Abstract
Purpose
This study was designed to examine the correlation of resistance exercise (RE)-induced myostatin (MSTN) with insulin resistance and plasma cytokines in healthy young men.
Methods
Twenty-four healthy men were randomly divided into RE (n = 12) and control (n = 12) group. After a session of familiarization, one repetition maximum (1-RM) was calculated. Circuit RE program involved 3 sets of 15 repetitions at 55 % of 1-RM. Blood samples were collected before and 24 h after the exercise. Paired t test, independent t test, and Pearson’s correlation were used for analyzing data.
Results
A significant decrease in plasma level of MSTN, glucose, insulin, interleukin-6 (IL-6), and homeostasis model assessment of insulin resistance (HOMA-IR) and a significant increase in plasma interleukin-10 (IL-10) were found in RE group 24 h post-exercise versus pre-exercise (p < 0.05). Furthermore, except plasma IL-10, a significant decrease in metabolic variables was found in RE group versus control group (p < 0.05). A significantly positive correlation of plasma MSTN with HOMA-IR and plasma IL-6 and a significantly negative correlation of plasma MSTN with plasma IL-10 were found in RE group versus control group (p < 0.05).
Conclusions
It seems that a circuit RE bout by reducing HOMA-IR and changing plasma cytokines (decreased IL-6 and increased IL-10) can decrease plasma level of MSTN in healthy young men. In other word, the beneficial effect of acute RE may be reflected by changes in MSTN in healthy young individuals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skeletal muscle produces biologically active proteins or “myokines” that facilitate metabolic interaction between body systems [1]. Myostatin (MSTN) belongs to the TGF-β superfamily and is a negative regulator of muscle development and size [2, 3]. MSTN binds to activin receptor IIb (ActRIIb) and inhibits skeletal muscle growth [4]. Recently, the direct effect of MSTN on adipocytes has been explored, so that MSTN mRNA was expressed in adipose tissue, but at much lower levels than in skeletal muscle [2–5]. Furthermore, MSTN protein is found in circulation [6]. Thus, the physiological functions of MSTN are not restricted to suppressing skeletal muscle growth, so that loss of, or decrease in, MSTN levels leads to increased insulin sensitivity and decreased insulin resistance [7]. Hence, the novel strategies, such as MSTN inhibition, that target muscle and fat mass accumulation may be beneficial in preventing obesity and insulin resistance [8]. Moreover, skeletal muscle can function as an endocrine organ and play important roles in metabolic regulation through secreting several cytokines [8]. These cytokines, termed myokines, are exerted in a hormone-like fashion and have functions in regulating metabolism [8]. Both MSTN, as a muscle-secreted cytokine, and muscle inflammation-related cytokines (especifically interleukin-6 (IL-6) and interleukin-10 (IL-10)) contribute to insulin resistance [9] and response to exercise [9, 10].
Several studies have examined the effect of chronic exercise-induced MSTN on energy metabolism [11–13]; however, studies on the MSTN response to acute exercise [14] and correlation of acute exercise-induced MSTN with metabolic parameters are limited. The potential benefit of circuit resistance training (a form of conditioning combining resistance training and high-intensity aerobics) for body weight management, especially when using light weights with 40–60 % of one repetition maximum (1-RM) made circuit resistance training to become an accepted part of programs for health [15]. Thus, this study was designed to evaluate the MSTN response to acute circuit resistance exercise (RE) and its correlation with insulin resistance and plasma cytokines (IL-6 and IL-10) in healthy young men.
Materials and methods
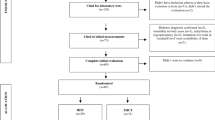
Twenty-four healthy young male students, placed on university dormitories boards, were selected in this study. In first session, subjects were asked to fill out questionnaires regarding to life style, past medical history, and physical activity to recruit eligible subject for the study. Inclusion criteria were age between 20 and 25 years and not to be smoker. Moreover, they should be free of any kind of disease that would prevent them from participating in this study such as cardiovascular, metabolic, and musculoskeletal disorders. Those who took any medication and supplements were excluded. In addition, all subjects experienced the weight training. The study protocol was approved by the Ethics Committee of the Shahid Beheshti University, Faculty of Physical Education and Sport Sciences. Verbal and written explanations of the experimental protocol were presented to the subjects prior to any data collection. Written consent also was obtained from all subjects before the participation in this study. Participants were randomly divided into circuit RE (n = 12) and control (n = 12) group. Subjects of RE group were familiarized with the procedures and equipment before the experiment. One week before the experiment, a pilot study is used to test the design of the full-scale experiment. Participants were asked to avoid exercise or strenuous physical activity for 48 h before the experiment.
