Abstract
Purpose
The dual antiproliferative mechanism of mycophenolate appears to be beneficial in Graves’ orbitopathy (GO).
Methods
Safety data from the two published mycophenolate trials and the original database of the European Group on Graves’ Orbitopathy (EUGOGO) trial were systematically analyzed. Treatment efficacy stratified by individual visual parameters of activity and severity were compared.
Results
A total of 129 adverse events (AE) involving 50 patients (29.4%) were noted among all mycophenolate-treated patients. Mycophenolate sodium plus intravenous glucocorticoid (MPS + GC) group of the EUGOGO trial recorded significantly more AE (55.4% versus 4.6% of patients affected) and serious adverse events (SAE) (12.5% versus 0%) than mycophenolate mofetil (MMF) group of the Chinese trial. None of those SAE was side effect (SE). Most SE in MPS + GC group were mild. Gastrointestinal disorders, infection and liver dysfunction affected 8.8%, 7.1% and 1.2% of all mycophenolate-treated patients (versus 5.4%, 5.4% and 1.2% of all patients on GC monotherapy, respectively). MPS + GC did not significantly increase the risk of infection or liver dysfunction when compared to GC monotherapy. No cytopenia, serious infection or treatment-related mortality was reported. The much higher AE rates of mycophenolate trials in other autoimmune diseases or transplantations suggested that major mycophenolate toxicities were mostly dose- and duration dependent. Mycophenolate, either as monotherapy or as combination, achieved better overall response than GC monotherapy.
Conclusion
The risk–benefit ratio of low-dose mycophenolate treatment in active moderate-to-severe GO is highly favorable given its reassuring safety profile with low rate of mild-to-moderate SE and promising efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Graves’ orbitopathy (GO) is the most common extra-thyroidal manifestation of Graves’ disease (GD) [1, 2]. The prevalence of GO among patients with GD varies widely across different series [3]. The pathological processes within the orbit include inflammatory infiltration of retro-ocular tissues within the orbit, de novo adipogenesis and increased production of hydrophilic glycosaminoglycans by orbital fibroblasts. Detailed treatment recommendations of GO have been published and updated in recent years [4].
Mycophenolic acid (MPA) is a competitive, selective and reversible inhibitor of inosine monophosphate dehydrogenase (IMPDH) [5], which catalyzes the rate-limiting step in the de-novo synthesis of guanosine nucleotides in lymphocytes. Unlike other cell types, lymphocytes are unable to produce guanosine nucleotides via the salvage pathway. MPA, therefore, leads to the depletion of guanosine-triphosphate (GTP) resulting in the apoptosis of activated T-lymphocytes and reduction of adhesion molecules which recruit lymphocytes, monocytes and neutrophils into sites of inflammation [6, 7]. An antiproliferative effect on orbital target cells has also been discussed. Mycophenolate mofetil (MMF) acts as a pro-drug and is hydrolyzed by esterases to form the active metabolite MPA [6]. Enteric-coated mycophenolate sodium (MPS) contains MPA packed in a gastro-resistant capsule and it was designed to improve MPA-related gastrointestinal intolerance by delaying the release of MPA until reaching the small intestine [8]. Considering their difference in molecular weights, each 500 mg of MMF is equivalent to 360 mg of MPA or MPS.
A Chinese case series first reported that mycophenolate was an effective treatment of GO in 2004 [9]. Recently, two major randomized clinical trials, which reported promising efficacy of mycophenolate in patients with active moderate-to-severe GO, were published. As medication safety is of utmost importance in immunomodulation, the safety data in both trials were systematically evaluated. Mycophenolate efficacy in GO was also briefly analyzed.
Materials and methods
A search of the NCBI PubMed database (National Library of Medicine, Bethesda, MD, https://www.ncbi.nlm.nih.gov/pubmed) was conducted in order to obtain data on clinical trials evaluating mycophenolate in patients with GO with no temporal limit. The following keywords were used: “Graves’ orbitopathy”, “Graves’ ophthalmopathy”, “Thyroid associated ophthalmopathy”, “Thyroid eye disease”, “Mycophenolate” and “MMF”. All were searched as MeSH-Terms. Combining the keywords, we found four original articles:
The first article consisted of a Chinese case series [9]. (“Chinese case series”)
The second article described for the first time the safety profile in 53 consecutive patients with severe GO on mycophenolate [10]. It represented an interim assessment of the following European Group on Graves’ Orbitopathy trial (“EUGOGO trial”).
