Abstract
Introduction
Long-acting somatostatin analogs (SSAs) are most widely used to treat growth hormone (GH)-secreting pituitary adenoma. However, approximately 30 % of treated patients show resistance to SSAs, which may be associated with the reduction of somatostatin receptor subtype 2 (SSTR2) mRNA and protein expression.
Materials and methods
The present study used immunohistochemistry to detect the expression of SSTR2 and SSTR5 in twenty human GH-secreting adenoma samples treated with SSAs and seven normal pituitary samples.
Results
The staining intensities of SSTR2 and SSTR5 were stronger in most adenoma samples than in normal pituitary. The expression of SSTR2 tended to be lower in the SSA non-responder group than in responders. A search of the Bioinformatics data bank and the miRCURY™ LNA array confirmed miR-185 as the putative mircoRNA (miRNA) regulating the expression of SSTR2. An in vitro study using Dual Luciferase reporter assay demonstrated that miR-185 likely targets the 3′-UTR of SSTR2 mRNA in the rat pituitary adenoma GH3 cell line. MiR-185 also downregulated or upregulated the expression of SSTR2 mRNA and SSTR2 protein, following transfection with miR-185 mimics or inhibitors, respectively.
Conclusion
MiR-185 enhanced the cell proliferation and inhibited the apoptosis of GH3 cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Growth hormone (GH)-secreting pituitary adenoma is a common functional pituitary adenoma. Clinical symptoms are acromegaly and mass effect in adult. The oversecretion of GH and increase in circulating Insulin-like growth factor I (IGF-I) levels increase the mortality of GH-secreting pituitary adenoma to approximately 1.3–3.0 times higher than the general population, which is primarily due to cardiovascular and respiratory diseases and metabolic complications [1, 2]. Therefore, the purpose of treatment of GH-secreting pituitary adenoma is to reduce the mass effect and normalize growth hormone and insulin-like growth factor I levels [3, 4].
Current treatment strategies for acromegaly include surgery, drug, and radiotherapy. Long-acting somatostatin analogs (SSAs), including octreotide long-acting release (OCT-LAR), lanreotide slow release (LAN-SR), and lanreotide autogel (LAN-ATG) are most widely used to treat GH-secreting pituitary adenoma. SSAs reduce tumor mass, decrease GH and IGF-I, and alleviate patient complications [5–8].
Approximately 30 % of treated patients show resistance to SSAs, which may be explained by the concept of “biochemical resistance” and “tumor resistance” [9]. “Biochemical resistance” complicates the choice of treatment. Several researchers revealed a reduction of somatostatin receptor subtype 2 (SSTR2) protein or SSTR2 mRNA in SSA non-responder GH adenoma patients [10–14].
Somatostatin receptors (SSTRs) are G-protein-coupled receptors that are expressed in various tissues, including brain, gut, pituitary, endocrine and exocrine pancreas, adrenals, thyroid, kidney, and immune cells. SSTRs are enriched in many tumor cells [15]. The function of SSTR signaling includes the inhibition of adenylyl cyclase activity and the modulation of potassium and calcium channel activity, which are involved in the secretion processes [15, 16], and stimulation of phosphotyrosine phosphatase (PTP) or mitogen-activated protein kinase (MAPK) activity, which are involved in the regulation of cell growth and differentiation [15].
A quantitative evaluation of the SSTR subtype expression in GH-secreting pituitary adenomas demonstrated that SSTR5 was the most abundantly expressed subtype, followed by SSTR2, SSTR3, SSTR1, and SSTR4 [17]. The predominant expression of SSTR2 and SSTR5 in these tumors enables the clinical application of the octreotide and lanreotide, which display high affinity to SSTR2 and moderate affinity to SSTR5 [18]. A positive correlation between SSTR2 expression and sensitivity to SSAs was demonstrated in acromegalic patients [10, 13, 14]. The greatest expression of SSTR2 occurred in patients who had normalized GH and IGF-I levels compared to patients with abnormal levels [17, 19]. Moreover, immunohistochemistry and quantitative real-time PCR revealed a reduced or absent expression of SSTR2 protein and mRNA in patients with normalized GH and IGF-I levels [10–12, 20].
Some studies demonstrated that ghrelin, glucocorticoids, food deprivation, and diabetes mellitus reduced the expression of SSTR2 [21]. However, these factors cannot explain the decreased expression of SSTR2 in GH adenoma. Therefore, we suggest that the low expression of SSTR2 was induced by a change in oncogenes.
MicroRNAs (miRNAs) are a class of non-coding RNAs that post-transcriptionally regulate the expression of downstream mRNAs by targeting the 3′ untranslated regions (3′-UTR), negatively regulating genes and controlling a wide range of biological functions, e.g., cell proliferation, differentiation, signaling pathways, apoptosis, and metabolism [22].
