Abstract
Objective
To investigate the expression of miR-217 and HIF-1α/VEGF pathway in patients with diabetic foot ulcer (DFU) and its effect on angiogenesis in DFU rats.
Methods
The serum levels of miR-217, HIF-1α and VEGF were detected in DFU and simple diabetes mellitus (DM) patients, and healthy controls. DFU rat models were established and treated with miR-217 inhibitors and/or HIF-1α siRNA. The ulcer healing of DFU rats was observed. Besides, ELISA method was performed to detect the serum level of HIF-1α, VEGF and inflammatory factors, immunohistochemical (IHC) method to test the micro-vessel density (MVD), as well as qRT-PCR and Western blot to determine expressions of miR-217, HIF-1α, VEGF, VEGFR2, eNOS, MMP-2, and MMP-9 in tissues.
Results
The serum levels of miR-217 were up-regulated while HIF-1α and VEGF were down-regulated in DFU patients and rats when compared with DM and healthy controls (all P < 0.05). Dual-luciferase reporter gene assay confirmed that HIF-1α was the direct target gene of miR-217. DFU rats treated with miR-217 inhibitors had decreased foot ulcer area and accelerated ulcer healing, with significantly reduced inflammatory factors (IL-1β, TNF-α and IL-6), as well as elevated HIF-1α and VEGF (all P < 0.05); meanwhile, they remarkably increased the MVD in foot dorsum wound tissues and the protein expressions of HIF-1α, VEGF, VEGFR2, eNOS, MMP-2, and MMP-9 (all P < 0.05).
Conclusion
Inhibiting miR-217 could up-regulate HIF-1α/VEGF pathway to promote angiogenesis and ameliorate inflammation of DFU rats, thereby effectively advancing the healing of ulcerated area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic foot ulcer (DFU), as one of the most devastating complications of diabetes mellitus (DM), is an infection, ulcer or destruction of deep tissue usually found in middle- and end-stage DM patients [33, 51]. As estimated, approximately 15% of DM patients would simultaneously have foot ulcer during their lifetime, among whom 14–24% need to receive amputation surgery, greatly affecting the life quality of those sufferers [13, 54]. To date, the nosogenesis of DFU has not been clearly clarified and it is generally believed to be associated with peripheral neuropathy (PNP), vascular disorders and foot infections [3, 7]. On the other hand, angiogenesis is the process of blood vessels extending their vascular branching by sprouting and forming a vascular network [31, 46]. It was reported that the healing disorder of diabetic ulcer was closely associated with the decreased nutrient supply caused by poor angiogenesis in granulation tissues [21, 26, 38]. Therefore, promoting the local angiogenesis of wound surface could ameliorate the healing disorder of diabetic ulcer to a certain degree.
Endothelial cells are the single layer cells of the vascular intima, playing an important role in maintaining the structure and function of blood vessels [14]. While vascular endothelial dysfunction is the initiating agent of DM complications in DM patients, including DFU [24]. As reported, miR-217 is not only closely related to the proliferation and migration of tumor cells [9, 42], but also has a regulatory function in the aging of endothelial cells [32]. Besides, Zhang S et al. reported that miR-217 could target FOXO3A to promote the proliferation and migration of HCMV-infected endothelial cells and angiogenesis [53]. Also, Shao Y et al. found that miR-217 could mediate Sirt1/HIF-1α pathway to modulate the inflammation and fibrosis of high glucose-exposed rat glomerular mesangial cells [39]. More importantly, we identified that HIF-1α is the target gene of miR-217 using the target gene prediction website. As an oxygen concentration-dependent key transcription factor, HIF-1α can regulate multiple target genes, including vascular endothelial growth factor (VEGF), and thereby modulating a variety of hypoxia-induced adaptive reactions, such as angiogenesis, metabolism, and cell apoptosis [4, 5]. A previous study also confirmed that HIF-1α and VEGF were involved in the healing and angiogenesis of DFU [52], suggesting that miR-217 may affect HIF-1α/VEGF pathway to play the regulatory role in angiogenesis of DFU.
Given the above, we detected the expression level of miR-217 and HIF-1α/VEGF in DFU patients and established DFU rat models to investigate the impact of miR-217-mediated HIF-1α/VEGF pathway on ulcer healing and angiogenesis of DFU, thereby exploring its therapeutic effect and possible mechanism.
Materials and methods
Ethics statement
The clinical research in this study was approved by the Ethics Committee of The First Affiliated Hospital of Shantou University Medical College and conformed to the guidelines of Helsinki Declaration [48]. All samples were collected after obtaining the informed consent form signed by all the subjects. Besides, the animal experiments were performed according to local protocols on the care and use of laboratory animals, and in strict obedience to the Guide for the Care and Use of Laboratory Animals published by the National Institute of Health in the USA [17].