The third article was a clinical monocenter trial [11]. (“Chinese trial”)
The fourth article provided the final results of the multi-center randomized EUGOGO trial [12].
The original study database of the EUGOGO trial, but not the Chinese trial, was available to us. Additional data from the database were generated for further analysis and comparison. We critically appraised and compared both trials in the following key aspects:
-
1.
Trial design and baseline characteristics
-
2.
Adverse events
-
To systematically evaluate safety data of both trials, the number of all adverse events (AE)/side effects (SE)/selected AE (gastrointestinal disorders, infection, liver dysfunction, weight gain) and the proportions of patients affected in each treatment arm were compared.
-
In both trials, an AE was defined as any undesirable symptom or sign that occurred after the initiation of the treatment.
-
In the EUGOGO trial, AE were documented and coded in accordance with the standardized medical dictionary for regulatory affairs (MedDRA), and they were assigned to the appropriate System Organ Class (SOC) as recommended by the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). An AE was deemed SE if related to the study drugs. Every AE was classified as mild, moderate or severe according to ICH E6 guideline for clinical practice.
-
The Chinese trial did not mention its AE coding or classification system. Only AE, but not SE, were reported. Every AE was also classified as mild, moderate or severe, but the seriousness criteria were not disclosed.
-
-
3.
Treatment efficacy
-
Both trials defined treatment response rates at week 12 and week 24 using different composite outcomes (Supplementary Table 1). In the EUGOGO trial additional data concerning sustained response at week 36 were collected.
-
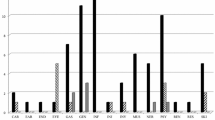
The Chinese trial further described the response rates in eight individual visual parameters, four concerning GO activity (improvement in pain, improvement in soft tissue involvement, improvement in clinical activity score (CAS) by at least two points, disease inactivation based on 10-point CAS ≤ 3/10 at 24 weeks) and the other four concerning GO severity (improvement in proptosis by at least 2 mm, improvement in eye movement, improvement in diplopia, increase in visual acuity by at least 2/10).
-
To evaluate treatment efficacy of both trials, we compared overall response rates and determined the proportion of patients (within the whole treatment arm) who achieved the above endpoints in the two mycophenolate treatment arms of both trials.
-
Results
The Chinese case series described 12 GO patients who received MMF monotherapy 1 g per day for 12 weeks and reported no AE during the study period. A high response rate of 92% (11/12) was demonstrated.
The following analysis mainly focused on the Chinese and EUGOGO trials:
-
1.
Trial design and baseline characteristics
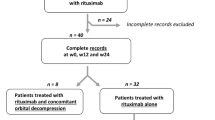
170 patients were randomized to mycophenolate, either as MMF monotherapy in the Chinese trial or MPS plus intravenous glucocorticoid (MPS + GC) in the EUGOGO trial. Each trial had a glucocorticoid (GC) monotherapy arm. An overview of the trial design and the reasons for trial discontinuation was displayed in Fig. 1. Compliance in both trials was good and only a few patients dropped out during the treatment.
The baseline characteristics of mycophenolate-treated patients were compared (Table 1). The patients in the EUGOGO trial were older and more often smokers. They had higher serum levels of thyroid hormones and were more often positive with thyroid stimulating hormone receptor autoantibody (TSH-R-Ab). Regarding the pre-treatment GO status, patients in the Chinese trial had higher baseline CAS but it was based on the 10-point instead of the 7-point scale used in the EUGOGO trial. Orbital pain was much less common in the Chinese trial. The baseline mean exophthalmometric measurements were similar at around 21 mm. However, as Chinese people have significantly lower upper limits of normal (18.6 mm versus 21 mm in Caucasians) [13,14,15], proptosis among patients in the Chinese trial probably was more severe and/or more prevalent. While the diplopia status was similar, much more patients in the EUGOGO trial suffered from ocular dysmotility. Intriguingly, patients in the Chinese trial were much more likely to have diplopia (80%) than decrease in eye movement (19%). The baseline characteristics of patients who discontinued in the Chinese trial (seven in MMF group) were not reported.
