Abstract
Background and aim
Dexamethasone Suppression Test (DST), recommended for Cushing’s Syndrome (CS) diagnosis, explores the pituitary feedback to glucocorticoids. Its diagnostic accuracy could be affected by dexamethasone bioavailability, and therefore, we have developed and validated a dexamethasone threshold after 1-mg DST.
Materials and methods
We studied 200 subjects: 125 patients were considered retrospectively and 75 were enrolled prospectively as the validation cohort. Serum dexamethasone, Late Night Salivary Cortisol (LNSC), and Urinary Free Cortisol (UFC) were measured with LC–MS/MS. Normal LNSC and UFC levels were used to exclude CS. The lower 2.5th percentile of dexamethasone distribution in non-CS patients with cortisol ≤ 50 nmol/L after 1-mg DST was used as threshold.
Results
16 patients were CS and 184 non-CS (108 adrenal incidentaloma and 76 excluded CS); 4.5 nmol/L resulted the calculated threshold. Cortisol after 1-mg DST confirmed high sensitivity (100% at 50 nmol/L cut-off) and moderate–low specificity (63%, increased to 91% at 138 nmol/L) to diagnose CS in the whole cohort of patients. We could reduce the number of false-positive results (from 10 to 6 and from 7 to 4 in AI and excluded CS) considering adequate dexamethasone levels. Dexamethasone levels were not affected by hypercortisolism, age, gender, smoke, weight, and creatinine. 6% of non-CS patients did not achieve adequate dexamethasone levels (40% of tests with serum cortisol > 138 nmol/L after 1-mg DST).
Conclusions
We developed and validated the routine dexamethasone measurement during 1-mg DST: it is independent from patient’s clinical presentation, and it should be used to increase the specificity of serum cortisol levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endogenous Cushing’s Syndrome (CS) is due to excessive and unregulated cortisol secretion [1, 2]. Its signs and symptoms are common among patients assessed for hypertension, diabetes, obesity, metabolic syndrome, osteoporosis, mood disorders, and so on [3,4,5]. However, CS is not frequent enough to support the use of routine screening in these high-risk conditions [4, 6, 7]. Moreover, the widespread use of abdominal scan leads to an increased incidental discovery of adrenal tumors (termed adrenal incidentaloma, AI) in up to 8–10% of geriatric patients, thus increasing the number of subjects to screen for CS, according to guidelines [8, 9]. In this scenario, diagnostic tests should present both high sensitivity (to discover a rare disease [10]) and high specificity (to avoid unnecessary and expensive further workup [11]).
The Endocrine Society’s Guidelines recommend to screen for CS using Late Nigh Salivary Cortisol (LNSC), Urinary Free Cortisol (UFC) or 1-mg Dexamethasone Suppression Test (1-mg DST) [1]. The high diagnostic accuracy of LNSC or UFC is well established [12,13,14]. Despite their similar sensitivity and specificity [10], fluctuations of cortisol production and secretion, measured by sequential LNSC or UFC, are common in CS [15, 16]. 1-mg DST is widely adopted in clinical practice: it represents one of the first choices among screening tests for hypercortisolism, is particularly useful in night-shift workers, and is considered the first test to define autonomous cortisol secretion in patients with AI [8, 9]. Unsuppressed serum cortisol (> 50 nmol/L) after 1-mg DST should be interpreted as a positive test [1, 10]; however, this cut-off provides a high diagnostic sensitivity and a moderate specificity [17], especially if other aspects of the hypothalamic–pituitary–adrenal (HPA) axis are not considered [18]. False-positive tests (especially considering 50 nmol/L as the cut-off) are a significant matter of concern, causing a burden to the healthcare system and to the patients, especially in those with AI. Besides autonomous cortisol secretion, the lack of specificity during 1-mg DST could be due to variable gastrointestinal absorption of dexamethasone, to its inter-individual differences in the metabolism, to interfering drugs, to decreased hypothalamic–pituitary sensitivity to glucocorticoids or to impaired renal function [19, 20]. Few studies investigated the bioavailability of serum dexamethasone in the routine setting, suggesting different threshold with different methods (old immunological assays as well as liquid chromatography with tandem mass spectrometry, LC–MS/MS) [20,21,22,23].
Therefore, our aims are to develop a method suitable for clinical purposes and to define the minimum level able to suppress cortisol after 1-mg DST.
