higher frequency of males (60 %) and Caucasians (88 %). The most common comorbidities were arterial hypertension (84 %) and diabetes (35 %), with a greater proportion of patients presenting baseline LVEF > 50 % (69 %). Baseline serum creatinine and eGFR were respectively 1.1 mg/dL and 68.5 mL/min. The mean serum creatinine and eGFR at ICU discharge were respectively 1.0 mg/dL and 71.2 mL/min and 1.2 mg/dL and 69.6 mL/min at hospital discharge. CPB was utilized in 77 % and CPB time was 102 ± 2 min. At ICU admission, arterial serum bicarbonate was 20.8 ± 0.2 mEq/L and CVP was 12.7 ± 0.1 cmH2O. The most frequent cardiac surgery was CABG (56 %), followed by valve surgery (22 %) and combined surgeries (22 %) (Table 1).
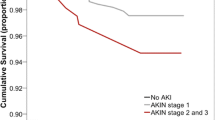
AKI incidence in this group was 41 % (n = 88), with 81 % (n = 174) on AKIN Stage 1, 14 % (n = 30) on AKIN Stage 2 and 5 % (n = 11) on AKIN Stage 3. The mean creatinine values in AKI and non-AKI groups were 1.4 and 1.1 mg/dl, respectively. The need for dialysis was observed in 8 % (n = 17) of patients and continuous veno-venous hemodialysis (CVVHD) with citrate anticoagulation was routinely prescribed to these patients. Most patients (84 %, n = 74) developed AKI within the first 02 days post-operative (Fig. 1). Incidence of CKD after 12 months of follow-up in non-AKI patients was 9 % (n = 19) compared to 25 % (n = 54) in AKI patients (p < 0.0001). The mean creatinine values of CKD patients at the end of 12 months follow-up was 1.8 mg/dL, compared to 1.2 mg/dL in non-CKD patients.
Statistically significant risk factors for CKD development at the univariate analysis (p < 0.05) were (Table 2):
Pre-operative
Age >60 years, heart failure, peripheral artery disease, hospitalization serum creatinine >0.8 mg/dL and hospitalization serum albumin <3.7 mg/dL.
Intra-operative
Hemorrhage, cardiopulmonary bypass time >130 min and fluid balance >4000 mL.
Post-operative
Low cardiac output, intra-aortic balloon pump use, AKI diagnosis and AKI duration >3 days.
The following factors remained as independent risk factors for long-term renal dysfunction development in the multivariate logistic regression model: age >60 years, hospitalization serum creatinine >0.8 mg/dL, peripheral artery disease, hemorrhage and AKI duration >3 days (Table 3).
Discussion
This study demonstrates that incident CKD is common in AKI patients following cardiac surgery and is strongly associated with the duration of AKI episode. To our knowledge, the association between the duration of an AKI episode and progression to CKD has not been previously described in cardiac surgery patients.
In large general population samples, retrospective analysis found that AKI development was associated with a significant risk for late CKD development in patients with preserved baseline renal function. In cardiac surgery patients, Ishani et al. found a greater risk of incident CKD associated with small post-operative increases in serum creatinine (HR 2.1 for incident CKD associated with an increase in serum creatinine of 25 %) [4]. In our study, the incidence of CKD after 12 months of follow-up in AKI patients with Egfr >60 mL/min/m2 at baseline was 25 %, a percent similar to previous studies. The novelty of this study is the finding that AKI duration >3 days was an independent risk factor for increasing incident CKD (HR of 13) in these patients. Similarly, Uchino et al. assessed the association of AKI duration and death in critically ill patients and demonstrated that those with sustained AKI (>3 days) had a sixfold increase in risk of mortality compared with patients without AKI [14].