-
2.
Overview of adverse events (Table 2)
Table 2 Overview of adverse events and side effects during treatment phase
A total of 285 AE affecting 99 of 338 patients (29.3%) were recorded during the treatment phase in both trials. 129 AE involving 50 patients (29.4%) were noted among all mycophenolate-treated patients and their detailed description was listed in Table 3. MPS + GC group of the EUGOGO trial recorded significantly more AE and serious adverse events (SAE) than MMF group of the Chinese trial. Those 10 SAE in the EUGOGO trial included seven patients with optic neuropathy (which were in fact cases of treatment failure or relapse), as well as anal fistula, edema and depression in one patient each. None of those SAE was classified as SE. Most SE in the MPS + GC group were mild in severity. There was no SAE reported in MMF group. No death or trial discontinuation due to AE or SE was reported in both studies. The safety data of patients who discontinued in the Chinese trial (seven in MMF group and nine in GC group) were not reported.
-
3.
Selected adverse events (Table 2)
Gastrointestinal disorders
Gastrointestinal (GI) disorders as AE were more common in MPS + GC group than MMF or GC groups (8.8% and 5.4% of all patients on mycophenolate and GC monotherapy, respectively) while half of them were graded as SE in MPS + GC group. All were mild in severity. Abdominal discomfort and dyspepsia accounted for most GI SE.
Infection
AE coded as infection were more frequent in MPS + GC group than MMF or GC groups (7.1% and 5.4% of all patients on mycophenolate and GC monotherapy, respectively), but only a minority were graded as SE in MPS + GC group. All infective episodes were mild to moderate in severity and they resolved uneventfully after appropriate antimicrobial treatments.
Liver dysfunction (liver enzyme more than three times upper limit of normal)
Liver dysfunction occurred in only two mycophenolate-treated patients (1.2% versus 1.2% of all patients on GC monotherapy), both from MPS + GC group, who had raised gamma-glutamyltransferase (GGT) level at week 24 (534 and 227 U/l). No severe hepatotoxicity or liver failure was noted.
Weight gain
No weight gain was observed among patients from MMF group. Weight gain of more than 0.5 kg was recorded in 36.3% of all patients on GC monotherapy and 49.4% of those in MPS + GC group. The proportions of patients with weight gain were similar between the two treatment arms in the EUGOGO trial.
-
4.
Treatment efficacy
A direct comparison between the two trials was limited by the different trial designs and drug regimens, as well as unavailability of the Chinese trial database. The overall responses rates at weeks 12, 24 and 36 were shown in Fig. 2. Overall, mycophenolate-treated groups demonstrated superior response rates at 12 weeks (Chinese trial), 24 weeks (both trials) and 36 weeks (EUGOGO trial) when compared to their respective GC monotherapy groups (Fig. 2). Approximately 70% (versus 90% in MMF group) and 30% (versus 60–70% in MMF group) of patients in MPS + GC group achieved endpoints in most individual visual parameters of activity (Fig. 3a) and severity (Fig. 3b), respectively. On the other hand, MPS + GC group of the EUGOGO trial performed better than MMF group in terms of improvement of pain and eye movement.
