Abstract
Background
Hyperphosphatemia is a common complication in dialysis patients that can be treated by oral phosphate binders. We investigated the efficacy and safety of oral ferric citrate as a phosphate binder for Taiwanese patients with end stage renal disease and with hyperphosphatemia who were undergoing hemodialysis.
Methods
This was a prospective, double-blind, placebo-controlled, randomized trial carried out in 5 hospitals in Taiwan. Ferric citrate (4 or 6 g/day) or placebo was administered for 56 days. Serum calcium, phosphorous levels, calcium × phosphorus product, serum ferritin level, transferrin saturation, and adverse events were recorded.
Results
A total of 166 patients completed the trial. The placebo group had relatively constant serum data. Serum phosphorus declined significantly in the 6 g/day group (p < 0.05 for 4 and 8 weeks) and the 4 g/day group (p < 0.05 for 4 and 8 weeks). There were similar changes in the calcium × phosphorus product. The serum ferritin level increased significantly in the 6 g/day group (p < 0.05) and the 4 g/day group (p < 0.05). There were similar changes in transferrin saturation. Most adverse events were mild to moderate and were comparable among the three groups.
Conclusions
A 56-day treatment with ferric citrate effectively controlled hyperphosphatemia and was well tolerated in maintenance hemodialysis patients. There were also moderate increases in serum ferritin and transferrin saturation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global burden of chronic kidney disease (CKD) has increased dramatically, and a recent study attributed 735,000 world-wide deaths per year to end-stage renal disease (ESRD) [1]. As of 2010, Taiwan had the highest prevalence of ESRD (2,584 patients per million population [pmp]), followed by Japan (2,260 pmp), and the United States (1,876 pmp) [2]. Demographic factors, especially the aging population and the rising incidence of diabetes and hypertension, have contributed to the increasing prevalence of ESRD [2, 3].
Hyperphosphatemia is a common complication of ESRD due to the reduced renal elimination of phosphate [4]. Untreated, hyperphosphatemia can lead to secondary hyperparathyroidism, decreased bone density, and most significantly to cardiovascular calcification and cardiovascular disease (CVD) [4, 5]. CVD is a major cause of death in dialysis patients [6, 7]. Hemodialysis (HD) and peritoneal dialysis (PD) provide only limited removal of phosphorus, so most dialysis patients are given oral phosphate binders to prevent intestinal absorption and maintain serum phosphorus at the recommended level of 3.5–5.5 mg/dl [8].
Several classes of drugs are used to control hyperphosphatemia, all of which act by decreasing phosphate absorption by the intestine [9–11]. Aluminum-based drugs are not recommended for long-term use because they are associated with bone toxicity, neurological damage, and other toxicities. Calcium-based drugs can lead to hypercalcemia, vascular calcification, and other complications. Sevelamer and lanthanum are more modern phosphate binders that do not contain calcium or aluminum, but these drugs are more expensive, and are also associated with certain adverse effects [11].
Ferric citrate is an iron-based drug currently under investigation for its efficacy and safety in the control of hyperphosphatemia in patients undergoing dialysis. A study has indicated that the phosphorus-lowering effect of ferric citrate is comparable to that of calcium carbonate [12]. Published studies of Asian and Western populations have documented the safety and efficacy of different doses of ferric citrate [12–15], but these studies had only a 28-day duration. One recently published study compared JTT-751, a phosphate binder with ferric citrate as the active ingredient, with sevelamer in patients undergoing HD with a treatment period of 12 weeks, and reported that JTT-751 was not inferior as regards changes in serum phosphate at the end of treatment, and that changes in serum calcium and parathyroid hormone (PTH) were similar between the groups [16]. In the present study, we assessed the efficacy and safety of 56 days of oral ferric citrate at 4 and 6 g/day as treatment for hyperphosphatemia in maintenance HD patients from five medical centers in Taiwan.