Our findings are in agreement with recent experimental data demonstrating that AKI can evolve to CKD in different animal models [15]. Recently, Bonventre et al redefined the role of the surviving epithelials cells after an episode of AKI, where the proximal tubule response is impaired with its proliferative response altered due to cell cycle arrest at the G2/M phase of the cell cycle, resulting in generation of profibrotic factors including cytokines, growth factors and matrix proteins [16]. Similarly, Golestaneh et al describes the concept of “uremic memory”, where permanent pathologic changes can be identified after an episode of AKI, with interstitial fibrosis and vascular denudation representing some histologic imprints of a past episode of AKI [15]. The assessment of biomarkers of structural renal tubular injury can predict progression to more severe stages of AKI in the early post-operative period after cardiac surgery and identify patients at risk of progression to CKD, further strengthening the relationship between anatomic alterations, AKI diagnosis and progression to CKD [17–21].
In the clinical field the key question to answer is at what point does decreased renal perfusion becomes irreversible and definitive in its insult to the tubules after AKI? The present results suggest that the duration of AKI episode may be one of the factors related to this adverse outcome. Recently, Brown et al. confirmed that the duration of AKI after cardiac surgery was directly proportional to long- term mortality, but the authors did not assess CKD progression in these patients [22]. In this study, we identified that the duration of AKI was a stronger predictor of CKD development than traditional risk factors, such as diabetes and hypertension.
The current findings stoutly reinforce the conception that all efforts should be made to correct reversible causes of kidney dysfunction in the installation phase of cardiac surgery-associated AKI, to avoid frank acute tubular necrosis development, which will impact in the subsequent incident CKD in long-term follow-up.
Whether non-modifiable factors such as age and gender are independently predictive of CKD after an AKI episode remains uncertain. Schiffl et al. reported results of a multivariate analysis that failed to demonstrate that age, gender, comorbidities, severity of illness at ICU admission, cause of AKI and mode or duration of renal replacement therapy were independent predictors of renal recovery at 1 year follow-up [23]. In our study, age >60 years was an independent predictor of CKD development after cardiac surgery, likely because of reduced renal function reserve and reduced tolerance to long-term kidney function impairment observed after an AKI episode.
This study presents some limitations. It was carried out in a single center with a large experience in cardiac surgery, which may not reflect the conditions of other institutions, since the clinical course of patients may be related to the number of surgeries performed in each center. Another limitation refers that only patients submitted to elective cardiac surgery were studied, which make uncertain the validity of these conclusions to AKI patients in other settings and post-operative fluid accumulation can determine inadequate AKI diagnosis based on serum creatinine alone [24]. Finally, we did not have data for 12-month follow-up in 370 patients. It should be noted that the pre and intra-hospital data from these patients were similar to the studied sample (data not shown).
In summary, patients who develop AKI following cardiac surgery presented high prevalence of long-term CKD. The duration of AKI was a strong independent risk factor for this late incident CKD development. Recognition of predictive factors for CKD development following cardiac surgery-associated AKI may help to develop strategies to prevent or halt CKD progression in this population.
References
Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA (2008) Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant 23(6):1970–1974
Machado MN, Nakazone MA, Maia LN (2014) Acute kidney injury based on KDIGO (Kidney Disease Improving Global Outcomes) criteria in patients with elevated baseline serum creatinine undergoing cardiac surgery. Rev Bras Cir Cardiovasc 29(3):299–307
Chawla LS, Amdur RL, Shaw AD, Faselis C, Palant CE, Kimmel PL (2014) Association between AKI and long-term renal and cardiovascular outcomes in united states veterans. Clin J Am Soc Nephrol 9(3):448–456