The height (cm) was measured using Stadiometer (Germany; SECA) and the weight (kg) by Balance (Germany; SECA). Body mass index (BMI) was calculated as the ratio of the body weight (kg) to the square of height (m2). WHR was calculated after measuring waist and hip circumferences. The skin folds were obtained using Harpenden skin fold caliper (Slim Guioe, US) on the right side of the body at the 3 sites: triceps, abdominal, and suprailiac. All measurements were taken in triplicate and average values at each point were used to estimate body fat percent [16].
Subjects arrived at the laboratory of physical activity and sport sciences faculty at 7:30 a.m. in an 8 h overnight fasted state and their height, weight, and body composition were measured.
Prior to calculating 1-RM, subjects performed a general warm-up (5 min running on a treadmill at moderate intensity) and a specific warm-up (2 sets of 7 repetitions of resistance training similar to the original protocol but at low intensity) to reduce the risk of injury. Thereafter, the circuit RE session targeted the upper and lower body, with 3 sets of 15 repetitions on 7 machines (chest press, pull up, leg press, shoulder press, knee extension, knee flexion, and elbow flexion) each lasting ~30 s. All exercises were performed at 55 % of the subjects’ 1-RM with 2-min rest intervals between sets and in total required ~20 min to complete. 1-RM was assessed by gradually increasing the weight, and the test was continued until the subject was not able to maintain proper form and fully lift the given weight. The last weight lifted fully was considered the 1-RM for each subject. Furthermore, subjects in control group performed any exercise (in a rest condition).
According to a previous study that indicated the response of plasma MSTN to a session of RE during 1–48 h after I-RM [14], blood samples were collected before and 24 h after the exercise. Blood samples were promptly centrifuged and the plasma was separated and stored at −70 °C. Plasma MSTN was analyzed by a Human MSTN ELISA kit (Cusabio Biotech, Wuhan, China), with intra-assay coefficient variation (InteraCV) of 6.3 %, respectively. Plasma IL-6 and IL-10 were measured using an ELISA kit (Diaclone, France; IntraCV: 7.5 and 6.8 %,), Plasma glucose was assessed by a colorimetric enzymatic method (glucose oxidase) (Pars Azmoon, Tehran, Iran; IntraCV: 7.2 %), and plasma insulin using an ELISA kit (Mercodia, Uppsala, Sweden; IntraCV: 5.1 %). Insulin resistance index was calculated using formula of homeostasis model assessment of insulin resistance: HOMA-IR = fasting insulin (μU/ml) × fasting glucose (mmol/l)/22.5 [17].
The Kolmogorov–Smirnov test was performed to define the presence of normality. Paired t test and independent t test were used for means comparison of metabolic parameters within (pre- and post-exercise) and between groups. The correlation between variables was analyzed by Pearson correlation. The level of significance was set at p < 0.05. The data are expressed as mean ± SD. All of the statistical tests were performed using the SPSS statistical package version 16 for Windows (Chicago, IL, USA).
Results
No significant difference was found in baseline characteristics of subjects (p > 0.05) (Table 1).
According to Table 2, a significant decrease in plasma level of MSTN, glucose, insulin, IL-6, and HOMA-IR and a significant increase in plasma IL-10 were found in RE group 24 h post-exercise versus pre-exercise (p < 0.05). Furthermore, no significant difference in metabolic variables was found in control group 24 h post-exercise versus pre-exercise (p > 0.05).
A significant decrease in plasma level of MSTN, glucose, insulin, IL-6, and HOMA-IR, and a significant increase in plasma IL-10 were found in RE group versus control group (p < 0.05) (Table 2).
A significantly positive correlation of changes in plasma MSTN (∆MSTN) with the changes in HOMA-IR (∆HOMA-IR) (Fig. 1a) and in plasma IL-6 (∆IL-6) (Fig. 2a) and a significantly negative correlation of changes in plasma MSTN (∆MSTN) with the changes in IL-10 (∆IL-10) (Fig. 3a) were found in RE group (p < 0.05). Furthermore, no significant correlation in changes of metabolic variables was found in control group (p > 0.05) (Figs. 1b, 2b, 3b).