Subjects and methods
Patient eligibility and enrollment
Eligible subjects were ≥18 years of age, had been receiving HD thrice weekly for at least 3 months, were on a stable dose (change ≤25 %) of a phosphate-binding agent for at least 1 month prior to study entry, had a urea reduction ratio (URR) >65 % within the 4 weeks prior to screening, hematocrit >20 %, and a serum calcium level of 8–10.5 mg/dl. Subjects given vitamin D supplementation or calcitriol were on stable doses (change ≤25 %) for at least 1 month prior to study entry, and all potentially childbearing women had negative pregnancy tests and used contraception during the study. The exclusion criteria were: pregnancy, lactating, gastrointestinal abnormality, tertiary hyperparathyroidism, congestive heart failure, diabetes mellitus with clinically relevant gastroparesis, unstable medical or psychiatric condition, clinically significant abnormality on screening ECG, active malignancy other than basal cell or squamous cell carcinoma, serum ferritin >800 ng/ml, history of iron allergy or hemochromatosis, or treatment with an investigational agent within 30 days of enrollment.
This study enrolled patients from five medical centers in Taiwan (Chang Gung Memorial Hospital, Kaohsiung Chang Gung Medical Hospital, Keelung Shin Kong Wu Ho-Su Memorial Hospital, Far Eastern Memorial Hospital, and Taichung Veterans General Hospital), and was conducted in compliance with the Declaration of Helsinki and the Guidelines for Good Clinical Practice of the International Conference on Harmonization. The study was approved by the Taiwan Food and Drug Administration and the local Institutional Review Boards. All subjects provided written informed consent. The study was registered at ClinicalTrials.gov; identifier: NCT01503736.
Study design
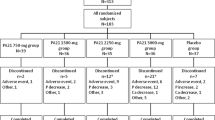
This was a randomized, double-blind, placebo-controlled, dose-ranging, multi-center, Phase III study to assess the effect of oral ferric citrate on serum phosphorus concentration in subjects with ESRD on maintenance hemodialysis. All subjects had serum phosphorus levels of 5.5–10 mg/dl after a 1- to 2-week washout period (during which all phosphate-binding agents were discontinued). Subjects were randomized in a 1:2:2 ratio to receive placebo, ferric citrate at 4 g/day, or ferric citrate at 6 g/day (Fig. 1). The medication (500 mg capsules tid) were given with meals for 8 weeks. Each capsule contains 105 mg elemental ferric iron, and this is equivalent to a daily dose of 840 mg elemental ferric iron in the 4 g/day group, and 1,260 mg elemental ferric iron in the 6 g/day group. Subjects with serum phosphorus levels of ≥9 mg/dl at two consecutive measurements after randomization were withdrawn from the study, and instructed to resume their pre-study medications. Subjects with transferrin saturation (TSAT) levels of ≥55 % were also withdrawn from the study.
Patient compliance was assessed at week 4 and week 8 (or the final visit). Daily intake of calcium and phosphorus was evaluated by a dietician during the screening period, and subjects were instructed to avoid changes in their diet during the study.
Determination of sample size
The results of our prior study [17] suggested that measurements of serum phosphorus concentration in our patient population could be assumed to have a standard deviation (SD) of 1.6 mg/dl. In that study, the 4 week decrease of serum phosphorus was approximately 1.0 mg/dl in the 4 g/day group and 1.5 mg/dl in the 6 g/day group (relative to placebo). Based on these assumptions, and a desired 80 % power to detect a significant difference between the 4 g/day and placebo groups at a 0.05 significance level, 155 efficacy-evaluable subjects were required to be randomized in a 1:2:2 ratio into three groups (placebo: n = 31; 4 g/day: n = 62; 6 g/day: n = 62). This sample size provided at least 80 % power for comparison of the 6 g/day and placebo groups. Assuming an approximate 15 % reduction from randomized subjects to efficacy evaluable subjects, it was estimated that 190 subjects would need to be randomized in order to have at least 155 efficacy evaluable subjects.