Study subjects and blood sample collection
From January 2015 to January 2018, 55 patients with DFU admitted to our hospital were recruited as DFU group, including 36 males and 19 females with the average age of 56.12 ± 12.93 years and a mean DM course of 3–24 years. Meanwhile, 42 simple DM patients without DFU were selected as DM group, consisting of 31 males and 11 females with the average age of 56.81 ± 13.52 years and the mean DM course of 4–25 years. All DM patients were diagnosed and confirmed based on the DM diagnostic criteria published by WHO [23], and the grading of DFU severity was performed according to the Wagner diabetic foot ulcer classification system [47]. All patients did not have complications such as malignant tumor, dysfunction of heart, liver and kidney, or history of cerebral hemorrhage, cerebral infarction and acute myocardial infarction (AMI). Moreover, 60 healthy individuals who undertook physical examination in our hospital during the same time period were recruited as healthy control (HC) group composed of 39 males and 21 females with the average age of 58.23 ± 15.48 years. There was no significant difference among the three groups in gender ratio and age (all P > 0.05). In the morning, 10 ml fasting blood was taken from cubital vein of all subjects into the heparin anticoagulant tube and centrifuged for 20 min at the rate of 3500 rpm/min, and the serum was separated and preserved in a refrigerator at − 80 °C.
Establishment of DFU rat models
Healthy male SD rats of SPF grade (purchased from Shanghai SLAC Laboratory Animal Co., LTD.), weighing 200–250 g, were intraperitoneally injected with streptozotocin (STZ, 55 mg/kg, Sigma) to construct DM models [54] after fasting for 12 h. After 72 h, blood samples were collected from the tail vein of rats in each group, and the fasting blood glucose was detected using One Touch blood sugar monitoring system and standard glucose test paper (Lifescan INC, Johnson & Johnson Company, Mil- pitas, CA, USA). Blood sugar concentration ≥ 16.7 mmol/L [10] was regarded as the criterion of successful DM models. To build DFU rat models, a 4 × 4 mm empyrosis on the dorsal hind foot of the DM rats was made at 10–12 weeks after treatment with STZ. First, thirty rats were randomly divided into three groups (n = 10 per group): Control group (rats injected with normal saline), DM group (rats injected with STZ to construct DM model) and DFU group (DM rats used to construct DFU model). Blood samples were collected from the rats and qRT-PCR was performed to detect the serum levels of miR-217. Then, forty DFU rats were randomly divided into four groups with ten per each: model group, miR-217 inhibitors group, miR-con (control) group, and miR-217 inhibitors + siHIF-1α group. Rats in the miR-217 inhibitors group, miR-con group and miR-217 inhibitors + siHIF-1α group were injected with miR-217 inhibitors, or miR-217 negative control, or siHIF-1α, respectively, mixed with the lipofectamine RNAiMAX Transfection Reagent (Invitrogen, USA) [16, 28]. Next, serum-free culture medium was added and placed for 4–6 h at room temperature. Then, rats of each group were injected with corresponding transfection reagents via the tail vein. miR-217 inhibitors, miR-217 negative control and HIF-1α siRNA were synthesized by Shanghai Genechem Co., Ltd.
Dual-luciferase reporter gene assay
Dual-luciferase reporter gene assay was conducted with human or rat wild-type plasmid HIF1α-3′UTR-WT and mutant-type plasmid HIF1α-3′UTR-MUT. First, 293T cells and skin fibroblast cell line CRL-1213 of Sprague–Dawley rats collected at the logarithmic growth phase were seeded onto 96-well plates. When cell confluence reached about 70%, 293T cells (ATCC) and CRL-1213 (ATCC) were co-transfected with HIF1α-3′UTR-WT or HIF1α-3′UTR-MUT plasmid and miR-217 mimic/NC (Shanghai Genechem Co., Ltd.) based on the instructions on the Lipofectamine 2000 kit. After 6 h of incubation in an incubator (Thermo, USA), the culture medium was replaced by medium containing 10% FBS for another 48 h of incubation. Dual-luciferase activity detection was performed by following the procedures provided by Promega. The culture solution was removed from 96-well plate, cells were rinsed with 100 μL PBS buffer, and 100 μL PLB solution was added into each well, and the plate was shaken for 15 min before the collection of cell lysis solution. The activity of ranilla luciferase was detected and the expression level of reporter gene was presented by firefly to ranilla luciferase activity ratio.
Enzyme-linked immunosorbent assay (ELISA) method
Frozen serum samples of patients and rats were taken and detected by strictly following the instructions on ELISA kit (Wuhan Boster Biological Technology Co. Ltd., Wuhan, Hubei, China). The optical density (OD) value was obtained at the wavelength of 450 nm with a Microplate reader 3550-UV (Bio-Rad). All samples and standard solution were tested by double hole detection to draw the standard curve and calculate the content of HIF-1α and VEGF in samples.