Materials and methods
Patients
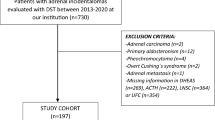
We considered a consecutive cohort of 200 patients referred to the Endocrinology Unit of Padova University-Hospital for suspected hypercortisolism between November 2017 and April 2019:
-
16 patients with confirmed CS (10 with pituitary-dependent Cushing’s Disease and 6 with cortisol-secreting adrenal adenoma). They presented both increased UFC and LNSC (respectively, > 168 nmol/24 h and > 2.6 nmol/L, in at least two collections [24, 25]).
-
108 patients with AI, discovered accidentally by abdominal imaging (computed tomography or magnetic resonance). As previously reported [17], patients with active malignancies or clear signs/symptoms of overt hypercortisolism (recent-onset easy bruising, facial plethora, proximal muscle weakness, or reddish purple striae > 1 cm wide) were excluded, as well as those with primary aldosteronism or pheochromocytoma.
-
76 subjects presented with medical conditions suggestive of hypercortisolism (termed excluded CS).
Normal LNSC and UFC levels (at least two collections) were used to rule out CS suspicion in all excluded CS and patients with AI.
To develop and validate the threshold, all patients taking drugs or medications that could affect dexamethasone bioavailability or metabolism were excluded from the study [26]. According to STARD (Standards for Reporting Diagnostic accuracy studies) criteria, we considered as reference standard the final diagnosis (CS, AI, or excluded CS) based on the above-mentioned criteria. The study was performed in accordance with the guidelines in the Declaration of Helsinki, the study was approved by the Ethics Committee of Padova University-Hospital, and all patients gave informed consent.
Serum dexamethasone measurement by LC–MS/MS
Dexamethasone (1 mg/mL), Dexamethasone 21-acetate (≥ 99% purity, internal standard, IS), and ammonium formate were supplied by Sigma-Aldrich (St. Louis, MO, USA). Formic acid and zinc sulphate heptahydrate were obtained from Carlo Erba (MI, Italy). LC–MS grade acetonitrile (Sigma–Aldrich, St. Louis, MO, USA) and Milli-Q organic free water (Millipore, Bedford, MA, USA) were used as HPLC solvents and for all solutions. Six-level calibrators are used for quantification of Dexamethasone (1.0, 2.5, 5.0, 10.0, 25.0 nmol/L). 100 µL of serum samples, calibrator, or control were added to 50-µL zinc sulphate 0.1 mol/L in a polypropylene micro-centrifuge tube; then, 250 µL of acetonitrile containing 4 µg/L IS were added. The tube was then mixed for 30 s. Finally, the tubes were centrifuged for 5 min at 16,600×g; 250 µL of the colourless supernatant was transferred into an appropriate HPLC vial and diluted with 250 µL of HPLC-grade water. A Waters UPLC-Acquity H class system (Milford, Massachusetts, USA) equipped with a binary pump, a vacuum degasser, auto-sampler, and a thermostated column compartment was used for chromatographic separation; the injection volume was 35 μL. An Acquity UPLC HSS C18 (1.8 µm; 2.1 × 150 mm; Waters) at 50 °C was used as the analytical column. Solvents A and B were 5-mM ammonium formate with 0.2% formic acid and acetonitrile with 0.1% formic acid, respectively. Mass spectrometric detection was carried out with a Waters TQD (Milford, Massachusetts, USA) and triple quadrupole detector with an electrospray ionization interface (ESI). The eluate was monitored by MS/MS detection set in multiple-reaction monitoring (MRM) mode. Hydrogen adducts of each analyte were used as precursor ions. In the positive-ion mode, two positive MRM transitions (m/z) were monitored for Dexamethasone and a single MRM transition was selected for Dexamethasone 21-acetate (IS) using optimized collision energies and cone voltage, as shown in Table 1. Further operative parameters were: source gas temperature 150 °C; desolvation gas temperature 450 °C, desolvation gas flow 800 L/h, cone gas flow 20 L/h, and capillary voltage 1.00 kV. MassLynx V 4.1 Software (Waters) was used for quantitative analysis. The assay was validated according to published acceptance criteria proposed by the Clinical and Laboratory Standards Institute (CLSI) [27]. Calibration curves were linear throughout the selected ranges (1–1000 nmol/L) and, in all cases (n = 10), the correlation coefficients (r2) were > 0.998. The lower limit of quantification was 1.0 nmol/L and corresponded to the lowest calibration point. No ion suppression was observed at the time of elution of either Dexamethasone or Dexamethasone 21-acetate. This effect was reproducible following the injection of different patient samples (n = 10) and water. Intra-assay imprecisions for three serum samples at 2.5, 5.0, and 10.0 nmol/L, repeated ten times in a single analytical run, were < 3.9%. Inter-assay CVs for the same three serum samples (repeated ten times in ten different runs) were < 5.3%. The recoveries’ percentage range of three serum samples spiked with 2.5, 5.0, and 10.0 nmol/L of Dexamethasone was 88–107%. All the obtained performance criteria meet the requirements of the CLSI method validation guideline [27].