Concomitant therapy
From the start of washout to the end of the study, all phosphate binding agents and other medications that could have potentially affected serum phosphorus or calcium concentrations were prohibited. Medications containing minimal amounts of aluminum, calcium, phosphorus, or magnesium, or used at a dose that would not interfere with phosphorus or calcium absorption, were allowed. From the start of washout until the end of the study, no iron-containing medications, and no oral or intravenous iron therapy was allowed. Vitamin D analogs were permitted during the study; however, the use and dose had to remain constant throughout the study. All patients were required to maintain their dialysis treatment at three times per week throughout the study.
Efficacy and safety assessment
The primary efficacy endpoint was change in serum phosphorus level at week 8 compared with placebo. The secondary efficacy endpoints were changes in serum phosphorus level at week 4, and in the calcium × phosphorus product at week 4 and week 8. Safety was evaluated based on the incidence of adverse events (AEs), serious AEs (SAEs), changes in hematological and biochemical laboratory parameters (including serum calcium and iron parameters), and other data including vital signs and ECG data. Each subject in the efficacy population had a pre-dose measurement, and at least one day-14 or later measurement of serum phosphorus. The safety population consisted of subjects who received at least one dose of the study medication.
Statistical analysis
Categorical data were presented as counts and percentages. Continuous data with a normal distribution were presented as mean ± SD, and continuous data with a skew distribution were presented as median and interquartile range. Data of the 3 treatment groups were compared with one-way ANOVA (continuous data with normal distribution), Kruskal–Wallis test (continuous data with skew distribution), and Fisher’s exact test (categorical data), and changes from baseline to week 8 of laboratory parameters with a normal distribution were assessed with an analysis of covariance (ANCOVA) model, with the baseline value as the covariate. Changes between two time points in each treatment group were tested by the paired t test for normally distributed data and by the Wilcoxon signed ranks test for non-normally distributed data. Analyses of dose–response effects were performed using a linear mixed model. The method of least significant difference (LSD) was used in cases of multiple comparisons. All statistical analyses were performed with SAS version 9.1.3 (SAS Institute, Inc., Cary, NC, USA), and the 2-sided significance level was set at 0.05.
Results
Patient characteristics and disposition
A total of 183 eligible subjects were randomized to 1 of 3 treatment groups. There were 113 males and 70 females with a mean age of 54.3 years, and these 183 subjects were used for the safety evaluation (safety population). The efficacy population consisted of 166 patients who had a pre-dose and at least one day-14 or later measurement of serum phosphorus. Twenty-four, 9, and 18 patients in the placebo, ferric citrate 4 g/day, and ferric citrate 6 g/day group terminated early the study; the reasons for early termination are summarized in Table 1. The dropout rate in the placebo group (66.7 %) was higher than that in the 4 and 6 g/day ferric citrate groups (12.0 and 25.0 %). Most of the early dropouts in the placebo group were due to voluntary withdrawal (Table 1).
The overall demographic and baseline characteristics of the efficacy and safety populations were similar (data not shown), and there were no significant differences in the baseline characteristics of the three treatment groups in the safety population (Table 2). There was no significant difference in dietary calcium and phosphorus intake among the three groups. Mean compliance rates were >80 % in the ferric citrate 6 and 4 g/day treatment groups expect for subjects who withdrew early, and were similar for each treatment period and between both treatment groups. Lower mean compliance rates, ranging from 70.9 to 77.8 %, were seen in the placebo group. Throughout the study, no clinically important changes in body weight or nutritional parameters, including serum albumin and total serum protein, were observed in the three groups.