Ulcer area observation in DFU rats
The ulcer healing of rats in each group was observed. On the 3rd, 7th, and 14th day of injection via tail veins, the ulcer area was photographed with a digital camera and analyzed with the software Image-Pro Plus. V6.0 to compare the ulcer healing of rats among different groups. After 14 days, the peripheral blood was taken from the venae angularis and centrifuged for 20 min at the rate of 3500 rpm/min to isolate the serum for the detection of HIF-1α and VEGF levels. At the same time, the changes in the levels of IL-1β, TNF-α and IL-6 were also measured by ELISA assay strictly in accordance with the manufacturer’s instructions. Later, rats were decapitated to get the wound tissues from the foot dorsum of rats, which were preserved in liquid nitrogen.
HE staining
Ulcer tissue samples were fixed in 10% neutral paraformaldehyde, embedded in paraffin, sliced into sections (each 5 μm in thickness), and dewaxed in a gradient manner. Then, sections were stained with hematoxylin for 8 min, followed by color separation with hydrochloric ethanol, rinsed with water, staining with eosin for 3 min, dehydration with gradient alcohol, hyalinization with xylene, and mounting with neutral resin. The pathological morphology changes of wound tissues were observed under a multi-functional fluorescence microscope BX60 (Olympus, Japan).
MVD detected by immunohistochemical method
The skin tissues were taken from foot dorsum of rats in each group, embedded in paraffin, made into sections, and baked at 68 °C for 20 min. After routine dewaxing with xylene, dehydration with gradient alcohol, and placement at room temperature for 15 min, tissues were rinsed with PBS buffer for 2–3 times × 5 min. Next, normal goat serum (NGS) blocking solution was added for 20 min at room temperature, and CD34 antibody (1:2000, BD Biosciences, San Jose, CA, USA) was added for overnight reaction at 37 °C. Then, secondary antibody was added for 1 h of incubation at 37 °C and tissues were washed with PBS buffer before DAB development, observation of chromogenic degree under a microscope, counterstaining with hematoxylin for 2 min, and routine dehydration, hyalinization, mounting and microscopic examination under an Olympus optical microscope. For quantitative analysis by immunohistochemical method, six high-power fields were randomly selected in each section to detect the integrated optical density (IOD) of each visual field.
qRT–PCR
Total RNA was extracted using the miRNeasy1 serum kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions with the exception that C. elegans synthetic miRNA Caenorhabditis elegans (cel)-miR-39 (Qiagen, Hilden, Germany) was added into serum samples prior to extraction and used to monitor RNA recovery, reverse transcription efficiency and for subsequent data normalization. The software Primer 5.0 was used to design the primers of miR-217, which were later synthesized by Sangon Biotech (Shanghai) Co., Ltd. and shown in Table 1. Next, the total RNA was reversely transcribed into cDNA using the PrimescriptTM RT reagent Kit (Takara, Japan), and qRT–PCR was performed with the SYBR® premix Ex Taq TM kit (Takara, Japan). The spiked-in syn-cel-miR-39 serves as endogenous control for data normalization. The expression of miR-217 in the foot dorsum skin tissues of rats was calculated with U6 as the internal reference gene. The 2−ΔΔCt method was used to analyze the relative expression of miR-217.
Western blot analysis
Foot dorsum skin tissues of rats were collected, lysis buffer was added by 100–200 μl to 20 mg tissues, and a glass homogenizer was used for homogenization, followed by 15 min of centrifugation at the rate of 12,000 rpm. The supernatant was collected for SDS–PAGE electrophoresis. Then, electronic transfer was employed to move isolated proteins to the nitrocellulose filter, which was placed in 5% skimmed milk PBS buffer for blocking for 1 h. Later, primary antibodies were added for overnight incubation at 4 °C, including HIF-1α (ab16066), VEGF (ab46154), VEGFR2 (ab11939), eNOS (ab5589), MMP-2 (ab151866), MMP-9 (ab73734) antibodies (1:1000, Abcam). Subsequently, the filter was rinsed with PBS buffer for three times and horseradish peroxidase (HRP)-conjugated secondary antibodies were added for 1 h of incubation at room temperature. Finally, the enhanced chemiluminescence method was used for development. Relative expression levels of proteins were presented as the gray value ratio of target band to reference band with GAPDH as the internal reference gene.
Statistical methods
Data analysis was conducted with the statistical software package SPSS 21.0 (IBM Corp., Armonk, NY, USA). Measurement data were presented as mean ± standard deviation, the comparison among multiple groups was conducted using one-way ANOVA, and intergroup comparison was performed by post hoc Tukey test. Above all, P < 0.05 indicated significant difference.
Results
Serum levels of miR-217 and HIF-1α/VEGF in DFU patients and rats
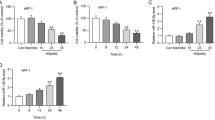
As shown in Fig. 1, compared with healthy controls, DM patients and rats were apparently increased in serum levels of miR-217, HIF-1α and VEGF, but DFU patients and rats had significantly higher miR-217 but lower HIF-1α and VEGF serum levels than DM and healthy controls (all P < 0.05). In DFU patients with different Wagner grades, serum miR-217 levels gradually went up, while the levels of HIF-1α and VEGF decreased with the increase of Wagner grade (all P < 0.05).