Response rates (by composite outcomes) of mycophenolate trials in GO. Response rates of GC monotherapy groups: 12 weeks: 51.3% (Chinese trial); 49% (EUGOGO trial); 24 weeks: 67.9% (Chinese trial); 53% (EUGOGO trial); 36 weeks: 46% (EUGOGO trial). The difference in response rate at 12 weeks of GC vs MPS + GC groups in EUGOGO was not statistically significant. Otherwise the differences in response rates at 12 weeks (Chinese trial MMF vs GC), at 24 weeks (both trial) and at 36 weeks (EUGOGO trial MPS + GC vs GC) were all statistically significant
Individual visual parameters of clinical disease activity at 24 weeks. #Defined as improvement by one grade in any of the following: eyelid swelling, eyelid erythema, conjunctival redness, conjunctival edema. @Based on 10-point and 7-point clinical activity score (CAS) in the Chinese trial and the EUGOGO trial, respectively. b Individual visual parameters of clinical disease severity at 24 weeks. *Not defined in the Chinese trial (measured by Hess Chart); defined in the EUGOGO trial as ≥ 8° improvement in eye muscle motility measured by perimeter arc
Discussion
This is the first systematic and comprehensive safety analysis evaluating 6-month courses of low-dose (equivalent of one gram daily) mycophenolate treatment in GO. AE were much more frequent in MPS + GC group when compared to MMF group. The excess of AE may partly be contributed by GC use. Nevertheless, the combination of mycophenolate and GC did not appear to incur significantly more SE when compared to GC monotherapy and, more importantly, most SE in the combination group were only mild.
Hepatotoxicity is a well-documented side effect of mycophenolate. Our analysis showed that mycophenolate-treated patients had similar risk of liver dysfunction as those on GC monotherapy. This finding is important because intravenous GC therapy itself carries a small risk of dose dependent acute liver damage [16,17,18,19]. The absence of significant hepatotoxicity reinforces the safety of low dose mycophenolate, both as monotherapy and as combination with GC.
The two most feared side effects of mycophenolate include bone marrow suppression and infection, which are potentially severe and life-threatening [20,21,22]. We did not observe any case of cytopenia among GO patients receiving mycophenolate. Only a small number of mycophenolate-treated patients developed infections of mild to moderate severity. Therefore, it is reassuring that significant myelosuppression did not occur at low dose of mycophenolate and the risk of infection was not potentiated even in combination with GC.
Furthermore, major mycophenolate toxicities are dose dependent as evidenced by the safety profiles of recent mycophenolate drug trials in other autoimmune diseases or transplantations (summarized in Table 4):
-
MMF monotherapy of daily dose 2–3 g was associated with higher risk of cytopenia, SAE and treatment-related death [23, 24]. In contrast, all these events were absent in the Chinese trial using low-dose MMF 1 g per day, and its rate of infections was also much lower.
-
When compared to the EUGOGO trial, the combination of mycophenolate at higher doses (daily dose of MMF 1.5–2 g or equivalent) and GC was associated with higher risk of cytopenia, infections, SAE and mortality [22, 25,26,27,28]. Of note, a significant proportion of those infections, ranging from 3 to 8.6%, were graded as serious or SAE and they were occasionally fatal [22, 26, 28].
-
3-drug combinations with high dose MMF (up to 4 g/day), glucocorticoid and tacrolimus/cyclosporin are common post-transplantation immunosuppressive regimens. As expected, they gave rise to even higher risk of leukopenia, infections, SAE and mortality [29,30,31]. GI intolerance was also significantly more common.
-
All the above trials did not report significant hepatotoxicity except one study which noted liver dysfunction in almost 80% of post-lung transplantation patients on MMF 2 g per day together with prednisolone and cyclosporin [30]. Intriguingly, a 3-year follow-up study of cardiac transplant recipients taking high-dose MMF of 3–4 g per day did not report any significant hepatotoxicity. Therefore, it raised the suspicion that hepatotoxicity may not be dose dependent.
-
The longer duration of mycophenolate treatment in those non-GO trials also impacts negatively on the safety as patients with certain autoimmune conditions (e.g. systemic lupus erythematosus with renal involvement [32]) or solid organ transplantations often need long-term mycophenolate as maintenance therapy. On the contrary, GO is typically characterized by an initial active inflammatory phase (during which immunomodulation is most effective) lasting for 6- to 12 months followed by a stabilization phase [33], and disease relapse or reactivation is much less common. Therefore, long-term immunosuppressive therapy is not a standard treatment in GO [34]. A finite and relatively short duration of 6-month mycophenolate treatment in GO further optimized its safety profile by avoiding more prolonged drug exposure.