Our previous studies found a differential expression of miRNAs between GH-secreting pituitary adenomas and normal pituitary and SSA responding and non-responding patients [23]. It is likely that these differentially expressed miRNAs play important roles in tumor pathogenesis, and some of these miRNAs may affect a patient’s response to SSA. Therefore, research on these miRNAs is necessary to elucidate the molecular mechanism of the resistance to SSA therapy and identify a potentially novel target for the treatment of GH adenoma. The present study examined SSTR2 expression in GH-secreting pituitary adenomas and normal pituitary tissue. We also predicted that the putative miRNA-185 plays a possible role in the regulation of SSTR2 in vitro using bioinformatics.
Materials and methods
Patient information
The Institutional Review Board of the First Affiliated Hospital, Sun Yat-sen University (Guangzhou, China) approved the study. The local ethics committee approved the pre-surgical medical treatment, and all participants provided informed written consent. Tissue samples were collected in accordance with the guidelines of local committee on human research. The biological diagnosis of acromegaly was based on the criteria that [1] plasma GH concentration was higher than 1 μg/l after oral administration of 75 g of glucose (oral glucose tolerance test, OGTT); [2] IGF-I concentration was increased compared to a normal population of the same age and sex; [3] relevant clinical features associated with acromegaly occurred; and [4] pituitary adenoma appeared on magnetic resonance imaging examination.
We examined 20 GH-secreting pituitary adenoma samples collected between July 2010 and October 2012 (10 men, 10 women; age range, 25–65 years) (Table 1). All patients were treated with lanreotide (Somatuline Autogel, Beaufour Ipsen, Paris, France) for 4 months prior to surgery. Previous conventional or gamma knife radiotherapy was not performed in any of the patients. Current criteria of defining resistance to SSAa are lacking [9]. Therefore, general criteria were used based on previous studies [12, 23]. Patients with a >50 % reduction of GH secretion after lanreotide treatment were considered SSA responders, and patients with a <50 % reduction in GH secretion were considered SSA non-responders. H&E and immunohistochemical staining confirmed the diagnosis of GH-secreting pituitary adenoma. A single dose of slow-release lanreotide treatment (starting with 30 mg/2 weeks i.m.) was used in all patients. All patients tolerated lanreotide well, and all patients completed the study. No adverse events were reported during the study. Magnetic resonance imaging of the pituitary and detection of plasma GH and IGF-I concentrations were performed after treatment. Seven normal pituitaries were obtained from the Department of Forensic Medicine, Sun Yat-sen University (Guangzhou, China). None of these cases had a recognized pituitary disease, and pituitaries exhibited normal gland appearance.
Immunohistochemistry
Immunohistochemistry was performed as previously described [24]. Briefly, formalin-fixed paraffin-embedded tissues from 20 GH-secreting pituitary adenomas and seven normal pituitary glands (see Table 1 for general and clinical characteristics of patients) were cut into 5 μm slices. Antigens were retrieved using a microwave in the presence of 10 mM citrate buffer (pH 6.0). Primary antibodies (SSTR2: Cat. 3582-1; SSTR5: Cat. 3619-1, Epitomics, USA) were applied in a 1:100 concentration. The secondary antibody was a goat anti-rabbit immunoglobulin (Kangwei, Beijjng). Antibody binding was visualized using HRP (DAKO, Zug, Switzerland). Staining was performed using DAB, and slices were counterstained using hemalum.
Adenomas were scored semiquantitatively using an immunoreactivity scoring system (IRS). The IRS was calculated as the product of the percentage of positive cells (4, 80 %; 3, 51–80 %; 2, 10–50 %; 1, 10 %; 0, 0 %) and staining intensity (3, strong; 2, moderate; 1, mild; and 0, no staining). The IRS scores ranged between 0 (no staining) and 12 (maximum staining) [13].
Two pathologists, who were not aware of the clinical data, independently performed score assessments. A senior pathologist rendered a final judgment if the assessments were not consistent.
MiRNA target predictions
Prediction for all miRNAs targeting SSTR2 was assessed using Targetscan (http://www.targetscan.org/) and our previous findings of the differential expression of miRNAs in GH-secreting pituitary adenomas [23]. The following rules of prediction were used: [1] If the putative miRNAs downregulated SSTR2 mRNA, then the putative miRNAs should be increased in the non-responder group compared to the responder group; [2] the seed sequence matches were conserved across species; and [3] the context scores of putative miRNAs in Targetscan were relatively higher. We obtained the DNA sequence of the 3′ UTR region of the putative target mRNA from GenBank (http:/www.ncbi.nlm.nih.gov/).
Cell culture
The regulation of miR-185 on SSTR2 was confirmed in vitro. We chose the rat pituitary adenoma GH3 cell line as a tool. The GH3 cell line (Xie-he Bank, Beijing, China) was cultured in RPMI1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 2.5 % fetal bovine serum (Gibco), 15 % horse serum, 100 mg ml−1 streptomycin, and 100 units ml−1 penicillin (Invitrogen) at 37 °C in a humidified atmosphere of 5 % CO2, as described previously [25].