The serum levels of miR-217 and HIF-1α/VEGF in DFU patients and rats. a–c The serum levels of miR-217 (a), HIF-1α (b) and VEGF (c) in healthy control (HC), diabetes mellitus (DM) and diabetic foot ulcer (DFU) patients *, P < 0.05 compared with HC group; #, P < 0.05 compared with DM group. d–f Comparison of the serum levels of miR-217 (d), HIF-1α (e) and VEGF (f) in DFU patients of different Wagner grade; *, P < 0.05 compared with grade I; #, P < 0.05 compared with grade II; &, P < 0.05 compared with grade III; %, P < 0.05 compared with grade IV. g–i The serum levels of miR-217 (g), HIF-1α (h) and VEGF (i) in control, DM and DFU rats
MiR-217 could target HIF-1α
With the platform TargetScan (http//www.targetscan.org/) shown in Fig. 2a, we found a binding site to miR-217 at the 3′-UTR region of HIF-1α from the human and rat. According to results of dual-luciferase reporter gene assay in 293T and CRL-1213 cells, the luciferase activity of HIF1α-3′UTR-MUT did not show observable difference after transfection with miR-217 mimic and NC (all P > 0.05). However, compared with NC, miR-217 mimic could dramatically reduce the luciferase activity of HIF1α-3′UTR-WT (all P < 0.05, Fig. 2b, c), suggesting that HIF-1α is a direct target gene of miR-217.
Verification of the target relationship between miR-217 and HIF-1α. a The binding site to miR-217 at the 3′-UTR region of HIF-1α shown by the target gene prediction website; b–c, miR-217 mimic reducing the luciferase activity of HIF1α-3′UTR-WT in 293T (b) and CRL-1213 cells (c) detected by dual-luciferase reporter gene assay; *, P < 0.05 indicated the significant differences
The ulcer healing of DFU rats
As illustrated by Fig. 3a, compared with the rats in the model group, those in the miR-con group did not show obvious changes in ulcer area at different time points (all P > 0.05), while rats in the miR-217 inhibitors group presented significant reduction in ulcer area and apparent accelerated ulcer healing on the 3d, 7d and 14d (all P < 0.05). Moreover, rats in the miR-217 inhibitors + siHIF-1α group had a larger ulcer area and slower healing than those in the miR-217 inhibitors group (all P < 0.05).
The ulcer healing of DFU rats in each group. a Comparison of ulcer area of rats in each group; *, P < 0.05 compared with model group; #, P < 0.05 compared with miR-217 inhibitors group; b, pathological morphology of ulcer area of rats on the 14th day of injection in each group observed after HE staining, with the arrow indicating neo-vessels
Additionally, HE staining (Fig. 3b) showed that rats in the model group had increased inflammatory cells, as well as decreased fibroblasts and neo-vessels, while those in the miR-217 inhibitors group presented typical new granulation tissues, which was demonstrated by the infiltration of massive fibroblasts and the formation of new capillary vessels. In comparison with miR-217 inhibitors group, the miR-217 inhibitors + siHIF-1α group was slower in healing and statistically reduced in fibroblasts and neo-vessels.
MVD of DFU rats in each group
As displayed in Fig. 4, CD34 protein was mainly expressed in cytoplasm of vascular endothelial cells with the brownish-yellow color. Compared with the model group, miR-con group was not significantly different in MVD of DFU rats (P > 0.05), while miR-217 inhibitors group went up remarkably in MVD (P < 0.05). Besides, rats in the miR-217 inhibitors + siHIF-1α group showed apparently decreased MVD compared with rats in the miR-217inhibitors group (P < 0.05).
Comparison of MVD in DFU rats from different groups. a Expression of CD34 in rats of each group detected by immunohistochemical assay on the 14th day of injection (red arrows indicate the newly developed blood vessels); b, comparison of MVD in DFU rats from different groups; *, P < 0.05 compared with model group; #, P < 0.05 compared with miR-217 inhibitors group
Expression of HIF-1α, VEGF and inflammatory factors in peripheral blood of rats
As shown in Fig. 5, compared with rats in the model group, those in the miR-217 inhibitors group had markedly decreased miR-217 serum levels and inflammatory factors (IL-1β, TNF-α and IL-6), but up-regulated levels of HIF-1α and VEGF (all P < 0.05); however, those in the miR-con group did not show any observable changes in these molecules (all P > 0.05). In comparison with rats in miR-217 inhibitors group, those in the miR-217 inhibitors + siHIF-1α group did not differ significantly in serum miR-217 levels, but declined dramatically in the levels of HIF-1α and VEGF and increased remarkably in inflammatory factors (IL-1β, TNF-α and IL-6) (all P < 0.05).