Conclusion
In present study, the ability of a single session of RE to change MSTN in healthy young men was examined. Several studies have indicated a decrease in muscle mRNA level of MSTN following a bout of RE [18–20], whereas a few studies have evaluated the response of plasma MSTN to RE [14]. In this study, a decrease in plasma level of MSTN was found 24 h after a single session of circuit RE in healthy young men. This result is in accordance with the finding of the only available study performed by Hulmi et al. [14] that reported a decrease in plasma MSTN 1–48 h after a session of heavy RE (5 sets of 10 repetitions with a load 75 % of 1-RM) in men. Notably, circulating MSTN protein is in relation to other factors, e.g., age, gender, and body mass [1, 10] and depends upon aspects of sampling time-points as well as exercise regimens (rest, repetition number, intensity, and duration of contraction). Furthermore, the effects of RE on MSTN may differ in timing or magnitude for muscle mRNA versus muscle protein versus plasma protein.
Other finding of this study suggests a decrease in plasma level of glucose and insulin and HOMA-IR 24 h after a circuit RE bout. In a similar study, Koopman et al. [21] showed that a single session of RE (8 sets of 10 repetitions at 75 % of 1-RM) improved whole-body insulin sensitivity (decreased HOMA-IR) for up to 24 h in healthy men. Furthermore, a positive correlation was found between plasma level of MSTN and HOMA-IR, indicating that a circuit RE bout may be sufficient to reduce insulin resistance as well as plasma level of MSTN and that exercise-induced decrease in insulin resistance may decrease plasma MSTN. Although the response of MSTN to exercise and the specific mechanisms and signaling events in skeletal muscle and adipose tissue that helps to overcome insulin resistance is not clearly understood [22], it is known that MSTN has functions in the regulation of metabolic homeostasis and potentially acts as a myokine for maintaining whole-body energy homeostasis [8]. MSTN regulates glucose metabolism by increasing glycolysis and glucose uptake, as well as decreasing glycogen content. It has been known that MSTN is an important part of signaling pathways that determine whole-body insulin sensitivity and that insulin action is related to circulating MSTN [1]. Recently, a mechanism has been proposed whereby the loss of MSTN will lead to the activation of AMP-activated protein kinase (AMPK). The activation of AMPK not only leads to increased fatty acid oxidation, but it also promotes glucose uptake by accelerating glucose transporter-4 (GLUT-4) trafficking to the membrane [5]. Similar to the effects of a single bout of endurance exercise, the stimulation of insulin sensitivity following RE may attribute to decreased muscle GLUT-4 translocation and/or increased GLUT-4 expression [21]. Since the increase in glucose transport in response to a single bout of exercise is mediated by activation of the AMPK pathway [23], the activation of AMPK may provide the underlying link between RE-mediated insulin sensitization and MSTN changes. Therefore, these data reveal that MSTN is a novel regulator of glucose metabolism and MSTN antagonists such as exercise may be beneficial in targeting insulin resistance.
Moreover, in present study, a decrease in plasma IL-6 and an increase in plasma IL-10 were found 24 h after a circuit RE bout. This study is the first suggesting that acute RE-induced MSTN relates to plasma cytokines. In addition, a positive correlation between plasma MSTN and IL-6 and a negative correlation between plasma MSTN and IL-10 were found in RE group, suggesting that decrease in plasma IL-6 and increase in plasma IL-10 following RE may reduce plasma MSTN. Skeletal muscle is an endocrine organ and upon contraction stimulates the production and release of cytokines that can affect metabolism. IL-6 as an inflammatory cytokine is released from skeletal muscle in response to RE [11]. IL-6 is the first cytokine present in the circulation during exercise and can elicit pro-inflammatory or anti-inflammatory effects [24]. Notably, the exercise-induced increase in plasma IL-6 is not linear over time; repeated measurements during exercise indicate an accelerating increase of plasma IL-6. In addition, the peak IL-6 level is reached at the end of the exercise or shortly, thereafter, followed by a rapid reduction toward pre-exercise levels [25]. A similar study also reported that plasma IL-6 increased immediately after the RE and went back to baseline levels at 6 h in men [26].