Transfection
GH3 cells were transfected in 6-well plates (6 × 104 cells/ml) with 50 nM of an miR-185 mimic or inhibitor (Ribobio, Guangzhou, China) using Lipofectamine 2000 (Invitrogen) and Opti-MEM medium (Invitrogen) without serum, according to the manufacturer’s protocol as described previously [25]. Cells were transfected simultaneously with the appropriate negative control (NC) (Ribobio, Guangzhou, China). miRNAs were extracted 24 h after transfection and the efficiency of transfection was determined using qRT-PCR.
SSTR2 3′-UTR reporter constructs and luciferase assay
The 3′-UTR of the rat SSTR2 gene was amplified from rat pituitary gland DNA using primers (SSTR2 Xho1 FW: 5′CCGCTCGAGAACAACCCGGGAACGCAAC3′ and SSTR2 Not1 RW: 5′ATAAGAATGCGGCCGCTCTTTTTACAAAAATGTATTATTATATG 3′) and cloned into the psiCHECK-2 vector (Ambion, Austin, TX, USA). The product was designated pGL3-SSTR2-wt after sequencing. Site-directed mutagenesis of the miR-185 target site in the SSTR2 3′-UTR was performed using a site-directed mutagenesis kit (Stratagene), and the product was named pGL3-SSTR2-mut (TGTGACC to ACACTGG) (primers: FW, 5′AGAGGACACGATGGCCTGGGACACTGGCGGTGGAAAGCAGCTACCCG 3′; RV, 5′CGGGTAGCTGCTTTCCACCGCCAGTGTCCCAGGCCATCGTGTCCTCT 3′).
Cells were seeded in 24-well plates 1 day before transfection. Cells were transiently cotransfected for reporter assays with 0.5 μg of pGL3-SSTR2-wt or mutant reporter plasmid and 50 nM control miRNA or miR-185 using Lipofectamine 2000 (Invitrogen). Firefly and Renilla luciferase activities were measured consecutively using the Dual Luciferase Assay (Promega) according to the manufacturer’s instructions, and the results were normalized for transfection efficiency by the control vector containing Renilla luciferase, pRL-TK (Promega). Experiments were performed in triplicate.
Quantitative real-time PCR (qRT-PCR)
Small RNA isolation from formalin-fixed, paraffin-embedded tissue slices approximately seventy-five micron thick was performed according to the manufacturer’s protocol. RNA isolation and reverse transcription from GH3 cells were performed as described previously [25]. Polymerase chain reaction was performed using the real-time PCR System (Bio-rad iQ5, Bio-Rad). U6 snRNA served as an endogenous control for paraffin-embedded tissue to normalize the expression levels of miR-185. The miR-185 and U6 primers (Ribobio, Guangzhou, China) were used under the following amplification conditions: 95 °C for 3 min, followed by 40 cycles of 95 °C for 15 s and 65 °C for 15 s. β-actin served as the endogenous control for SSTR2, and the amplification conditions were 58 °C for 2 min; 95 °C for 2 min, followed by 35 cycles of 95 °C for 15 s and 60 °C for 32 s. The expression of miR-185 and SSTR2 mRNA were determined using the comparative cycle threshold method of relative quantitation.
Western blotting
Western blotting was performed as previously described [25]. Cellular proteins were extracted after transfection with miR-185 mimics or inhibitors, and 30 µg of total protein was subjected to SDS-PAGE immunoblotting analysis. PVDF membranes (Bio-rad) were blocked with 5 % non-fat milk for 1 h at room temperature. Membranes were washed twice with TBST (5 min each) and incubated with the SSTR2 primary antibody (1:1000, Cat. 3582-1, Epitomics, USA) at 4 °C overnight. The next day, the membrane was incubated with an HRP-conjugated secondary antibody (Thermo Pierce) and signal detection was performed using Super ECL Plus Detection Reagent (Applygen Technologies Inc., China). The signals were quantified using a chemiluminescence detector and the accompanying densitometry software (UVP, Upland, CA). The Western blots showed in figures are representative blots and quantitative analysis was calculated based on three independent experiments.
Cell viability assay
MTT [3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assays were used to assess cell viability as previously described [25]. GH3 cells were seeded in 96-well plates at a density of 4 × 105/well 24 h after transfection with miR-185 mimics and negative controls and cultured in serum-free RPMI 1640 medium for 24 h. A volume of 10 μl of MTT (5 mg ml−1 in PBS, Sigma) was added to each well and incubated at 37 °C in a humidified atmosphere of 5 % CO2 for 4 h. Acidified isopropyl alcohol (100 µl) was added overnight at 37 °C to dissolve the formazan generated in living GH3 cells. The absorbance was measured using an absorbance reader (Sunrise, TECAN) at 570 nM. The cell viability rate was calculated as follows: Cell viability rate = [(OD of treated cells − OD of blank)/(OD of control cells − OD of blank)] × 100 %.