Expression of miR-217 and HIF-1α/VEGF pathway-related proteins in foot dorsum skin tissues of DFU rats
Compared with rats in the model group, those in the miR-217 inhibitors group demonstrated a decrease in the expression of miR-217 and a dramatic increase in the protein expressions of HIF-1α, VEGF, VEGFR2, eNOS, MMP-2, and MMP-9 (all P < 0.05, Fig. 6), while rats in the miR-con group showed no significant difference in these indexes (all P > 0.05). Meanwhile, miR-217 inhibitors + siHIF-1α group was remarkably lower than the miR-217 inhibitors group in the protein expression levels of HIF-1α, VEGF, VEGFR2, eNOS, MMP-2 and MMP-9 (all P < 0.05), but has no significance in the expression of miR-217 (P > 0.05).
The expression of miR-217and HIF-1α/VEGF pathway-related proteins in foot dorsum skin tissues of DFU rats. a Expression of miR-217 in foot dorsum skin tissues of DFU rats detected by qRT–PCR; b, expression of HIF-1α/VEGF pathway-related proteins detected by Western blot; c–h, Protein expressions of HIF-1α (c), VEGF (d), VEGFR2 (e), eNOS (f), MMP-2 (g), and MMP-9 (h) in DFU rats of each group. *, P < 0.05 compared with model group; #, P < 0.05 compared with miR-217 inhibitors group
Discussion
By detecting the serum level of miR-217, we found DFU patients had higher miR-217 serum levels as compared with healthy controls and simple DM patients, and this indicator was positively correlated to the severity of DFU. Consistent with our findings, miR-217 expression was apparently up-regulated in patients with diabetes mellitus type 2 (T2DM) and positively correlated to the DM severity, as indicated by Shao Y et al. [40]. Meanwhile, high glucose exposure induced the increase of miR-217, but silencing miR-217 can improve cell viability, reduce cell apoptosis, and mitigate high glucose-induced foot tissue injury in the study of Sun J et al. [43]. These evidences provided a possibility that the up-regulation of miR-217 in DFU patients was likely to be caused by the sustained high glucose stimulation. Besides, we also found apparently higher levels of HIF-1α and VEGF in DM patients than in healthy controls, while these two indicators were obviously reduced in the serum of DFU patients with the increase of disease severity. Also, Pichu S et al. also discovered that the HIF-1α expression level in DFU patients was dramatically lower than that in T2DM patients and healthy controls [35]. And the serum level of VEGF-A in DFU patients was remarkably lower than that in simple DM patients and the increase of VEGF-A concentration was associated with the reduction of risk for DFU in the study of Chen Z et al. [8]. Mechanically, the up-regulated expression of HIF-1α and VEGF in DM patients may be caused by the hypoxia and ischemia of local tissues, which activated the endogenous defense and repair mechanism, and thereby leading to the increase of HIF-1α (a transcriptional complex responding to hypoxia) to affect glucose metabolism and up-regulate the expression of HIF-1α inducible genes, like VEGF [25, 36]. Indeed, high glucose can suppress the protein expression and transcriptional function of HIF-1α in the previous work [12, 45], which may explain the reduction of HIF-1α and VEGF in DFU patients. Long-term high glucose exposure would lower the stability of HIF-1α, reduce the response of tissues and cells to hypoxia, and cause the decreased angiogenesis of DM patients, and thus inhibiting the wound healing of DM patients and even aggravating DFU [6, 49]. All the above suggested that long-term high glucose in DM patients caused up-regulation of miR-217 and downregulation of HIF-1α and VEGF, which may participate in DFU development.