Our analysis of the two trials demonstrated that combination treatment gave rise to slightly more GI intolerance, albeit mild in severity, than mycophenolate or GC alone. No mycophenolate-treated patient required dosage reduction or medication discontinuation because of GI intolerance. Although diarrhea is believed to be the most common GI adverse reaction to mycophenolate, we did not observe the same phenomenon. Only one out of 10 GI SE in MPS + GC group was diarrhea, while the remaining ones were mostly nausea and abdominal discomfort. MPS may have less GI intolerance than MMF, although to date this theoretical advantage is not proven by high-quality clinical evidence [5].
Mycophenolate has been shown to be more efficacious than GC monotherapy in active moderate-to-severe GO, either as monotherapy or as combination with intravenous GC. However, as the Chinese trial employed an unconventional steroid regimen, whether MMF monotherapy remains superior to the standard weekly intravenous GC regimen remains to be determined. Several key differences in patients’ baseline characteristics, which include higher mean age [35], more prevalent smoking [36], longer duration of GO [37] and greater proportion of patients with positive TSH-R-Ab [38,39,40,41,42,43,44], are predictive of more severe GO or less favorable response to immunosuppressive treatment among patients in the EUGOGO trial. These factors may explain the lower response rates (overall response by composite outcomes and most individual visual parameters) in the EUGOGO trial in comparison to the Chinese trial. In addition, as patients in the Chinese trial probably had more severe or more prevalent proptosis at baseline, unsurprisingly more patients in MMF group had improvement in proptosis. It was difficult to comment on the improvement in eye movement as this parameter was not clearly defined in the Chinese trial. The more prevalent orbital pain at baseline in the EUGOGO trial probably explained why pain improvement was more common in MPS + GC group.
Conclusion
Mycophenolate, either as monotherapy or as combination with intravenous GC, is associated with low rate of mild to moderate SE and absence of major toxicities. Therefore, the risk–benefit ratio of low-dose mycophenolate treatment in active moderate-to-severe GO is highly favorable given its reassuring safety profile and promising efficacy.
References
Bartalena L, Fatourechi V (2014) Extrathyroidal manifestations of Graves’ disease: a 2014 update. J Endocrinol Invest 37(8):691–700
Hai YP, Lee ACH, Frommer L, Diana T, Kahaly GJ (2019) Immunohistochemical analysis of human orbital tissue in Graves’ orbitopathy. J Endocrinol Invest 19:15
Piantanida E, Tanda ML, Lai A, Sassi L, Bartalena L (2013) Prevalence and natural history of Graves’ orbitopathy in the XXI century. J Endocrinol Invest 36(6):444–449
Bartalena L, Macchia PE, Marcocci C, Salvi M, Vermiglio F (2015) Effects of treatment modalities for Graves’ hyperthyroidism on Graves’ orbitopathy: a 2015 Italian Society of Endocrinology Consensus Statement. J Endocrinol Invest 38(4):481–487
Staatz CE, Tett SE (2014) Pharmacology and toxicology of mycophenolate in organ transplant recipients: an update. Arch Toxicol 88(7):1351–1389
Allison AC, Eugui EM (2000) Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 47(2–3):85–118
Eugui EM, Allison AC (1993) Immunosuppressive activity of mycophenolate mofetil. Ann N Y Acad Sci 685:309–329
Budde K, Durr M, Liefeldt L, Neumayer HH, Glander P (2010) Enteric-coated mycophenolate sodium. Expert Opin Drug Saf 9(6):981–994
Wang J, Wang YT, Shao JQ, Wang X, Du H (2004) Immunosuppressive therapies in patients with Graves’ ophthalmopathy. Zhonghua Nei Ke Za Zhi 43(2):125–127
Riedl M, Kuhn A, Kramer I, Kolbe E, Kahaly GJ (2016) Prospective, systematically recorded mycophenolate safety data in Graves’ orbitopathy. J Endocrinol Invest 39(6):687–694