Ishani A, Nelson D, Clothier B, Schult T, Nugent S (2011) The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med 171(3):226–233
Ponte B, Felipe C, Muriel A, Tenorio MT, Liano F (2008) Long-term functional evolution after an acute kidney injury: a 10-year study. Nephrol Dial Transplant 23(12):3859–3866
Schiffl H, Lang SM, Fischer R (2012) Long-term outcomes of survivors of ICU acute kidney injury requiring renal replacement therapy: a 10-year prospective cohort study. CKJ Clin Kidney J 5(4):297–302
Chalmers J, Mediratta N, McShane J, Shaw M, Pullan M, Poullis M (2013) The long-term effects of developing renal failure post-coronary artery bypass surgery, in patients with normal preoperative renal function. Eur J Cardiothorac Surg 43(3):555–559
Cooper DS, Claes D, Goldstein SL, Bennett MR, Ma Q, Devarajan P (2016) Follow-up renal assessment of injury long-term after acute kidney injury (FRAIL-AKI). Clin J Am Soc Nephrol 11(1):21–29
Gameiro J, Neves JB, Rodrigues N, Bekerman C, Melo MJ, Pereira M (2016) Acute kidney injury, long-term renal function and mortality in patients undergoing major abdominal surgery: A cohort analysis. Clin Kidney J 9(2):192–200
Helgadottir S, Sigurdsson MI, Palsson R, Helgason D, Sigurdsson GH, Gudbjartsson T (2016) Renal recovery and long-term survival following acute kidney injury after coronary artery surgery: a nationwide study. Acta Anaesthesiol Scand 60(9):1230–1240
Pickering JW, Frampton CM, Endre ZH (2009) Evaluation of trial outcomes in acute kidney injury by creatinine modeling. Clin J Am Soc Nephrol 4(11):1705–1715
Pistolesi V, Di Napoli A, Fiaccadori E, Zeppilli L, Polistena F, Sacco MI (2016) Severe acute kidney injury following cardiac surgery: short-term outcomes in patients undergoing continuous renal replacement therapy (CRRT). J Nephrol 29(2):229–239
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG (2007) Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11(2):R31
Uchino S, Bellomo R, Bagshaw SM, Goldsmith D (2010) Transient azotaemia is associated with a high risk of death in hospitalized patients. Nephrol Dial Transplant 25(2):1833–1839
Golestaneh L, Melamed ML, Hostetter TH (2009) Uremic memory: the role of acute kidney injury in long-term outcomes. Kidney Int 76(8):813–814
Yang L, Humphreys BD, Bonventre JV (2011) Pathophysiology of acute kidney injury to chronic kidney disease: maladaptive repair. Contrib Nephrol 174:149–155
Koyner JL, Garg AX, Coca SG, Sint K, Thiessen-Philbrook H, Patel UD (2012) Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol 23(5):905–914
McIlroy DR, Wagener G, Lee HT (2010) Neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery: the effect of baseline renal function on diagnostic performance. Clin J Am Soc Nephrol 5(2):211–219
Parikh CR, Thiessen-Philbrook H, Garg AX, Kadiyala D, Shlipak MG, Koyner JL (2013) Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of aki after cardiac surgery. Clin J Am Soc Nephrol 8(7):1079–1088
Pilarczyk K, Edayadiyil-Dudasova M, Wendt D, Demircioglu E, Benedik J, Dohle DS (2015) Urinary [TIMP-2]*[IGFBP7] for early prediction of acute kidney injury after coronary artery bypass surgery. Ann Intensive Care 5(1):50
Alge JL, Arthur JM (2015) Biomarkers of AKI: a review of mechanistic relevance and potential therapeutic implications. Clin J Am Soc Nephrol 10(1):147–155
Brown JR, Kramer RS, Coca SG, Parikh CR (2010) Duration of acute kidney injury impacts long-term survival after cardiac surgery. Ann Thorac Surg 90(4):1142–1148
Schiffl H (2006) Renal recovery from acute tubular necrosis requiring renal replacement therapy: a prospective study in critically ill patients. Nephrol Dial Transplant 21(5):1248–1252
Macedo E, Bouchard J, Soroko SH, Chertow GM, Himmelfarb J, Ikizler TA (2010) Fluid accumulation, recognition and staging of acute kidney injury in critically-ill patients. Crit Care 14(3):R82
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
For this type of study formal consent is not required. The ethical Institutional Review Board approved the study and waived informed consent due to the pure observational character of the study.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Emmanuel A. Burdmann and Luis Yu share the senior authorship of this manuscript.
Rights and permissions
About this article
Cite this article
Palomba, H., Castro, I., Yu, L. et al. The duration of acute kidney injury after cardiac surgery increases the risk of long-term chronic kidney disease. J Nephrol 30, 567–572 (2017). https://doi.org/10.1007/s40620-016-0351-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-016-0351-0