Beyond that, the DFU rat models were also constructed, and we found the reduced ulcer area and the increased MVD in DFU rats after treated with miR-217 inhibitors. Since MVD is one of the most useful indicators in reflecting angiogenesis [15], we can hypothesize that inhibition of miR-217 could accelerate the ulcer healing and promote angiogenesis of DFU rats. In agreement with our research, inhibiting miR-217 could up-regulate SirT1 expression to suppress endothelial cell senescence and enhance angiogenic activity, as suggested by Menghini et al. [32]. Another important finding in this study was that DFU rats treated with miR-217 inhibitors presented an elevated expression of HIF-1α and VEGF, decreased expression of inflammatory factors (IL-1β, TNF-α, and IL-6), as well as dramatically increased eNOS, MMP-2 and MMP-9. To our knowledge, a variety of inflammatory factors, like IL-1β, TNF-α and IL-6 secreted by monocytes and neutrophils, are involved in the wound healing process in DFU patients [1, 34, 41]. In addition, IL-1β could also induce the expression of MMPs [2], and the abnormal expression of MMPs in the wound healing process could trigger the extensive hydrolysis of matrix, thus hindering the healing of ulcers [27, 37]. Furthermore, the enhanced VEGF, and reduced levels of IL-1β, TNF-1α, as well as the decreased protein expressions of eNOS, MMP2 and MMP9, would promote the wound healing of DFU rats, ameliorate angiogenesis and inhibit inflammation in another study [30], which demonstrated that miR-217 inhibitors could suppress the inflammation and ameliorate the abnormal expression of MMPs to promote ulcer healing. At the same time, HIF-1α has been identified to be able to bind to HIF-1β subunit in endothelial cells to increase transcription and the binding to VEGF initiator, elevating the expression level of VEGF [11, 19], which is an angiogenic factor closely related to neovascularization (NV) [20], and can effectively promote the proliferation and migration of endothelial cells with the elevated level of endothelial nitric oxide synthase (eNOS) [22], triggering a series of reactions to induce angiogenesis and increase the blood supply to local ulcer area, and promoting wound healing in DFU patients [29, 50]. Moreover, the DFU mice treated with 20(S)-protopanaxadiol (PPD) could stimulate angiogenesis by upregulating the expression of HIF-1α-mediated VEGF to accelerate wound healing in the study of Zhang EY et al. [52]. Sunkari et al. also revealed that over-expressed HIF-1α could enhance the proliferation of endothelial cells and promote the wound healing of DFU rats [44]. Worth mentioning, HIF-1α was confirmed to be the target gene of miR-217 through utilizing dual-luciferase reporter gene assay in our investigation. Similar to our results, miR-217 could modulate the proliferation, EMT and apoptosis of renal cell carcinoma (Rcc) cells by targeting HIF-1α [18]. More importantly, our animal experiments revealed that HIF-1α siRNA could reverse the role of miR-217 inhibitors in promoting ulcer healing, suggesting that suppressing miR-217 could enhance VEGF expression and promote angiogenesis by upregulating the expression of its target gene HIF-1α, and thus accelerating wound healing.
To sum up, our study found up-regulated miR-217 expression and down-regulated HIF-1α/VEGF expression in DFU patients. Moreover, inhibiting miR-217 could promote angiogenesis and ameliorate inflammation by up-regulation of HIF-1α/VEGF pathway, thereby accelerating the ulcer healing of DFU rats.
References
Ahmad J, Zubair M, Malik A, Siddiqui MA, Wangnoo SK (2012) Cathepsin-D, adiponectin, TNF-alpha, IL-6 and hsCRP plasma levels in subjects with diabetic foot and possible correlation with clinical variables: a multicentric study. Foot (Edinb) 22:194–199
Aida Y, Maeno M, Suzuki N, Shiratsuchi H, Motohashi M, Matsumura H (2005) The effect of IL-1beta on the expression of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in human chondrocytes. Life Sci 77:3210–3221
Aumiller WD, Dollahite HA (2015) Pathogenesis and management of diabetic foot ulcers. JAAPA 28:28–34
Baptista RB, Souza-Castro N, Almeida-Val VM (2016) Acute hypoxia up-regulates HIF-1alpha and VEGF mRNA levels in Amazon hypoxia-tolerant Oscar (Astronotus ocellatus). Fish Physiol Biochem 42:1307–1318
Berchner-Pfannschmidt U, Tug S, Kirsch M, Fandrey J (2010) Oxygen-sensing under the influence of nitric oxide. Cell Signal 22:349–356
Botusan IR, Sunkari VG, Savu O, Catrina AI, Grunler J, Lindberg S, Pereira T, Yla-Herttuala S, Poellinger L, Brismar K et al (2008) Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci USA 105:19426–19431
Brem H, Sheehan P, Boulton AJ (2004) Protocol for treatment of diabetic foot ulcers. Am J Surg 187:1S–10S