Ye X, Bo X, Hu X, Cui H, Lu B, Shao J et al (2017) Efficacy and safety of mycophenolate mofetil in patients with active moderate-to-severe Graves’ orbitopathy. Clin Endocrinol (Oxf) 86(2):247–255
Kahaly GJ, Riedl M, Konig J, Pitz S, Ponto K, Diana T et al (2018) Mycophenolate plus methylprednisolone versus methylprednisolone alone in active, moderate-to-severe Graves’ orbitopathy (MINGO): a randomised, observer-masked, multicentre trial. Lancet Diabetes Endocrinol 6(4):287–298
Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL et al (2016) 2016 American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 26(10):1343–1421
Tsai CC, Kau HC, Kao SC, Hsu WM (2006) Exophthalmos of patients with Graves’ disease in Chinese of Taiwan. Eye (Lond) 20(5):569–573
de Juan E Jr., Hurley DP, Sapira JD (1980) Racial differences in normal values of proptosis. Arch Intern Med 140(9):1230–1231
Weissel M, Hauff W (2000) Fatal liver failure after high-dose glucocorticoid pulse therapy in a patient with severe thyroid eye disease. Thyroid 10(6):521
Marino M, Morabito E, Brunetto MR, Bartalena L, Pinchera A, Marocci C (2004) Acute and severe liver damage associated with intravenous glucocorticoid pulse therapy in patients with Graves’ ophthalmopathy. Thyroid 14(5):403–406
Salvi M, Vannucchi G, Sbrozzi F, Del Castello AB, Carnevali A, Fargion S et al (2004) Onset of autoimmune hepatitis during intravenous steroid therapy for thyroid-associated ophthalmopathy in a patient with Hashimoto’s thyroiditis: case report. Thyroid 14(8):631–634
Moleti M, Giuffrida G, Sturniolo G, Squadrito G, Campenni A, Morelli S et al (2016) Acute liver damage following intravenous glucocorticoid treatment for Graves’ ophthalmopathy. Endocrine 54(1):259–268
Rerolle JP, Szelag JC, Le Meur Y (2007) Unexpected rate of severe leucopenia with the association of mycophenolate mofetil and valganciclovir in kidney transplant recipients. Nephrol Dial Transplant 22(2):671–672
Mendes MM, Carminatti M, Pinheiro HS (2017) Severe sepsis from a Ciprofloxacin resistant salmonellosis in a kidney transplant recipient. J Bras Nefrol 39(1):82–85
Ordi-Ros J, Saez-Comet L, Perez-Conesa M, Vidal X, Mitjavila F, Castro Salomo A et al (2017) Enteric-coated mycophenolate sodium versus azathioprine in patients with active systemic lupus erythematosus: a randomised clinical trial. Ann Rheum Dis 76(9):1575–1582
Rathinam SR, Babu M, Thundikandy R, Kanakath A, Nardone N, Esterberg E et al (2014) A randomized clinical trial comparing methotrexate and mycophenolate mofetil for noninfectious uveitis. Ophthalmology 121(10):1863–1870
Tashkin DP, Roth MD, Clements PJ, Furst DE, Khanna D, Kleerup EC et al (2016) Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med 4(9):708–719
Hou JH, Le WB, Chen N, Wang WM, Liu ZS, Liu D et al (2017) Mycophenolate mofetil combined with prednisone versus full-dose prednisone in IgA nephropathy with active proliferative lesions: a randomized controlled trial. Am J Kidney Dis 69(6):788–795
Remy P, Audard V, Natella PA, Pelle G, Dussol B, Leray-Moragues H et al (2018) An open-label randomized controlled trial of low-dose corticosteroid plus enteric-coated mycophenolate sodium versus standard corticosteroid treatment for minimal change nephrotic syndrome in adults (MSN Study). Kidney Int 94(6):1217–1226
Yunyun F, Yu P, Panpan Z, Xia Z, Linyi P, Jiaxin Z et al (2019) Efficacy and safety of low dose Mycophenolate mofetil treatment for immunoglobulin G4-related disease: a randomized clinical trial. Rheumatology (Oxf) 58(1):52–60
Tuin J, Stassen PM, Bogdan DI, Broekroelofs J, van Paassen P, Cohen Tervaert JW et al (2019) Mycophenolate mofetil versus cyclophosphamide for the induction of remission in nonlife-threatening relapses of antineutrophil cytoplasmic antibody-associated vasculitis: randomized, controlled trial. Clin J Am Soc Nephrol 14(7):1021–1028