Data and statistical analysis
We have performed Shapiro–Wilk test of normality to assess data distribution. Dexamethasone levels in the whole cohort did not present a symmetrically-skewed distribution (p < 0.001); however, considering the three groups, its levels distribution achieved normality only in CS (p = 0.073), remaining non-normal in excluded CS (p = 0.008) and in AI (p < 0.001). A normal distribution was reached only in CS also considering only those tests with adequate dexamethasone levels (p = 0.244). Therefore, since the majority of data deviate from normality, we adopted only non-parametric tests, and groups were compared by Chi-square test for categorical variables and by the Wilcoxon rank sum test for quantitative variables.
We considered an indirect sampling technique, according to the CLSI guidelines [28], to select those subjects to study to calculate the dexamethasone level able to suppress serum cortisol. We selected only those non-CS patients with adequate cortisol suppression (≤ 50 nmol/L) after 1-mg DST. Receiver Operating Curve (ROC) was computed to study Sensitivity (SE) and Specificity (SP) of serum cortisol after 1-mg DST. Moreover, we calculated the Likelihood Ratio (LR), to indicate how much the probability of having CS increases if the test is positive (LRpos), and how much this probability decreases if the 1-mg DST test is negative (LRneg), according to the Simel method and 95% Confidence Interval (95% CI) previously reported [29].
Statistical analysis was performed using the SPSS 17 software package (SPSS, Inc, Chicago, IL). The significance level was set at a p value < 0.05 for all the tests.
Results
Cut-off development and clinical picture
We considered 200 patients: 125 were analyzed to calculate the threshold (up to October 2018), while 75 were considered in the prospective part of the study (validation cohort).
The retrospective cohort consists in 15 CS and 110 non-CS patients (66 patients with AI and 44 excluded CS), considered as a single group of non-CS with normal UFC and LNSC levels (respectively, median 60 nmol/24 h and 1.4 nmol/L). The 2.5th percentile of dexamethasone level distribution after 1-mg DST in those non-CS subjects of the retrospective cohort with adequate (≤ 50 nmol/L) cortisol suppression was 4.5 nmol/L: it was considered as the threshold. The cut-off has been confirmed also in the prospective series, considering 2.5th percentile of dexamethasone distribution in both 114 non-CS patients with suppressed (≤ 50 nmol/L) serum cortisol after 1-mg DST and in the whole cohort of 184 non-CS patients, achieving a high SE (98%). Overall, as reported in Table 2, 94% of patients (187 out of 200 cases) revealed sufficient dexamethasone levels after 1-mg DST: 115 out of 125 cases (92% of tests) in the retrospective part of the study, and 96% in the prospective cohort (72 out of 75 tests).
The 97.5th percentile of dexamethasone distribution was 27 nmol/L: seven non-CS subjects had serum dexamethasone above this level (4 AI and 3 excluded CS), three presented cortisol after 1-mg DST ≤ 50 nmol/L; three patients with CS presented high dexamethasone concentration and unsuppressed cortisol. Other clinical or biochemical data were similar considering patients with dexamethasone levels < 27 nmol/L.
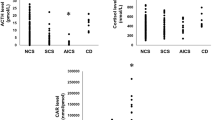
Serum dexamethasone levels were similar among CS, AI, and excluded CS (reported in Fig. 1), and the percentage of patients with adequate dexamethasone levels after DST was not different between the three groups (p = 0.404). As expected, patients with AI were older than CS and excluded CS. Overweight was one of the main reasons to refer for suspect hypercortisolism; however, BMI levels were similar in the cohort considered. Dexamethasone levels were not related to weight or BMI in the whole cohort of patients (p = 0.872 and p = 0.741), as well as in CS, AI, or in excluded CS (respectively p = 0.197, p = 0.5 and p = 0.277). Considering renal function, dexamethasone and creatinine levels were correlated in CS (regression line y = 0.6136x − 34.128; R2 = 0.5306; p = 0.007); this correlation was not confirmed in AI and excluded CS (respectively, p = 0.78 and 0.661), as well as in the whole cohort of subjects (p = 0.344). According to selection criteria, UFC and LNSC levels were higher in patients with CS than excluded CS and AI. Gender and active smoking did not affect dexamethasone levels (respectively, p = 0.459 and p = 0.524), also considering the three groups of patients.