Chen Z, Fu S, Wu Z, Chen J, Huang Y, Wang Y, Fu M (2018) Relationship between plasma angiogenic growth factors and diabetic foot ulcers. Clin Chim Acta 482:95–100
de Yebenes VG, Bartolome-Izquierdo N, Nogales-Cadenas R, Perez-Duran P, Mur SM, Martinez N, Di Lisio L, Robbiani DF, Pascual-Montano A, Canamero M et al (2014) miR-217 is an oncogene that enhances the germinal center reaction. Blood 124:229–239
Frank RN (2004) Diabetic retinopathy. N Engl J Med 350:48–58
Galasso G, Schiekofer S, Sato K, Shibata R, Handy DE, Ouchi N, Leopold JA, Loscalzo J, Walsh K (2006) Impaired angiogenesis in glutathione peroxidase-1-deficient mice is associated with endothelial progenitor cell dysfunction. Circ Res 98:254–261
Gao W, Ferguson G, Connell P, Walshe T, Murphy R, Birney YA, O’Brien C, Cahill PA (2007) High glucose concentrations alter hypoxia-induced control of vascular smooth muscle cell growth via a HIF-1alpha-dependent pathway. J Mol Cell Cardiol 42:609–619
Gibbons GW (2003) Lower extremity bypass in patients with diabetic foot ulcers. Surg Clin North Am 83:659–669
Glynn JJ, Hinds MT (2014) Endothelial outgrowth cells: function and performance in vascular grafts. Tissue Eng Part B Rev 20:294–303
Han ZG, Yu TT, Shan L (2012) Expression of erythropoietin and erythropoietin receptor in non-small cell lung cancer and its correlation with microvessel density. Zhonghua Zhong Liu Za Zhi 34:605–608
He B, Xiao J, Ren AJ, Zhang YF, Zhang H, Chen M, Xie B, Gao XG, Wang YW (2011) Role of miR-1 and miR-133a in myocardial ischemic postconditioning. J Biomed Sci 18:22
Health USNIo (1985) Laboratory animal welfare: public health service policy on humane care and use of laboratory animals by awardee institutions; notice. Fed Regist 50:19584–19585
Hong Q, Li O, Zheng W, Xiao WZ, Zhang L, Wu D, Cai GY, He JC, Chen XM (2017) LncRNA HOTAIR regulates HIF-1alpha/AXL signaling through inhibition of miR-217 in renal cell carcinoma. Cell Death Dis 8:e2772
Jeon O, Song SJ, Bhang SH, Choi CY, Kim MJ, Kim BS (2007) Additive effect of endothelial progenitor cell mobilization and bone marrow mononuclear cell transplantation on angiogenesis in mouse ischemic limbs. J Biomed Sci 14:323–330
Keating AM, Jacobs DS (2011) Anti-VEGF treatment of corneal neovascularization. Ocul Surf 9:227–237
Kondo T, Ishida Y (2010) Molecular pathology of wound healing. Forensic Sci Int 203:93–98
Kuhlencordt PJ, Rosel E, Gerszten RE, Morales-Ruiz M, Dombkowski D, Atkinson WJ, Han F, Preffer F, Rosenzweig A, Sessa WC et al (2004) Role of endothelial nitric oxide synthase in endothelial activation: insights from eNOS knockout endothelial cells. Am J Physiol Cell Physiol 286:C1195–C1202
Kuwabara M (2016) Hyperuricemia, cardiovascular disease, and hypertension. Pulse (Basel) 3:242–252
Ladeia AM, Sampaio RR, Hita MC, Adan LF (2014) Prognostic value of endothelial dysfunction in type 1 diabetes mellitus. World J Diabetes 5:601–605
Lee JH, Gao Z, Ye J (2013) Regulation of 11beta-HSD1 expression during adipose tissue expansion by hypoxia through different activities of NF-kappaB and HIF-1alpha. Am J Physiol Endocrinol Metab 304:E1035–E1041
Lee YC, Hung MH, Liu LY, Chang KT, Chou TY, Wang YC, Wu YC, Lai CL, Tsai CC, Su KC et al (2011) The roles of transforming growth factor-beta(1) and vascular endothelial growth factor in the tracheal granulation formation. Pulm Pharmacol Ther 24:23–31
Li N, Luo HC, Yang C, Deng JJ, Ren M, Xie XY, Lin DZ, Yan L, Zhang LM (2014) Cationic star-shaped polymer as an siRNA carrier for reducing MMP-9 expression in skin fibroblast cells and promoting wound healing in diabetic rats. Int J Nanomed 9:3377–3387
Li XQ, Chen FS, Tan WF, Fang B, Zhang ZL, Ma H (2017) Elevated microRNA-129-5p level ameliorates neuroinflammation and blood-spinal cord barrier damage after ischemia-reperfusion by inhibiting HMGB1 and the TLR3-cytokine pathway. J Neuroinflammation 14:205
Losi P, Briganti E, Errico C, Lisella A, Sanguinetti E, Chiellini F, Soldani G (2013) Fibrin-based scaffold incorporating VEGF- and bFGF-loaded nanoparticles stimulates wound healing in diabetic mice. Acta Biomater 9:7814–7821
Lv Y, Ge L, Zhao Y (2017) Effect and mechanism of SHED on ulcer wound healing in Sprague-Dawley rat models with diabetic ulcer. Am J Transl Res 9:489–498
Manzke E, Katchburian E, Faria FP, Freymuller E (2005) Structural features of forming and developing blood capillaries of the enamel organ of rat molar tooth germs observed by light and electron microscopy. J Morphol 265:335–342
Menghini R, Casagrande V, Cardellini M, Martelli E, Terrinoni A, Amati F, Vasa-Nicotera M, Ippoliti A, Novelli G, Melino G et al (2009) MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation 120:1524–1532