Annexin V-FITC/PI staining
The apoptosis rate of GH3 cells was determined using Annexin V-FITC (Annexin V-fluorescein isothiocyanate) and PI (propidium iodide) staining (eBioscience, USA). Acquisition and analyses were performed using a Beckman Coulter Epics XL-MCL and Expo32ADC 1.2 Software, as previously described [25]. Propidium iodide was used as the live/dead discriminator. GH3 cells were washed with PBS three times 24 h after transfection with miR-185 mimics or negative controls and resuspended in 500 μl binding buffer. Annexin V-FITC (5 μl) and 10 μl PI were added to each sample, and the samples were incubated for 10 min in the dark. The FITC signal detector FL1 and PI staining signal detector FL3 were used to detect cells using the flow cytometer (Ex = 488 nM; Em = 530 nM). The number of analyzed cells was 5 × 104 − 8 × 104. The compensation and compensation matrix was set as follows: FL1 − %FL2 = 8.3, FL2 − %FL1 = 0.0. The experiment was performed three times and the mean values were calculated.

Statistical analysis
Each experiment was performed in triplicate. All data are expressed as the mean ± SD. The statistical significance of differences between groups was analyzed using one-way analysis of variance (ANOVA) followed by Student’s t test using SPSS 13.0 for Windows. P < 0.05 was considered statistically significant.
Results
Immunohistochemistry of SSTR2 and SSTR5 in normal samples and GH-secreting pituitary adenomas
SSTR2 staining (Table 1)
Eleven cases (11/16) in the SSA-responder group (p1–16) were scored as 12; 1 case (1/16) was scored 9, and 4 cases (4/16) were scored 8. One case (1/4) in the SSA non-responder group (p17–20) was scored as 9; 2 cases (2/4) were scored 8; and 1 case (1/4) was scored 6. All 7 cases (7/7) in the normal pituitary gland group were scored as 4 (Figs. 1, 2).
Representative immunohistochemical scores of SSTR2 (a–e) and SSTR5 (f–i) in normal pituitary and GH-secreting pituitary adenoma (×200). a Normal pituitary gland, score 4; b–e GH-secreting pituitary adenomas, b patient no. 19, score 6; c patient no. 5, score 8; d patient no. 4, score 9; e patient no. 8, score 12; f normal pituitary, score 8; GH-secreting pituitary adenomas, g patient no. 13, score 8; h patient no. 9, score 9; i patient no. 2, score 12
SSTR5 staining (Table 1)
Thirteen cases (13/16) in the SSA-responder group (p1–16) were scored as 12; 2 cases (2/16) were scored 9, and 1 case (1/16) was scored 8. All 4 cases (4/4) in the SSA non-responder group (p17–20) were scored as 12, and all 7 cases (7/7) in the normal pituitary gland group were scored 8 (Figs. 1, 2).
The staining intensity of SSTR2 in all adenoma samples (20/20) was stronger than normal pituitaries, even in p4, p5, and p19 in which SSTR2 labeling was relatively weak compared with the other adenoma samples. The staining intensity of SSTR5 in tumors was stronger than normal pituitary, except for one sample (p13) that was scored the same as normal pituitary. There were no significant differences between non-responder group and responders. This finding is consistent with previous studies [14]. Therefore, we primarily investigated the expression and regulation of SSTR2 in pituitary tumors.
Bioinformatic predictions
A total of 202 miRNAs were predicted to bind sites within SSTR2 mRNA using TargetScan (6.2, 2012), and only six miRNAs (miR-155, miR-185, miR-297, miR-519d, miR-766, and miR-934) were expressed higher in the non-responder group than the responder group on the basis of our miRCURY™ LNA Arrays. MiR-155, miR-185, and miR-519d were conserved, but miR-185 obtained the highest context score. Therefore, miR-185 mostly met our requirements (Table 2).
Expression of miR-185 in tumor sections
The bioinformatics suggested that miR-185 may regulate SSTR2 expression. Therefore, we examined the results of the miRCURY™ LNA Arrays in our tumor samples. Eighteen RNAs were extracted from 20 GH-secreting pituitary adenomas (Fig. 3a). Two samples (P2, P4) were excluded because of low amounts of RNA, which was insufficient for replication. The expression level of miR-185 was 5.85 ± 1.92 in the non-responder group, 0.37 ± 0.07 in the responder group, and 2.48 ± 1.00 in the normal pituitary gland group. The differences between groups were statistically significant (Fig. 3b). These results confirmed our finding in the miRCURY™ LNA arrays that miR-185 was upregulated in SSA non-responders.
a Relative expression of miR-185 in GH-secreting pituitary adenomas and normal pituitary. P1–16, SSA-responder group; P17–20, SSA non-responder group; N1–7, normal pituitary. b Reduced expression of miR-185 in responder adenomas compared to non-responder adenomas and normal pituitary glands. *P < 0.05, **P < 0.01
The putative 3′-UTR target site of SSTR2 is directly regulated by miR-185
Transient transfection of GH3 cells with pGL3-SSTR2-wt and miR-185 mimics significantly decreased luciferase activity compared to the miR-185 mimics NC (P < 0.05). Luciferase activity after transfection with miR-185 inhibitors was unaffected (P > 0.05) (Fig. 4). MiR-185 directly targeted and degraded SSTR2 mRNA.