Efficacy evaluation
Serum phosphorus
During the 8-week study period, the mean serum phosphorus concentration remained high in the placebo group (range 7.37–7.65 mg/dl) compared to the two ferric citrate groups, in which serum phosphorus declined rapidly at week 1 and remained low for the following 7 weeks (Fig. 1; Table 3). At baseline, there was no significant difference among the groups (p > 0.05), the serum phosphorus levels in the placebo, 4 and 6 g/day groups being 7.37 ± 1.26, 6.96 ± 1.08 and 6.95 ± 1.15 mg/dl, respectively. At week 8, serum phosphorus was 7.42 ± 1.89, 5.38 ± 1.45 and 4.69 ± 1.25 mg/dl, with a mean change from baseline of 0.08, −1.60 and −2.27 mg/dl, in the placebo, 4 and 6 g/day groups, respectively. The mean change from baseline in both ferric citrate groups was significantly different compared to that of the placebo group (p < 0.001). Analysis of these data by a linear mixed model with adjustments for age, weight, and baseline serum phosphorus showed a significant dose–response effect; a lower serum phosphorus concentration was associated with a higher dose of ferric citrate (p < 0.001). The percentage of patients reaching the treatment target of phosphorus ≤5.5 mg/dl at week 8 was 16.7, 57.6, and 74.1 % in the placebo, 4 and 6 g/day groups, respectively.
Change in serum calcium
There were minor increases in the serum calcium concentration in all groups (range 0.15–0.18 mg/dl). The increases in both ferric citrate groups were not statistically significant relative to the placebo group, but they were statistically significant relative to the baseline at week 4 and week 8. The minor increases in the ferric citrate groups were not considered clinically significant, and the serum calcium concentration remained within the normal range.
Changes in serum ferritin and TSAT
Ferritin and TSAT levels showed a trend to increase in both ferric citrate groups (Fig. 2a, b). At week 8, the change from baseline level of ferritin in the ferric citrate groups was significantly different from that of the placebo group (median −41.75, 73.90, and 103.40 ng/ml for the placebo, 4 and 6 g/day groups, respectively); while no significant changes were found in TSAT compared to placebo group (Table 4). In addition, the increase of TSAT appeared to be slower in the second month of treatment. From week 4 to week 8, the changes of TSAT were fairly small in the ferric citrate groups (2.4 and 0.48 % in the 4 and 6 g/day groups, respectively).
Trends of ferritin (a), TSAT (b), hemoglobin (c), and bicarbonate (d) over the 8 week study period. Data are presented as median (inter-quartile range). *Significant difference compared to the placebo group: p = 0.034 and 0.017 for week 4 TSAT in the ferric citrate 4 and 6 g/day groups, respectively; p = 0.028 for week 8 bicarbonate in the ferric citrate 6 g/day group. ‡Significant difference compared to baseline within a group: p < 0.001 for the ferritin level of ferric citrate 4 and 6 g/day groups, TSAT level of the ferric citrate 4 g/day group, and hemoglobin level of the ferric citrate 6 g/day group, for both week 4 and week 8; p = 0.002 and 0.003 for week 4 and week 8, respectively, TSAT of the ferric citrate 6 g/day group. §Significant difference compared to week 4 within a group: p = 0.015 and 0.004 for week 8 ferritin within the ferric citrate 4 and 6 g/day groups, respectively. TSAT transferrin saturation
Eight subjects were withdrawn as a result of TSAT levels >55 % (2, 3, and 3 in the placebo, 4 and 6 g/day groups, respectively). Three subjects in the 6 g/day group showed high serum ferritin values that were clinically significant, but none of these changes necessitated study withdrawal. No other iron-related test values or iron-related safety issue led to discontinuation of treatment.
Changes in serum hemoglobin and bicarbonate
The changes of hemoglobin from baseline to week 8 were not different between the three groups (p = 0.104, Table 4). However, as compared to baseline, the hemoglobin level in the 6 g/day group showed a significant increase at week 4 and week 8 (median 11.1 g/dl at week 4 and 11.3 g/dl at week 8 vs. 10.6 g/dl at baseline, p ≤ 0.001 for both) (Fig. 2c).
The changes of bicarbonate from baseline to week 8 were not different between the three groups (p = 0.365, Table 4), and the bicarbonate level of each group did not change significantly with time. However, the ferric citrate 6 g/day group showed an increase in bicarbonate level compared to the placebo group at week 8 (median 23.75 vs. 21.8 mmol/l, p = 0.028, Fig. 2d). Trends of ferritin, TSAT, hemoglobin, and bicarbonate over the 8-week study period are summarized in the Supplemental Table.