Dexamethasone levels and diagnostic accuracy
In Table 3, we reported the result of tests according to the different cut-off levels for CS diagnosis (50 and 138 nmol/L) and to the dexamethasone level calculated as threshold. Overall, 11 non-CS patients (5 AI and 6 excluded CS, and 6% of all non-CS cohort) presented insufficient serum dexamethasone levels (< 4.5 nmol/L) after 1-mg DST. It is worth noting that four patients confessed a posteriori that they did not consume the prescribed oral dexamethasone dose (and their serum dexamethasone levels were undetectable). Regarding AI, 4 out of 5 patients revealed unsuppressed serum cortisol after 1-mg DST (> 138 nmol/L), suggesting a misleading autonomous cortisol secretion. In four cases (1 AI and 3 excluded CS), serum cortisol after 1-mg DST was suppressed (< 50 nmol/L) despite insufficient dexamethasone levels.
In the whole cohort, serum cortisol levels were not suppressed ≤ 138 nmol/L in 17 non-CS patients (7 excluded CS and 10 AI): in 7 cases (41%), their dexamethasone levels were insufficient. Overt CS was excluded with normal UFC and LNSC levels, and 1-mg DST was repeated in five cases (achieving adequate suppression in 3 and sufficient dexamethasone levels in 5).
The diagnostic accuracy (SE, SP, LRpos, and LRneg, in different groups) of serum cortisol levels after 1-mg DST was increased if we considered patients with sufficient dexamethasone levels (≥ 4.5 nmol/L), as summarized in Table 4. A significant LRpos value (> 10, depicting the high likelihood to be a CS if the test is positive) was achieved only in those with adequate dexamethasone levels and considering 138 nmol/L as the threshold for serum cortisol.
Discussion
Since 2008, the Endocrine Society’s Guidelines suggest to diagnose CS in suspected population using as screening tests free cortisol measurement (LNSC or UFC, with an accurate method as mass spectrometry, if available) or total serum cortisol after overnight suppression test (1-mg DST) [1]. Some experts proposed simultaneous measurement of both cortisol and dexamethasone to ensure adequate serum dexamethasone concentrations, to properly evaluate false-positive results [1, 20,21,22]. We considered two collections of UFC and LNSC to rule out overt CS, because high variability of cortisol levels is well known in patients with CS [15, 30], and we have previously validated in an independent cohort the diagnostic accuracy of our thresholds [24, 25].
We have developed and validated a threshold for serum dexamethasone measurement during 1-mg DST, in a series of patients with suspected hypercortisolism (AI and subjects with clinical features suggestive of cortisol excess). We have decided, according to CLSI guidelines, to consider an indirect sampling technique to create the cut-off [28], because our aim was to develop the threshold of serum dexamethasone able to suppress serum cortisol in case of suspected CS. To exclude a diagnostic bias, in all cases, CS was confirmed if both LNSC and UFC levels were increased, and the inclusion criteria of the cohort considered to calculate the cut-off (non-CS subjects with serum cortisol ≤ 50 nmol/L after 1-mg DST) were similar to another recent work [20]. We calculated a threshold based upon the 2.5th percentile of dexamethasone distribution in subjects without CS and adequate cortisol suppression (≤ 50 nmol/L after 1-mg DST), and then, we validated this cut-off in a prospective cohort, confirming its diagnostic accuracy, feasible in clinical practice and similar to that recently proposed with similar method and statistical analyses [20, 31]. Several different thresholds or reference ranges developed with an LC–MS method are proposed in the literature [20, 31, 32]; therefore, an effort to consider a harmonization study between laboratories is mandatory.
Besides cut-off validation, the gain in diagnostic accuracy achieved with dexamethasone measurement is effective, especially in specificity, therefore increasing LRpos. A screening test should be at least as sensitive as possible, to reduce the number of false-negative results and, therefore, misdiagnosis [10]; however, an acceptable specificity is mandatory [11], at least to reduce unnecessary tests. In our cohort, only patients with unsuppressed serum cortisol (> 138 nmol/L) and adequate dexamethasone levels present a high likelihood to be a CS. The reduced SP in patients with AI was not increased considering dexamethasone measurement, probably due to the subtle autonomous cortisol secretion [17]. Nevertheless, up to 40% of inadequate cortisol suppression after 1-mg DST with the high-specificity cut-off (138 nmol/L) in patients without CS could be explained with insufficient dexamethasone levels.