Transfection efficiency of miR-185
The relative expression of miR-185 24 h after transfection in GH3 cells was 8.66 ± 0.41 in the miR-185 mimics group, 1.69 ± 0.11 in the mimic NC group, 1.58 ± 0.23 in the control group, 0.12 ± 0.13 in the inhibitor group, and 1.45 ± 0.05 in the inhibitor NC group (iNC). There were significant differences between the miR-185 mimic group and NC group and the miR-185 inhibitor group and inhibitor NC group (Fig. 5). The successful transfections were used in the following experiments.
Expression of SSTR2 mRNA after transfection of miR-185 mimics and inhibitors in GH3 cells
The expression of SSTR2 mRNA in GH3 cells transfected with miR-185 mimics for 24 h was 0.12 ± 0.03 in the miR-185 mimics group, 1.15 ± 0.16 in the mimic NC group, and 1.17 ± 0.05 in the control group. There was a significant difference between the miR-185 mimic group and NC group (P < 0.01) (Fig. 6). SSTR2 mRNA was downregulated in GH3 cells 24 h after transfection with miR-185 mimics.
The expression of SSTR2 mRNA in GH3 cells transfected with miR-185 inhibitors for 24 h was 1.91 ± 0.29 in the miR-185 inhibitors group, 1.13 ± 0.31 in the inhibitors iNC group, and 1.17 ± 0.05 in the control group. There was a significant difference between the miR-185 inhibitor group and iNC group (P < 0.05) (Fig. 6). SSTR2 mRNA was upregulated in GH3 cells 24 h after transfection with miR-185 inhibitors.
Expression of SSTR2 protein after transfection of miR-185 mimics and inhibitors in GH3 cells
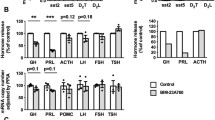
Western blots and densitometry revealed that the expression of SSTR2 protein was significantly decreased in the miR-185 mimic group compared to the NC group (mimics 0.63 ± 0.06 vs. NC 0.85 ± 0.01, P < 0.001) (Fig. 7a). The expression of SSTR2 protein was downregulated in GH3 cells transfected with miR-185 mimic.
Western blots and densitometry revealed that the expression of SSTR2 protein was significantly increased in the miR-185 inhibitors group compared to the NC group (inhibitors 1.73 ± 0.25 vs. NC 1.35 ± 0.10, P < 0.01) (Fig. 7b). SSTR2 protein expression was upregulated in GH3 cells transfected with miR-185 inhibitors.
Transfection of miR-185 mimics enhanced proliferation and inhibited apoptosis of GH3 cells
MTT assays demonstrated increased proliferation of GH3 cells transfected with miR-185 mimics for 24 h compared to the NC group (mimics 0.23 ± 0.0051 vs. NC 0.21 ± 0.0044, P < 0.05). MiR-185 mimics exhibited increased proliferation of GH3 cells compared with controls, but this difference did not reach statistical significance (mimics 0.23 ± 0.0051 vs. control 0.22 ± 0.0069, P > 0.05) (Fig. 8).
Flow cytometry demonstrated that transfection with miR-185 mimics did not affect early apoptosis in GH3 cells (mimics 11.97 ± 1.53 % vs. NC 10.27 ± 0.49 %, P > 0.05). However, mimics decreased late period apoptosis in GH3 cells (mimics 38.87 ± 1.21 % vs. NC 48.54 ± 1.46 %, P < 0.05) (Fig. 9).
Discussion
A recent study evaluating the quantitative expression of SSTR2 at molecular levels demonstrated that the amount of SSTR2 gene product in GH-secreting pituitary adenomas was higher than normal pituitaries [26]. Consistent with this finding, our immunohistochemical study revealed an increased expression of SSTR2 in SSA-responder adenomas compared to normal pituitaries and reduced expression of SSTR2 in SSA non-responder adenomas.