Safety evaluation
Physical examinations, clinical and laboratory evaluations, vital signs, and ECG
There were no deaths during the study. In the placebo group, there were no significant changes in clinical parameters related to patient safety. In the ferric citrate groups, there were no clinically significant changes in vital signs, physical examination, or ECG that appeared related to ferric citrate administration. A total of 16 subjects (1 in the placebo group, 4 in the 4 g/day group, and 11 in the 6 g/day group) had laboratory values that were clinically significant after study onset, but not at baseline. None of these values were reported as an AE or SAE, with the exception of two subjects with low serum phosphorus concentrations and 1 with low hemoglobin (all in the 6 g/day group). These three cases were reported as AEs, which led to drug withdrawal, and which were classified as related to treatment (data not shown).
Adverse events
Four subjects in the 6 g/day group and 1 in the 4 g/day group experienced a total of seven SAEs, all of which were considered unlikely to be related to ferric citrate treatment. Twelve subjects (3 in the placebo group, 2 in the 4 g/day group, and 7 in the 6 g/day group) terminated participation because of AEs. There was no discernible relationship between dose and type of AE that led to withdrawal.
The most common AEs were gastro-intestinal (GI) disorder, occurring in 27.8, 61.3 and 58.3 % of the placebo, 4 and 6 g/day groups, respectively. Table 5 shows treatment-related AEs in the three groups. Most of treatment-related AEs in the ferric citrate groups were discolored feces (ferric 37.3 and 37.5 % in the 4 and 6 g/day groups), followed by diarrhea (6.7 and 4.2 % in the 4 and 6 g/day groups). In the placebo group, five treatment-related AEs were reported; two cases of discolored feces, two cases of diarrhea, and one case of abdominal pain. Overall, ferric citrate was well tolerated at both doses.
Discussion
Our study demonstrated that administration of oral ferric citrate at 4 or 6 g/day for 56 days effectively and safely reduced the serum phosphorus levels of patients with ESRD on maintenance hemodialysis. Patients given a dose of 6 g/day had significantly lower serum phosphorus than those given 4 g/day. In addition, ferric citrate also led to moderate increases in serum ferritin and TSAT, indicating improved iron storage, a potential benefit for patients with CKD who often are anemic [18]. Most patients tolerated the study drug well, and the most common treatment-related AEs were gastrointestinal complaints.
Management of hyperphosphatemia is critically important for the control of bone and mineral disorders in CKD, and previous studies have confirmed that an increased level of serum phosphorous is associated with poor long-term outcome [4–7]. However, it is usually difficult to achieve the recommended serum phosphorus level of 3.5–5.5 mg/dl. Dietary phosphate restriction typically provides just a minor contribution to phosphorus control [19]. Previous animal studies showed that ferric citrate decreased intestinal phosphate absorption in normal and uremic rats [20].
In this clinical study, ferric citrate administration of 4 g and 6 g/day lowered serum phosphorus by 1.60 and 2.27 mg/dl in a Taiwanese population. The results of this clinical study are in line with those of prior studies and show that ferric citrate provides a consistent and stable phosphorus lowering effect throughout an 8-week time frame [12–15]. In another 4-week study in the United States, ferric citrate of 1, 6 g, and 8 g/day lowered serum phosphorus by 0.1, 1.9, and 2.1 mg/dl, respectively [13]. It has been found that patients in Asian countries seem to require lower doses of ferric citrate to achieve the target serum phosphorus level of 3.5–5.5 mg/dl than patients from Western countries. This may be due to the lower average body weight, and lower dietary phosphate intake of patients from Asian countries.
Unlike calcium-based phosphate binders, ferric citrate does not appear to notably affect serum calcium or to induce hypercalcemia [13]. In our study, there were minor increases in the serum calcium level in the ferric citrate groups, but they were not significantly different from those of the placebo group. The simultaneous change of calcium and phosphate should take serum PTH level into account. However, PTH was not measured in this study. Future studies should monitor PTH to identify how ferric citrate affects the balance of calcium, phosphorus, and PTH, and its effect on renal-associated osteopathy.