In our series, data regarding dexamethasone levels were not normally (skewed) distributed and not influenced by age, gender, smoking, or final diagnosis, suggesting that dexamethasone bioavailability is not affected by the patients’ cortisol levels, as previously reported [20]. Also renal function did not alter dexamethasone metabolism, and the observation that higher dexamethasone levels have been measured only in CS patients with high creatinine could be related to the protein catabolism and sarcopenia, common in hypercortisolism, rather than to chronic kidney disease [33]. Therefore, 1-mg DST should be used in all cases, irrespective of their clinical presentation.
Considering recent guidelines proposed by the European Society of Endocrinology [9], mainly based on the increased cardiovascular risk and mortality in patients with AI and autonomous cortisol secretion [34,35,36], the critical hormonal test to assess the integrity of HPA axis in patients with AI is cortisol after 1-mg DST. In our series, FOUR patients with AI presented unsuppressed serum cortisol levels after 1-mg DST (> 138 nmol/L), without reaching sufficient dexamethasone levels. Therefore, we excluded autonomous cortisol secretion on the basis of normal UFC and ACTH levels, as well as preserved cortisol rhythm (assessed with LNSC, despite its limited role to define subclinical hypercortisolism in AI [37,38,39]). The diagnosis of CS among patients with AI is a matter of concern [8, 40]. We have decided to define overt CS in case of both UFC and LNSC increased levels (their diagnostic accuracy for overt CS is well established [10]), because in the selection criteria, we did not consider 1-mg DST to define CS, to avoid a selection bias. Moreover, considering AI and autonomous cortisol secretion (the so-called subclinical hypercortisolism), serum cortisol after 1-mg DST could be not adequately suppressed (< 50 nmol/L) or completely unsuppressed (< 138 nmol/L) in 10–40% of cases [8, 17, 20]; therefore, the role of 1-mg DST test to define overt CS in patients with AI has yet to be defined.
Some patients (n = 4) presented an unexpected result: suppressed serum cortisol after 1-mg DST with an insufficient dexamethasone level. We could speculate that the glucocorticoid receptor (GR) activity may be at least in part related to the different clinical behavior of patients with CS [41], as well as the response of the HPA axis to dexamethasone. In particular, some polymorphisms of the GR gene (BclI, N363S) have been associated in with an enhanced sensitivity to glucocorticoids and a worse metabolic or cardiovascular profile (closer to CS) [42,43,44]; in such patients, an increased “suppressive” response to dexamethasone may explain the adequate cortisol suppression with insufficient dexamethasone levels. Nevertheless, their adequately suppressed serum cortisol after 1-mg DST was sufficient to exclude CS diagnosis, which is the first aim of 1-mg DST. In our series, also two patients with CS presented low dexamethasone levels after 1-mg DST: their diagnosis of hypercortisolism has been established upon increased UFC and LNSC levels; however, their unsuppressed serum cortisol may further confuse the final diagnosis if 1-mg DST test has been used as the first and single screening procedure. We encourage that such patients refer preferentially to dedicated tertiary center, with a proved expertise in the diagnosis of CS.
Intriguingly, among 11 patients with insufficient dexamethasone levels, 4 admitted that they did not consume dexamethasone the night before cortisol sample, because they were afraid of “powerful” glucocorticoid. Obviously, their serum cortisol levels were not suppressed, and it can be questioned if the patients who did not take the dexamethasone tablet should be included in the calculations. Nevertheless, we could unmask these cases only through dexamethasone measurement. It is well known that patient’s compliance is one of the main causes of false-positive or false-negative results due to pre-analytical errors [45, 46]; however, physicians are encouraged to closely assess the adherence of patients to the diagnostic pathway proposed.