To our knowledge, the mechanism and significance of differential expression of SSTR2 between GH-secreting pituitary adenoma and normal pituitary are not known. A series of in vitro studies demonstrated that SSTR2 and SSTR5 exhibit constitutive non-ligand-dependent activity for the regulation of ACTH secretion in AtT-20 cells [27, 28], which indicates that SSTRs may act as functional proteins in tumor cells. Increased expression of SSTR2 and SSTR5 in pituitary adenoma in the present study further suggests an important role of SSTRs in tumor cells. Once a pituitary adenoma forms, the oversecretion of GH and cell proliferation triggers feedback responses of the hypothalamic-anterior pituitary axis and the homeostatic control system of pituitary cells, which may negatively affect the maintenance of cell homeostasis. A hypothalamus-independent mechanism increases SSTR expression, which may improve the sensitivity of external SST binding to their receptors. Increased SSTRs may suppress intracellular cAMP levels and GH secretion as constitutively functional proteins and inhibit cell proliferation [27, 28]. Further, the oversecretion of GH induces feedback of the hypothalamic-anterior pituitary axis, which results in an increase of somatostatin release-inhibiting factors (SRIFs), SST14 and SST28, which bind five SSTR subtypes with nanomolar affinity. SSTR1–4 bind SST-14 > SST-28, whereas SSTR5 exhibits 10- to 15-fold selectivity for SST-28 compared to SST-14 [15]. SSTR2 and SSTR5 are major endogenous SRIF ligands in the regulation of GH secretion and cell proliferation [29, 30]. Increased SSTR2 and SSTR5 expressions in adenoma fit the regulation of hypothalamic-anterior pituitary axis hypothesis. Therefore, the increase in SSTR2 and SSTR5 in adenomas that we observed is reasonable. Notably, there may be a downregulation of SSTR2 after octreotide treatment, which supports our hypothesis [31]. This hypothesis may explain the increase in SSTR2 and SSTR5 expressions in GH-secreting pituitary adenomas. However, further research is needed to elucidate the molecular mechanisms.
The role of miR-185 in oncogenesis or tumor suppressor varies depending on the tumor type [32–37]. Our study found that miR-185 targeted SSTR2 mRNA to downregulate SSTR2 protein expression, promote proliferation, and inhibit apoptosis of tumor cells, which would be classified as an oncogene in pituitary adenoma cells. A differential expression of miR-185 was also observed in SSA-responder and non-responder GH-secreting pituitary adenoma. These results demonstrate that the different responses of GH-secreting pituitary adenomas to SSA are related to the expression of SSTR2, which is likely attributed to genetic changes. Tumor cells in SA non-responder adenomas exhibited the characteristic of evading growth suppressors, which is identical to “cancer” [38]. Therefore, the treatment strategy should be more aggressive for patients whose GH levels decrease less than 50 % after receiving drug treatment for more than 4 months. It is unknown whether miR-185 may directly increase cell viability, as it was non-transfected cells, but not mock-transfected control cells that were employed in the present study, further studies are therefore warranted.
The present study demonstrated that miR-185 was likely involved in drug resistance and the pathogenesis of GH-secreting pituitary adenomas. Similarly, reduced dopamine-receptor subtype 2 (D2) receptors were also found in prolactinomas that are resistant to dopamine agonists [39]. Therefore, different types of pituitary gland tumors may share the same molecular mechanisms and pathogenesis.
Not all of the miRNAs that could regulate SSTR2 were studied because of the limited availability of miRNAs arrays, biochemical database, and cell lines. There was also no standard definition of resistance to SSAs, but we defined the admittance standard of non-responders as a GH value decrease of less than 50 % after treatment with SSA. This definition resulted in fewer numbers of recruited patients, and the sample size may not have been large enough. Also, it would be interesting to check whether the expression of miR-185 might be different in microadenoma vs. macroadenoma together with SSTR expression. Obviously more work is needed to clarify whether expression of miR-185 is correlated with tumor size.
In conclusion, the expression of SSTR2 in GH-secreting pituitary adenomas was greater than that in normal pituitary glands. MiR-185 may downregulate SSTR2 expression by targeting SSTR2 mRNA. MiR-185 may promote the cell proliferation and reduce the apoptosis of GH3 cells.
Abbreviations
- SSA:
-
Somatostatin analogs
- SSTR:
-
Somatostatin receptor subtype
- GH:
-
Growth hormone
- IGF-I:
-
Insulin-like growth factor I
- SRIF:
-
Somatostatin release-inhibiting factors
References