Patients with CKD on maintenance dialysis typically develop iron deficiency, a common contributing cause of anemia. Continued blood loss associated with routine lab tests and regular dialysis procedures also contribute to iron deficiency. Correction of iron deficiency through iron replacement by oral or intravenous supplementation is essential for optimization of erythropoiesis therapy.
In this study, we observed increases in ferritin and TSAT level, and a small trend to increased hemoglobin levels in the ferric citrate groups, which may be due to partial absorption of ferric citrate. Iron absorption is a tightly regulated process that occurs in the duodenal enterocyte. The process is carefully regulated by factors such as total iron stores and hemoglobin level, and it is down-regulated during inflammation. This regulation system in the human body may explain the gradual rises in serum ferritin and TSAT in the ferric citrate treatment groups in this study, and why the TSAT level showed a trend to reach a plateau at the second month of treatment. From a safety point of view, there were three out of 147 (2 %) subjects in the ferric citrate groups who had an elevated serum ferritin level that was considered clinically significant, but none of them had to be withdrawn for safety reasons. Eight subjects were withdrawn as a result of TSAT levels >55 %, which included 2 out of 36 (5.6 %) subjects in the placebo group, and 6 out of 147 (4.1 %) in the ferric citrate groups. No other subject discontinued due to iron-related safety concerns. It should be noted that serum ferritin and TSAT are known to be affected by several factors other than iron increase, including inflammation and nutrition status. For iron management in CKD patients with anemia, the Kidney Disease/Improving Global Outcomes (KDIGO) guidelines suggest goal-directed intravenous iron use for a TSAT ≤30 % and serum ferritin ≤500 ng/ml [21]. Currently, an evidence-based standard for the upper limit of serum ferritin is not available. International treatment guidelines generally accept an upper limit of serum ferritin values as high as 500–1,000 ng/ml [22]. In this 8-week study, the increases of ferritin and TSAT were considered modest as these iron parameters are generally within normal limits for dialysis patients. Consistent with previous studies, we did not observe signs of iron toxicity during the treatment period. In the clinical settings, as both serum phosphate and iron are routinely tested, use of ferric citrate can be carefully titrated to minimize the risk of iron overload.
Overall, our patients exhibited good tolerance to ferric citrate with nearly 82 % of them completing the study. Not surprisingly, the most common adverse effects of ferric citrate were stool discoloration and mild to moderate gastrointestinal problems, which are also common with other drugs used to control hyperphosphatemia. On the contrary, there was a high withdrawal rate of 66 % in the placebo group due to voluntary withdrawal. This was likely because the subjects experienced no change in serum phosphorus level with the placebo treatment. Further large-scale and long-term studies are needed to confirm the efficacy and safety of ferric citrate for the treatment of hyperphosphatemia and its potential benefit for iron-deficiency anemia.
Conclusions
In conclusion, during the 8-week treatment period, it was demonstrated that ferric citrate was safe and effective for the control of serum phosphorus in a dose-dependent manner in CKD patients on maintenance HD. Ferric citrate treatment was also associated with a moderate increase in ferritin and TSAT, suggesting that it might also be beneficial for anemia in these patients. Ferric citrate may be a beneficial alternative for the treatment of hyperphosphatemia.