Furthermore, IL-6 is secreted by skeletal muscle during exercise and exercise-induced increases in plasma level of IL-6 are followed by increased circulating level of anti-inflammatory cytokine IL-10 [9], indicating that IL-6 has an anti-inflammatory effect and may negatively regulate the inflammation of acute phase response by increasing IL-10 [27]. Although the exercise-induced IL-6 and IL-10 response is dependent on intensity, duration, and mode of the exercise, [25, 27, 28], in present study, the adaptations induced by RE training in subjects who having experienced weight training may be sufficient to decrease plasma IL-6 and increase IL-10 24 h after acute RE. In other word, the anti-inflammatory effects of regular RE may be mediated by induction of an anti-inflammatory environment with each bout of circuit RE in healthy men.
Notably, although IL-6 has been related to low-grade inflammation and insulin resistance, in muscle cells, it may have a metabolic role with particular influences involving glycogen levels of the muscle [24]. IL-6 alone can increase glycolysis and glucose uptake [8], suggesting that lowered glycogen stores following the exercise bout may affect plasma level of IL-6. IL-6 is implicated in skeletal muscle insulin resistance [9] and in a previous study, plasma IL-6 was inversely related to insulin sensitivity in healthy subjects [29]. Moreover, IL-10 increases insulin sensitivity and protects skeletal muscle from obesity-associated macrophage infiltration, increases in inflammatory cytokines, and the deleterious effects of these cytokines on insulin signaling and glucose metabolism [27]. Collectively, these data indicate that IL-6 and IL-10 may play an important role in MSTN-mediated glucose metabolism. In addition, RE-induced decrease in MSTN in parallel with decreasing IL-6 and increasing IL-10 may be effective in preventing insulin resistance.
This study has some limitations. First, because of the narrow selection criteria, the sample size was small. However, it appears that the design of our study was a requirement for the goals to be achieved. Second, although it is simple, noninvasive, and known to be correlated well with clamp test, the HOMA-IR formula that is used to calculate insulin resistance in this work is only an estimate and cannot be as accurate as the euglycemic-hyperinsulinemic clamp method. Third, post-loading analysis was limited to one time-point (24 h).
In conclusions, this study showed that plasma MSTN decreased following acute circuit RE and it correlated with HOMA-IR and plasma cytokines such as IL-6 and IL-10. It seems that a circuit RE bout by reducing HOMA-IR and changing plasma cytokines (decreased IL-6 and increased IL-10) can decrease plasma level of MSTN in healthy young men. Thus, targeting and MSTN inhibition by exercise may be useful method for prevention of insulin resistance. Moreover, MSTN level should be evaluated further as a target for defining the optimal mode, dose and intensity of exercise for health benefits. More research is warranted to elucidate the exact mechanisms responsible for the change in MSTN and insulin resistance following exercise.
References
Hittel DS, Axelson M, Sarna N, Shearer J, Huffman KM, Kraus WE (2010) Myostatin decreases with aerobic exercise and associates with insulin resistance. Med Sci Sports Exerc 42:2023–2092
Dilger AC, Spurlock ME, Grant AL, Gerrard DE (2010) Myostatin null mice respond differently to dietary-induced and genetic obesity. Anim Sci J 81:586–593
McPherron AC, Lawler AM, Lee SJ (1997) Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387:83–90
Allen DL, Cleary AS, Speaker KJ, Lindsay SF, Uyenishi J, Reed JM, Madden MC, Mehan RS (2008) Myostatin, activin receptor IIb, and follistatin-like-3 gene expression are altered in adipose tissue and skeletal muscle of obese mice. Am J Physiol Endocrinol Metab 294:E918–E927
Zhang C, McFarlane C, Lokireddy S, Bonala S, Ge X, Masuda S, Gluckman PD, Sharma M, Kambadur R (2011) Myostatin-deficient mice exhibit reduced insulin resistance through activating the AMP-activated protein kinase signalling pathway. Diabetologia 54:1491–1501
Guo T, Jou W, Chanturiya T, Portas J, Gavrilova O, McPherron AC (2009) Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS One 4:e4937
Zhao B, Wall RJ, Yang J (2005) Transgenic expression of myostatin propeptide prevents diet-induced obesity and insulin resistance. Biochem Biophys Res Commun 337:248–255
Chen Y, Ye J, Cao L, Zhang Y, Xia W, Zhu D (2010) Myostatin regulates glucose metabolism via the AMP-activated protein kinase pathway in skeletal muscle cells. Int J Biochem Cell Biol 42:2072–2081