Moura LI, Dias AM, Carvalho E, de Sousa HC (2013) Recent advances on the development of wound dressings for diabetic foot ulcer treatment—a review. Acta Biomater 9:7093–7114
Pakyari M, Farrokhi A, Maharlooei MK, Ghahary A (2013) Critical role of transforming growth factor beta in different phases of wound healing. Adv Wound Care (New Rochelle) 2:215–224
Pichu S, Sathiyamoorthy J, Krishnamoorthy E, Umapathy D, Viswanathan V (2015) Impact of the hypoxia inducible factor-1alpha (HIF-1alpha) pro582ser polymorphism and its gene expression on diabetic foot ulcers. Diabetes Res Clin Pract 109:533–540
Rahtu-Korpela L, Karsikas S, Horkko S, Blanco Sequeiros R, Lammentausta E, Makela KA, Herzig KH, Walkinshaw G, Kivirikko KI, Myllyharju J et al (2014) HIF prolyl 4-hydroxylase-2 inhibition improves glucose and lipid metabolism and protects against obesity and metabolic dysfunction. Diabetes 63:3324–3333
Rohani MG, Parks WC (2015) Matrix remodeling by MMPs during wound repair. Matrix Biol 44–46:113–121
Saaristo A, Tammela T, Farkkila A, Karkkainen M, Suominen E, Yla-Herttuala S, Alitalo K (2006) Vascular endothelial growth factor-C accelerates diabetic wound healing. Am J Pathol 169:1080–1087
Shao Y, Lv C, Wu C, Zhou Y, Wang Q (2016) Mir-217 promotes inflammation and fibrosis in high glucose cultured rat glomerular mesangial cells via Sirt1/HIF-1alpha signaling pathway. Diabetes Metab Res Rev 32:534–543
Shao Y, Ren H, Lv C, Ma X, Wu C, Wang Q (2017) Changes of serum Mir-217 and the correlation with the severity in type 2 diabetes patients with different stages of diabetic kidney disease. Endocrine 55:130–138
Shaw TJ, Martin P (2009) Wound repair at a glance. J Cell Sci 122:3209–3213
Su J, Wang Q, Liu Y, Zhong M (2014) miR-217 inhibits invasion of hepatocellular carcinoma cells through direct suppression of E2F3. Mol Cell Biochem 392:289–296
Sun J, Li ZP, Zhang RQ, Zhang HM (2017) Repression of miR-217 protects against high glucose-induced podocyte injury and insulin resistance by restoring PTEN-mediated autophagy pathway. Biochem Biophys Res Commun 483:318–324
Sunkari VG, Lind F, Botusan IR, Kashif A, Liu ZJ, Yla-Herttuala S, Brismar K, Velazquez O, Catrina SB (2015) Hyperbaric oxygen therapy activates hypoxia-inducible factor 1 (HIF-1), which contributes to improved wound healing in diabetic mice. Wound Repair Regen 23:98–103
Thangarajah H, Vial IN, Grogan RH, Yao D, Shi Y, Januszyk M, Galiano RD, Chang EI, Galvez MG, Glotzbach JP et al (2010) HIF-1alpha dysfunction in diabetes. Cell Cycle 9:75–79
Urra H, Hetz C (2014) A novel ER stress-independent function of the UPR in angiogenesis. Mol Cell 54:542–544
Wagner FW Jr (1981) The dysvascular foot: a system for diagnosis and treatment. Foot Ankle 2:64–122
World Medical A (2013) World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310:2191–2194
Xiao H, Gu Z, Wang G, Zhao T (2013) The possible mechanisms underlying the impairment of HIF-1alpha pathway signaling in hyperglycemia and the beneficial effects of certain therapies. Int J Med Sci 10:1412–1421
Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J (2000) Vascular-specific growth factors and blood vessel formation. Nature 407:242–248
Yazdanpanah L, Nasiri M, Adarvishi S (2015) Literature review on the management of diabetic foot ulcer. World J Diabetes 6:37–53
Zhang EY, Gao B, Shi HL, Huang LF, Yang L, Wu XJ, Wang ZT (2017) 20(S)-Protopanaxadiol enhances angiogenesis via HIF-1alpha-mediated VEGF secretion by activating p70S6 kinase and benefits wound healing in genetically diabetic mice. Exp Mol Med 49:e387
Zhang S, Liu L, Wang R, Tuo H, Guo Y, Yi L, Wang D, Wang J (2013) MicroRNA-217 promotes angiogenesis of human cytomegalovirus-infected endothelial cells through downregulation of SIRT1 and FOXO3A. PLoS One 8:e83620
Zhao QS, Xia N, Zhao N, Li M, Bi CL, Zhu Q, Qiao GF, Cheng ZF (2013) Localization of human mesenchymal stem cells from umbilical cord blood and their role in repair of diabetic foot ulcers in rats. Int J Biol Sci 10:80–89
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
The clinical research in this study was approved by the Ethics Committee of The First Affiliated Hospital of Shantou University Medical College and conformed to the guidelines of Helsinki Declaration.
Informed consent
All samples were collected after obtaining the informed consent form signed by all the subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, CJ., Lan, YM., Ou, MQ. et al. Expression of miR-217 and HIF-1α/VEGF pathway in patients with diabetic foot ulcer and its effect on angiogenesis of diabetic foot ulcer rats. J Endocrinol Invest 42, 1307–1317 (2019). https://doi.org/10.1007/s40618-019-01053-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-019-01053-2