References
Lozano R, Naghavi M, Foreman K et al (2012) Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2095–2128
US Renal Data System (2013) USRDS 2013 Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda
Thodis ED, Oreopoulos DG (2011) Home dialysis first: a new paradigm for new ESRD patients. J Nephrol 24:398–404
Coladonato JA (2005) Control of hyperphosphatemia among patients with ESRD. J Am Soc Nephrol 16(Suppl 2):S107–S114
Qunibi WY (2004) Consequences of hyperphosphatemia in patients with end-stage renal disease (ESRD). Kidney Int Suppl 90:S8–S12
Kusano E (2011) Mechanism by which chronic kidney disease causes cardiovascular disease and the measures to manage this phenomenon. Clin Exp Nephrol 15:627–633
Miller LM, Sood MM, Sood AR et al (2010) Cardiovascular disease in end-stage renal disease: the challenge of assessing and managing cardiac disease in dialysis patients. Int Urol Nephrol 42:1007–1014
Qunibi W, Kalantar-Zadeh K (2011) Target levels for serum phosphorus and parathyroid hormone. Semin Dial 24:29–33
Gutiérrez OM, Wolf M (2010) Dietary phosphorus restriction in advanced chronic kidney disease: merits, challenges, and emerging strategies. Semin Dial 23:401–406
Tonelli M, Pannu N, Manns B (2010) Oral phosphate binders in patients with kidney failure. N Engl J Med 362:1312–1324
Hutchison AJ, Smith CP, Brenchley PE (2011) Pharmacology, efficacy and safety of oral phosphate binders. Nat Rev Nephrol 7:578–589
Yang WC, Yang CS, Hou CC, Wu TH, Young EW, Hsu CH (2002) An open-label, crossover study of a new phosphate-binding agent in haemodialysis patients: ferric citrate. Nephrol Dial Transplant 17:265–270
Dwyer JP, Sika M, Schulman G et al (2013) Dose-response and efficacy of ferric citrate to treat hyperphosphatemia in hemodialysis patients: a short-term randomized trial. Am J Kidney Dis 61:759–766
Sinsakul M, Sika M, Koury M et al (2012) The safety and tolerability of ferric citrate as a phosphate binder in dialysis patients. Nephron Clin Pract 121:c25–c29
Yokoyama K, Hirakata H, Akiba T, Sawada K, Kumagai Y (2012) Effect of oral JTT-751 (ferric citrate) on hyperphosphatemia in hemodialysis patients: results of a randomized, double-blind, placebo-controlled trial. Am J Nephrol 36:478–487
Yokoyama K, Akiba T, Fukagawa M et al (2014) A randomized trial of JTT-751 versus sevelamer hydrochloride in patients on hemodialysis. Nephrol Dial Transplant 29(5):1053–1060
Panion & BF Biotech, Inc. (2006) A randomized, double-blind, placebo-controlled, dose-ranging study of the effects of ferric citrate on serum phosphate in patients with end stage renal disease (ESRD). Panion & BF Biotech, Inc., Report No. PBB00101
Babitt JL, Lin HY (2012) Mechanisms of anemia in CKD. J Am Soc Nephrol 23:1631–1634
Calvo MS, Uribarri J (2013) Contributions to total phosphorus intake: all sources considered. Semin Dial 26:54–61
Hsu CH, Patel SR, Young EW (1999) New phosphate binding agents: ferric compounds. J Am Soc Nephrol 10:1274–1280
Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group (2012) KDIGO Clinical Practice Guideline for anemia in chronic kidney disease. Kidney Int 2(Suppl):279–335
Fishbane S (2008) Upper limit of serum ferritin: misinterpretation of the 2006 KDOQI anemia guidelines. Semin Dial 21:217–220
Acknowledgement
We would like to thank all participating investigators including Kuo CC, Wu CH, Ng HY, and Chiou YT (Chang Gung Memorial Hospital, Kaohsiung, Taiwan); Lee CC, Sun CY, Hsu HJ, and Tsai CJ (Chang Gung Medical Hospital, Keelung, Taiwan); Leu JG, and Lu LC (Shin Kong Wu Ho-Su Memorial Hospital, Taiwan); Hsu SP, Pai MF, Wu HY, and Chen HY (Far Eastern Memorial Hospital, Taiwan); Lin BS as the Medical Monitor, and Panion & BF Biotech Inc. as the sponsor of the study.
Conflict of interest
All authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
ClinicalTrials.gov; identifier: NCT01503736.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, CT., Wu, IW., Chiang, SS. et al. Effect of oral ferric citrate on serum phosphorus in hemodialysis patients: multicenter, randomized, double-blind, placebo-controlled study. J Nephrol 28, 105–113 (2015). https://doi.org/10.1007/s40620-014-0108-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-014-0108-6