Akpan I, Goncalves MD, Dhir R, Yin X, Pistilli EE, Bogdanovich S, Khurana TS, Ucran J, Lachey J, Ahima RS (2009) The effects of a soluble activin type IIB receptor on obesity and insulin sensitivity. Int J Obes 33:1265–1273
Louis E, Raue U, Yang Y, Jemiolo B, Trappe S (2007) Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol 103:1744–1751
LeBrasseur NK, Schelhorn TM, Bernardo BL, Cosgrove PG, Loria PM, Brown TA (2009) Myostatin inhibition enhances the effects of exercise on performance and metabolic outcomes in aged mice. J Gerontol A 64:940–948
Bernardo BL, Wachtmann TS, Cosgrove PG, Kuhn M, Opsahl AC, Judkins KM, Freeman TB, Hadcock JR, LeBrasseur NK (2010) Postnatal PPARdelta activation and myostatin inhibition exert distinct yet complimentary effects on the metabolic profile of obese insulin-resistant mice. PLoS One 5:e11307
Saremi A, Parastesh M (2011) Twelve-week resistance training decreases myostatin level and improves insulin sensitivity in overweight-obese women. Int J Diabetes Metab 19:63–68
Hulmi JJ, Tannerstedt J, Selänne H, Kainulainen H, Kovanen V, Mero AA (2009) Resistance exercise with whey protein ingestion affects mTOR signaling pathway and myostatin in men. J Appl Physiol 106:1720–1729
Alcaraz P, Sánchez-Lorente J, Blazevich A (2008) Physical performance and cardiovascular responses to an acute bout of heavy resistance circuit training versus traditional strength training. J Strength Cond Res 22:667–671
Jackson AS, Pollock ML (1978) Generalized equations for predicting body density of men. Br J Nutr 40:497–504
Dill DB, Costill DL (1974) Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37:247–248
Kim JS, Cross JM, Bamman MM (2005) Impact of resistance loading on myostatin expression and cell cycle regulation in young and older men and women. Am J Physiol Endocrinol Metab 288:E1110–E1119
Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S (2006) Myogenic gene expression at rest and after a bout of resistance exercise in young (18–30 yr) and old (80–89 yr) women. J Appl Physiol 101:53–59
Coffey VG, Shield A, Canny BJ, Carey KA, Cameron-Smith D, Hawley JA (2006) Interaction of contractile activity and training history on mRNA abundance in skeletal muscle from trained athletes. Am J Physiol Endocrinol Metab 290:E849–E855
Koopman R, Manders RJ, Zorenc AH, Hul GB, Kuipers H, Keizer HA, van Loon LJ (2005) A single session of resistance exercise enhances insulin sensitivity for at least 24 h in healthy men. Eur J Appl Physiol 94:180–187
Allen DL, Hittel DS, McPherron AC (2012) Expression and function of myostatin in obesity, diabetes, and exercise adaptation. Med Sci Sports Exerc 43:1828–1835
Hawley JA, Lessard SJ (2008) Exercise training-induced improvements in insulin action. Acta Physiol (Oxf) 192:127–135
Brandt C, Pedersen BK (2010) The role of exercise-induced myokines in muscle homeostasis and the defense against chronic diseases. J Biomed Biotechnol 2010:520258
Fischer CP (2006) Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev 12:6–33
Phillips MD, Mitchell JB, Currie-Elolf LM, Yellott RC, Hubing KA (2010) Influence of commonly employed resistance exercise protocols on circulating IL-6 and indices of insulin sensitivity. J Strength Cond Res 24:1091–1101
Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA (2011) The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 11:607–615
Febbraio MA, Pedersen BK (2002) Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J 16:1335–1347
Heliövaara MK, Teppo AM, Karonen SL, Tuominen JA, Ebeling P (2005) Plasma IL-6 concentration is inversely related to insulin sensitivity, and acute-phase proteins associate with glucose and lipid metabolism in healthy subjects. Diabetes Obes Metab 7:729–736
Acknowledgments
The author thanks all the subjects for their participation in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author has no conflict of interests.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the Ethics Committee of the Shahid Beheshti University, Faculty of Physical Education and Sport Sciences.
Informed consent
Informed consent was obtained from all individual participants included in this study.
Rights and permissions
About this article
Cite this article
Kazemi, F. The correlation of resistance exercise-induced myostatin with insulin resistance and plasma cytokines in healthy young men. J Endocrinol Invest 39, 383–388 (2016). https://doi.org/10.1007/s40618-015-0373-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-015-0373-9