Beside strengths, our work presents some limitations. First, the lack of a normal reference range: we did not consider a cohort of healthy subjects recruited for that purpose, considering that an indirect sampling technique could be used to create the lower cut-off able to suppress serum cortisol (which is more useful in patients with suspected CS rather than in normal healthy controls). Dexamethasone measurement is expensive and not feasible in all laboratories; therefore, a careful cost–benefit balance should be considered. Dexamethasone could be measured only in patients that did not suppress serum cortisol after 1-mg DST, albeit the cost of a repeated test must be considered in this case. Moreover, mass spectrometry is required to measure dexamethasone: in this scenario, only referral center should adopt this method, to measure also urinary and salivary cortisol, hence reducing the final cost. Moreover, we have calculated the cut-off only in patients with adequate cortisol suppression after 1-mg DST, thus probably affecting the final sensitivity of the test. To conclude, we excluded those patients taking selected drugs that may interfere with dexamethasone metabolism: a dedicated study should be considered to evaluate the diagnostic accuracy of 1-mg DST test combined with dexamethasone measurement.
Routine measurement of dexamethasone level is feasible in clinical practice, it is independent from patient’s clinical presentation, and is able to increase the diagnostic accuracy of serum cortisol after 1-mg DST for suspected hypercortisolism.
References
Nieman LK, Biller BMK, Findling JW et al (2008) The diagnosis of cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 93(5):1526–1540. https://doi.org/10.1210/jc.2008-0125
Ceccato F, Boscaro M (2016) Cushing’s syndrome: screening and diagnosis. High Blood Press Cardiovasc Prev 23(3):209–215. https://doi.org/10.1007/s40292-016-0153-4
Boscaro M, Arnaldi G (2009) Approach to the patient with possible cushing’s syndrome. J Clin Endocrinol Metab 94(9):3121–3131. https://doi.org/10.1210/jc.2009-0612
Shimon I (2015) Screening for cushing’s syndrome: is it worthwhile? Pituitary 18(2):201–205. https://doi.org/10.1007/s11102-015-0634-9
Ceccato F, Marcelli G, Martino M et al (2018) The diagnostic accuracy of increased late night salivary cortisol for Cushing’s syndrome: a real-life prospective study. J Endocrinol Invest 42(3):327–335. https://doi.org/10.1007/s40618-018-0921-1
Omura M, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T (2004) Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens Res 27(3):193–202. https://doi.org/10.1291/hypres.27.193
Terzolo M, Reimondo G, Chiodini I et al (2012) Screening of Cushing’s syndrome in outpatients with type 2 diabetes: results of a prospective multicentric study in Italy. J Clin Endocrinol Metab 97(10):3467–3475. https://doi.org/10.1210/jc.2012-1323
Terzolo M, Stigliano A, Chiodini I et al (2011) AME position statement on adrenal incidentaloma. Eur J Endocrinol 164(6):851–870. https://doi.org/10.1530/EJE-10-1147
Fassnacht M, Arlt W, Bancos I et al (2016) Management of adrenal incidentalomas: European society of endocrinology clinical practice guideline in collaboration with the European network for the study of adrenal tumors. Eur J Endocrinol 175(2):G34. https://doi.org/10.1530/EJE-16-0467
Elamin MB, Murad MH, Mullan R et al (2008) Accuracy of diagnostic tests for Cushing’s syndrome: a systematic review and metaanalyses. J Clin Endocrinol Metab 93(5):1553–1562. https://doi.org/10.1210/jc.2008-0139
Pecori Giraldi F, Ambrogio AG, De Martin M, Fatti LM, Scacchi M, Cavagnini F (2007) Specificity of first-line tests for the diagnosis of Cushing’s syndrome: assessment in a large series. J Clin Endocrinol Metab 92(11):4123–4129. https://doi.org/10.1210/jc.2007-0596
Raff H (2009) Utility of salivary cortisol measurements in Cushing’s syndrome and adrenal insufficiency. J Clin Endocrinol Metab 94(10):3647–3655. https://doi.org/10.1210/jc.2009-1166
Nunes M-L, Vattaut S, Corcuff J-B et al (2009) Late-night salivary cortisol for diagnosis of overt and subclinical Cushing’s syndrome in hospitalized and ambulatory patients. J Clin Endocrinol Metab 94(2):456–462. https://doi.org/10.1210/jc.2008-1542