Orme SM, McNally RJ, Cartwright RA, Belchetz PE (1998) Mortality and cancer incidence in acromegaly: a retrospective cohort study. United Kingdom Acromegaly Study Group. J Clin Endocrinol Metab 83:2730–2734
Dekkers OM, Biermasz NR, Pereira AM, Romijn JA, Vandenbroucke JP (2008) Mortality in acromegaly: a metaanalysis. J Clin Endocrinol Metab 93:61–67
Biermasz NR, Dekker FW, Pereira AM, van Thiel SW, Schutte PJ, van Dulken H, Romijn JA, Roelfsema F (2004) Determinants of survival in treated acromegaly in a single center: predictive value of serial insulin-like growth factor I measurements. J Clin Endocrinol Metab 89:2789–2796
Swearingen B, Barker FG 2nd, Katznelson L, Biller BM, Grinspoon S, Klibanski A, Moayeri N, Black PM, Zervas NT (1998) Long-term mortality after transsphenoidal surgery and adjunctive therapy foracromegaly. J Clin Endocrinol Metab 83:3419–3426
Melmed S, Colao A, Barkan A, Molitch M, Grossman AB, Kleinberg D, Clemmons D, Chanson P, Laws E, Schlechte J, Vance ML, Ho K, Giustina A (2009) Guidelines for acromegaly management: an update. J Clin Endocrinol Metab 94:1509–1517
Freda PU, Katznelson L, van der Lely AJ, Reyes CM, Zhao S, Rabinowitz D (2005) Long-acting somatostatin analog therapy of acromegaly: a meta-analysis. J Clin Endocrinol Metab 90:4465–4473
Bronstein M, Musolino N, Jallad R, Cendros JM, Ramis J, Obach R, Leselbaum A, Catus F (2005) Pharmacokinetic profile of lanreotide Autogel in patients with acromegaly after four deep subcutaneous injections of 60, 90 or 120 mg every 28 days. Clin Endocrinol (Oxf) 63:514–519
Jallad RS, Musolino NR, Salgado LR, Bronstein MD (2005) Treatment of acromegaly with octreotide-LAR: extensive experience in a Brazilian institution. Clin Endocrinol (Oxf) 63:168–175
Colao A, Auriemma RS, Lombardi G, Pivonello R (2011) Resistance to somatostatin analogs in acromegaly. Endocr Rev 32:247–271
Ferone D, de Herder WW, Pivonello R, Kros JM, van Koetsveld PM, de Jong T, Minuto F, Colao A, Lamberts SW, Hofland LJ (2008) Correlation of in vitro and in vivo somatotropic adenoma responsiveness tosomatostatin analogs and dopamine agonists with immunohistochemical evaluation ofsomatostatin and dopamine receptors and electron microscopy. J Clin Endocrinol Metab 93:1412–1417
Taboada GF, Luque RM, Neto LV, Machado EO, Sbaffi BC, Domingues RC, Marcondes JB, Chimelli LM, Fontes R, Niemeyer P, de Carvalho DP, Kineman RD, Gadelha MR (2008) Quantitative analysis of somatostatin receptor subtypes (1–5) gene expressionlevels in somatotropinomas and correlation to in vivo hormonal and tumor volume responses to treatment with octreotide LAR. Eur J Endocrinol 158:295–303
Plockinger U, Albrecht S, Mawrin C, Saeger W, Buchfelder M, Petersenn S, Schulz S (2008) Selective loss of somatostatin receptor 2 in octreotide-resistant growth hormone-secreting adenomas. J Clin Endocrinol Metab 93:1203–1210
Gatto F, Feelders RA, van der Pas R, Kros JM, Waaijers M, Sprij-Mooij D, Neggers SJ, van der Lelij AJ, Minuto F, Lamberts SW, de Herder WW, Ferone D, Hofland LJ (2013) Immunoreactivity score using an anti-sst2A receptor monoclonal antibody strongly predicts the biochemical response to adjuvant treatment with somatostatin analogs in acromegaly. J Clin Endocrinol Metab 98:E66–E71
Takei M, Suzuki M, Kajiya H, Ishii Y, Tahara S, Miyakoshi T, Egashira N, Takekoshi S, Sanno N, Teramoto A, Osamura RY (2007) Immunohistochemical detection of somatostatin receptor (SSTR) subtypes 2A and 5 in pituitary adenoma from acromegalic patients: good correlation with preoperative response to octreotide. Endocr Pathol 18:208–216
Patel YC (1999) Somatostatin and its receptor family. Front Neuroendocrinol 20:157–198
Tsaneva-Atanasova K, Sherman A, van Goor F, Stojilkovic SS (2007) Mechanism of spontaneous and receptor-controlled electrical activity in pituitarysomatotrophs: experiments and theory. J Neurophysiol 98:131–144
Taboada GF, Luque RM, Bastos W, Guimaraes RF, Marcondes JB, Chimelli LM, Fontes R, Mata PJ, Filho PN, Carvalho DP, Kineman RD, Gadelha MR (2007) Quantitative analysis of somatostatin receptor subtype (SSTR1-5) gene expression levels in somatotropinomas and non-functioning pituitary adenomas. Eur J Endocrinol 156:65–74
Hofland LJ, Lamberts SW (2003) The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocr Rev 24:28–47
Casarini AP, Jallad RS, Pinto EM, Soares IC, Nonogaki S, Giannella-Neto D, Musolino NR, Alves VA, Bronstein MD (2009) Acromegaly: correlation between expression of somatostatin receptor subtypes and response to octreotide-lar treatment. Pituitary. 12:297–303
Saveanu A, Gunz G, Dufour H, Caron P, Fina F, Ouafik L, Culler MD, Moreau JP, Enjalbert A, Jaquet P (2001) Bim-23244, a somatostatin receptor subtype 2- and 5-selective analog with enhanced efficacy in suppressing growth hormone (GH) from octreotide-resistant human GH-secreting adenomas. J Clin Endocrinol Metab 86:140–145