Eisen HJ, Kobashigawa J, Keogh A, Bourge R, Renlund D, Mentzer R et al (2005) Three-year results of a randomized, double-blind, controlled trial of mycophenolate mofetil versus azathioprine in cardiac transplant recipients. J Heart Lung Transplant 24(5):517–525
Strueber M, Warnecke G, Fuge J, Simon AR, Zhang R, Welte T et al (2016) Everolimus versus mycophenolate mofetil de novo after lung transplantation: a prospective, randomized, open-label trial. Am J Transplant 16(11):3171–3180
Qazi Y, Shaffer D, Kaplan B, Kim DY, Luan FL, Peddi VR et al (2017) Efficacy and safety of everolimus plus low-dose tacrolimus versus mycophenolate mofetil plus standard-dose tacrolimus in de novo renal transplant recipients: 12-month data. Am J Transplant 17(5):1358–1369
Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN et al (2019) 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 78(6):736–745
Bartley GB (2011) Rundle and his curve. Arch Ophthalmol 129(3):356–358
Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C et al (2016) The 2016 European thyroid association/european group on Graves’ orbitopathy guidelines for the management of Graves’ orbitopathy. Eur Thyroid J. 5(1):9–26
Perros P, Crombie AL, Matthews JN, Kendall-Taylor P (1993) Age and gender influence the severity of thyroid-associated ophthalmopathy: a study of 101 patients attending a combined thyroid-eye clinic. Clin Endocrinol (Oxf) 38(4):367–372
Wiersinga WM (2013) Smoking and thyroid. Clin Endocrinol (Oxf) 79(2):145–151
Terwee CB, Prummel MF, Gerding MN, Kahaly GJ, Dekker FW, Wiersinga WM (2005) Measuring disease activity to predict therapeutic outcome in Graves’ ophthalmopathy. Clin Endocrinol (Oxf) 62(2):145–155
Lytton SD, Ponto KA, Kanitz M, Matheis N, Kohn LD, Kahaly GJ (2010) A novel thyroid stimulating immunoglobulin bioassay is a functional indicator of activity and severity of Graves’ orbitopathy. J Clin Endocrinol Metab 95(5):2123–2131
Ponto KA, Kanitz M, Olivo PD, Pitz S, Pfeiffer N, Kahaly GJ (2011) Clinical relevance of thyroid-stimulating immunoglobulins in Graves’ ophthalmopathy. Ophthalmology 118(11):2279–2285
Ponto KA, Diana T, Binder H, Matheis N, Pitz S, Pfeiffer N et al (2015) Thyroid-stimulating immunoglobulins indicate the onset of dysthyroid optic neuropathy. J Endocrinol Invest 38(7):769–777
Kampmann E, Diana T, Kanitz M, Hoppe D, Kahaly GJ (2015) Thyroid stimulating but not blocking autoantibodies are highly prevalent in severe and active thyroid-associated orbitopathy: a prospective study. Int J Endocrinol 2015:678194
Kahaly GJ (2015) Bioassays for TSH receptor antibodies: quo vadis? Eur Thyroid J. 4(1):3–5
Diana T, Wuster C, Olivo PD, Unterrainer A, Konig J, Kanitz M et al (2017) Performance and specificity of 6 immunoassays for TSH receptor antibodies: a multicenter study. Eur Thyroid J. 6(5):243–249
Kahaly GJ, Wuster C, Olivo PD, Diana T (2019) High titers of thyrotropin receptor antibodies are associated with orbitopathy in patients with Graves disease. J Clin Endocrinol Metab 104(7):2561–2568
Author information
Authors and Affiliations
Contributions
Literature search and data interpretation were performed by Riedl and Lee. The manuscript was written by Lee, Riedl and Kahaly. Kahaly, Diana and Frommer critically reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors stated that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional ethics committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, A.C.H., Riedl, M., Frommer, L. et al. Systemic safety analysis of mycophenolate in Graves’ orbitopathy. J Endocrinol Invest 43, 767–777 (2020). https://doi.org/10.1007/s40618-019-01161-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-019-01161-z