Ceccato F, Barbot M, Zilio M et al (2013) Performance of salivary cortisol in the diagnosis of Cushing’s syndrome, adrenal incidentaloma, and adrenal insufficiency. Eur J Endocrinol 169(1):31–36. https://doi.org/10.1530/EJE-13-0159
Petersenn S, Newell-Price J, Findling JW et al (2014) High variability in baseline urinary free cortisol values in patients with Cushing’s disease. Clin Endocrinol 80(2):261–269. https://doi.org/10.1111/cen.12259
Sandouk Z, Johnston P, Bunch D et al (2018) Variability of late-night salivary cortisol in Cushing disease: a prospective study. J Clin Endocrinol Metab 103(3):983–990. https://doi.org/10.1210/jc.2017-02020
Ceccato F, Antonelli G, Frigo AC et al (2017) First-line screening tests for Cushing’s syndrome in patients with adrenal incidentaloma: the role of urinary free cortisol measured by LC-MS/MS. J Endocrinol Invest 40(7):753–760. https://doi.org/10.1007/s40618-017-0644-8
Morelli V, Scillitani A, Arosio M, Chiodini I (2017) Follow-up of patients with adrenal incidentaloma, in accordance with the European society of endocrinology guidelines: could we be safe? J Endocrinol Invest 40(3):331–333. https://doi.org/10.1007/s40618-016-0558-x
Guthrie S (1991) The impact of dexamethasone pharmacokinetics on the DST: a review. Psychopharmacol Bull 27(4):565–576. https://www.ncbi.nlm.nih.gov/pubmed/1813902
Ueland GÅ, Methlie P, Kellmann R et al (2017) Simultaneous assay of cortisol and dexamethasone improved diagnostic accuracy of the dexamethasone suppression test. Eur J Endocrinol 176(6):705–713. https://doi.org/10.1530/EJE-17-0078
Meikle AW, Lagerquist LG, Tyler FH (1975) Apparently normal pituitary-adrenal suppressibility in Cushing’s syndrome: dexamethasone metabolism and plasma levels. J Lab Clin Med 86(3):472–478. https://www.ncbi.nlm.nih.gov/pubmed/1151162
Meikle AW (1982) Dexamethasone suppression tests: usefulness of simultaneous measurement of plasma cortisol and dexamethasone. Clin Endocrinol 16(4):401–408. https://www.ncbi.nlm.nih.gov/pubmed/7094363
Sasaki Y, Katabami T, Asai S, Fukuda H, Tanaka Y (2017) In the overnight dexamethasone suppression test, 1.0 mg loading is superior to 0.5 mg loading for diagnosing subclinical adrenal Cushing’s syndrome based on plasma dexamethasone levels determined using liquid chromatography-tandem mass spectrometry. Endocr J 64(9):833–842. https://doi.org/10.1507/endocrj.EJ17-0083
Ceccato F, Antonelli G, Barbot M et al (2014) The diagnostic performance of urinary free cortisol is better than the cortisol: cortisone ratio in detecting de novo Cushing’s syndrome: the use of a LC-MS/MS method in routine clinical practice. Eur J Endocrinol 171(1):1–7. https://doi.org/10.1530/EJE-14-0061
Antonelli G, Ceccato F, Artusi C, Marinova M, Plebani M (2015) Salivary cortisol and cortisone by LC-MS/MS: validation, reference intervals and diagnostic accuracy in Cushing’s syndrome. Clin Chim Acta 451:247–251. https://doi.org/10.1016/j.cca.2015.10.004
Valassi E, Swearingen B, Lee H et al (2009) Concomitant medication use can confound interpretation of the combined dexamethasone-corticotropin releasing hormone test in Cushing’s syndrome. J Clin Endocrinol Metab 94(12):4851–4859. https://doi.org/10.1210/jc.2009-1500
A clinical and laboratory standards institute (CLSI), liquid chromatography-mass spectrometry methods; approved guidelines for Clinical Chemistry, available online at https://clsi.org/media/1346/c62a_sample.pdf
Horowitz GL In: Lewis MA (ed) Defining, establishing, and verifying reference intervals in the clinical laboratory; Approved guidelines for Clinical Chemistry, available online https://clsi.org/media/1421/ep28a3c_sample.pdf
Ceccato F, Barbot M, Zilio M et al (2015) Screening tests for cushing’s syndrome: urinary free cortisol role measured by LC-MS/MS. J Clin Endocrinol Metab 100(10):3856–3861. https://doi.org/10.1210/jc.2015-2507
Raff H (2012) Cushing’s syndrome: diagnosis and surveillance using salivary cortisol. Pituitary 15(1):64–70. https://doi.org/10.1007/s11102-011-0333-0
de Graaf AJ, Mulder AL, Krabbe JG (2019) Retrospective analysis of repeated dexamethasone suppression tests—the added value of measuring dexamethasone. Ann Clin Biochem Int J Lab Med 56(6):708–710. https://doi.org/10.1177/0004563219870834