Ben-Shlomo A, Melmed S (2010) Pituitary somatostatin receptor signaling. Trends Endocrinol Metab 21:123–133
Esquela-Kerscher A, Slack FJ (2006) Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer 6:259–269
Mao ZG, He DS, Zhou J, Yao B, Xiao WW, Chen CH, Zhu YH, Wang HJ (2010) Differential expression of microRNAs in GH-secreting pituitary adenomas. Diagn Pathol 5:79
Schulz S, Schulz S, Schmitt J, Wiborny D, Schmidt H, Olbricht S, Weise W, Roessner A, Gramsch C, Hollt V (1998) Immunocytochemical detection of somatostatin receptors sst1, sst2A, sst2B, and sst3 in paraffin-embedded breast cancer tissue using subtype-specific antibodies. Clin Cancer Res 4:2047–2052
Chen CH, Xiao WW, Jiang XB, Wang JW, Mao ZG, Lei N, Fan X, Song BB, Liao CX, Wang HJ, She ZG, Zhu YH (2013) A novel marine drug, SZ-685C, induces apoptosis of MMQ pituitary tumor cells by downregulating miR-200c. Curr Med Chem 20:2145–2154
Neto LV, Machado EO, Luque RM, Taboada GF, Marcondes JB, Chimelli LM, Quintella LP, Niemeyer P Jr, de Carvalho DP, Kineman RD, Gadelha MR (2009) Expression analysis of dopamine receptor subtypes in normal human pituitaries, nonfunctioning pituitary adenomas and somatotropinomas, and the association between dopamine and somatostatin receptors with clinical response to octreotide-LAR in acromegaly. J Clin Endocrinol Metab 94:1931–1937
Ben-Shlomo A, Zhou C, Pichurin O, Chesnokova V, Liu NA, Culler MD, Melmed S (2009) Constitutive somatostatin receptor activity determines tonic pituitary cell response. Mol Endocrinol 23:337–348
Ben-Shlomo A, Pichurin O, Barshop NJ, Wawrowsky KA, Taylor J, Culler MD, Chesnokova V, Liu NA, Melmed S (2007) Selective regulation of somatostatin receptor subtype signaling: evidence for constitutive receptor activation. Mol Endocrinol 21:2565–2578
Shimon I (2003) Somatostatin receptors in pituitary and development of somatostatin receptor subtype-selective analogs. Endocrine 20:265–269
Shimon I, Melmed S (1997) Structure and function of somatostatin receptors in growth hormone control. J Endocrinol 155(l):S3–S6 (discussion S7–8)
Fougner SL, Borota OC, Berg JP, Hald JK, Ramm-Pettersen J, Bollerslev J (2008) The clinical response to somatostatin analogues in acromegaly correlates to the somatostatin receptor subtype 2a protein expression of the adenoma. Clin Endocrinol (Oxf) 68:458–465
Tang H, Wang Z, Liu X, Liu Q, Xu G, Li G, Wu M (2012) LRRC4 inhibits glioma cell growth and invasion through a miR-185-dependent pathway. Curr Cancer Drug Targets 12:1032–1042
Zhi Q, Zhu J, Guo X, He S, Xue X, Zhou J, Hu B, Li H, Chen S, Zhao H, Kuang Y (2013) Metastasis-related miR-185 is a potential prognostic biomarker for hepatocellular carcinoma in early stage. Biomed Pharmacother 67:393–398
Qadir XV, Han C, Lu D, Zhang J, Wu T (2014) MicroRNA-185 inhibits hepatocellular carcinoma growth by targeting the DNMT1/PTEN/Akt pathway. Am J Pathol 184(8):2355–2364
Li X, Chen YT, Josson S, Mukhopadhyay NK, Kim J, Freeman MR, Huang WC (2013) MicroRNA-185 and 342 inhibit tumorigenicity and induce apoptosis through blockade of the SREBP metabolic pathway in prostate cancer cells. PLoS One 8:e70987
Qu F, Cui X, Hong Y, Wang J, Li Y, Chen L, Liu Y, Gao Y, Xu D, Wang Q (2013) MicroRNA-185 suppresses proliferation, invasion, migration, and tumorigenicity of human prostate cancer cells through targeting androgen receptor. Mol Cell Biochem 377:121–130
Imam JS, Buddavarapu K, Lee-Chang JS, Ganapathy S, Camosy C, Chen Y, Rao MK (2010) MicroRNA-185 suppresses tumor growth and progression by targeting the Six1 oncogene in human cancers. Oncogene 29:4971–4979
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Oh MC, Aghi MK (2011) Dopamine agonist-resistant prolactinomas. J Neurosurg 114:1369–1379
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No. 41076092, 30971538), the Guangdong Provincial 125 Key Laboratory Foundation of Medicine (K970401302), Science and Technology Foundation of Guangzhou (2013J4100055), and the Science and Technology Foundation of Zhuhai (2014D0401990011).
Conflict of interest
The authors report no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board of the First Affiliated Hospital, Sun Yat-sen University and of the local ethics committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Tissue samples were collected in accordance with the guidelines of local committee on human research. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study and all participants provided informed written consent.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fan, X., Mao, Z., He, D. et al. Expression of somatostatin receptor subtype 2 in growth hormone-secreting pituitary adenoma and the regulation of miR-185. J Endocrinol Invest 38, 1117–1128 (2015). https://doi.org/10.1007/s40618-015-0306-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-015-0306-7