Mayo clinic. https://www.mayocliniclabs.com/test-catalog/Overview/91956
Miller BS, Ignatoski KM, Daignault S et al (2011) A quantitative tool to assess degree of sarcopenia objectively in patients with hypercortisolism. Surgery 150(6):1178–1185. https://doi.org/10.1016/j.surg.2011.09.020
Di Dalmazi G, Vicennati V, Garelli S et al (2014) Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing’s syndrome: a 15-year retrospective study. Lancet Diabetes Endocrinol 2(5):396–405. https://doi.org/10.1016/S2213-8587(13)70211-0
Debono M, Bradburn M, Bull M, Harrison B, Ross RJ, Newell-Price J (2014) Cortisol as a marker for increased mortality in patients with incidental adrenocortical adenomas. J Clin Endocrinol Metab 99(12):4462–4470. https://doi.org/10.1210/jc.2014-3007
Morelli V, Reimondo G, Giordano R et al (2014) Long-term follow-up in adrenal incidentalomas: an Italian multicenter study. J Clin Endocrinol Metab 99(3):827–834. https://doi.org/10.1210/jc.2013-3527
Masserini B, Morelli V, Bergamaschi S et al (2009) The limited role of midnight salivary cortisol levels in the diagnosis of subclinical hypercortisolism in patients with adrenal incidentaloma. Eur J Endocrinol 160(1):87–92. https://doi.org/10.1530/EJE-08-0485
Palmieri S, Morelli V, Polledri E, et al (2013) The role of salivary cortisol measured by liquid chromatography-tandem mass spectrometry in the diagnosis of subclinical hypercortisolism. Eur J Endocrinol 168(3):289. https://www.ncbi.nlm.nih.gov/pubmed/23211572
Ceccato F, Barbot M, Albiger N et al (2017) Daily salivary cortisol and cortisone rhythm in patients with adrenal incidentaloma. Endocrine. https://doi.org/10.1007/s12020-017-1421-3
Fassnacht M, Dekkers OM, Else T et al (2018) European society of endocrinology clinical practice guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European network for the study of adrenal tumors. Eur J Endocrinol 179(4):G1–G46. https://doi.org/10.1530/EJE-18-0608
Trementino L, Appolloni G, Concettoni C, Cardinaletti M, Boscaro M, Arnaldi G (2012) Association of glucocorticoid receptor polymorphism A3669G with decreased risk of developing diabetes in patients with Cushing’s syndrome. Eur J Endocrinol 166(1):35–42. https://doi.org/10.1530/EJE-11-0722
van Rossum EFC, Koper JW, van den Beld AW, et al (2003) Identification of the BclI polymorphism in the glucocorticoid receptor gene: association with sensitivity to glucocorticoids in vivo and body mass index. Clin Endocrinol 59(5):585–592. https://www.ncbi.nlm.nih.gov/pubmed/14616881
Di Blasio AM, van Rossum EFC, Maestrini S, et al (2003) The relation between two polymorphisms in the glucocorticoid receptor gene and body mass index, blood pressure and cholesterol in obese patients. Clin Endocrinol 59(1):68–74. https://www.ncbi.nlm.nih.gov/pubmed/12807506
Tzanela M, Mantzou E, Saltiki K et al (2012) Clinical and biochemical impact of BCL1 polymorphic genotype of the glucocorticoid receptor gene in patients with adrenal incidentalomas. J Endocrinol Invest 35(4):395–400. https://doi.org/10.3275/7840
Plebani M (2010) The detection and prevention of errors in laboratory medicine. Ann Clin Biochem 47(Pt 2):101–110. https://doi.org/10.1258/acb.2009.009222
Plebani M (2016) Towards a new paradigm in laboratory medicine: the five rights. Clin Chem Lab Med 54(12):1881–1891. https://doi.org/10.1515/cclm-2016-0848
Funding
This study did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest that might be perceived as influencing the impartiality of the reported research.
Ethical approval
The study was performed in accordance with the guidelines in the Declaration of Helsinki, the study was approved by the Ethics Committee of Padova University-Hospital.
Informed consent
All patients gave informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ceccato, F., Artusi, C., Barbot, M. et al. Dexamethasone measurement during low-dose suppression test for suspected hypercortisolism: threshold development with and validation. J Endocrinol Invest 43, 1105–1113 (2020). https://doi.org/10.1007/s40618-020-01197-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-020-01197-6