Abstract
Purpose
Recognition of dysthyroid optic neuropathy (DON) requires sensitive diagnostic tools. Clinical assessment may fail to reliably evaluate the acuteness of DON especially if signs for inflammation are missing. Aim of this cross-sectional study was to assess the relationship between thyroid-stimulating immunoglobulins (TSI) and onset of DON.
Methods
At a multidisciplinary orbital center, serum TSI levels were measured in 180 consecutive patients with thyroid eye disease (TED) and 302 healthy controls with a FDA-cleared cell-based bioassay using a chimeric TSH receptor and a CRE-dependent luciferase.
Results
Thirty of 180 (16.7 %) patients with TED had DON of recent onset or a past history of DON (post-DON). Optic disk swelling was present and visual-evoked potentials were pathologic in all eyes with DON of recent onset, but in one of 13 (7.7 %) with post-DON, only (p = 0.005). 19/20 (96 %) patients with DON of recent onset were TSI-positive. TSI was associated with DON of recent onset (OR: 20.96; 95 % CI 1.064–412.85, p = 0.045). All controls were TSI negative. TSI correlated with the clinical activity score (R = 0.70, p < 0.001) and higher TSI-levels were noted in active vs. inactive TED (485.1 ± 132.3 vs. 277.7 ± 143.7 %, cut-off < 140 %; p < 0.001). Six of seven (85.7 %) patients with inactive TED with recent onset DON versus one of four (25 %) with active post-DON were TSI-positive (p = 0.006). A discriminatory cut-point of 377 SRR % for TSI was determined based on a ROC analysis (sensitivity: 0.95, specificity: 0.8).
Conclusions
Serum TSI levels identify patients with DON of recent onset requiring urgent therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid eye disease (TED) is the most common extrathyroidal manifestation of Graves’ disease [1, 2]. Besides autoimmune processes oxygen-free radicals and cytokines play a pathogenic role in TED [3]. There is a spectrum of ocular involvement in Graves’ disease, from complete absence of symptoms and signs-to-sight-threatening conditions [4]. Dysthyroid optic neuropathy (DON) is a major sight-threatening complication affecting 4–8 % of patients with TED [4–7]. It is defined as neuropathy caused by compression or stretch of the optic nerve associated with edema, volume increase and inflammation of orbital tissue due to deposition of excess glycosaminoglycans. Impaired vision leads to quality of life impairment [8, 9] and might have socio-economic consequences [10]. DON of recent onset requires urgent medical treatment (high-dose intravenous steroid pulses [11, 12]) and/or orbital bony decompression to avoid permanent or progressive visual loss [13–19]. Because these procedures carry the risk of serious complications [20–24], it is essential that a timely and correct diagnosis is made [25]. Controversy still exists regarding the diagnostic features of DON [26, 27]. Also, recognition of DON might be delayed, especially if the presentation is atypical [28, 29]. Furthermore, in newly presenting patients, it can be particularly difficult to correctly evaluate whether DON has developed recently and thus warrants urgent therapy. Finally, alternative causes for visual impairment can be present in patients with TED [30–33] highlighting the fact that serious efforts should be undertaken to improve diagnostics and accurate management of DON. Several studies have suggested that serum levels of the functional thyroid stimulating immunoglobulins (TSI), measured with a novel cell-based thyroid stimulating hormone (TSH) receptor (TSHR) bioassay, may correlate with symptoms and signs of TED [34–39]. Analytical performance, validation, clinical utility and standardization of this FDA-cleared bioassay have been demonstrated [40–44]. Since recognition of DON requires sensitive diagnostic tools and clinical assessment may fail to reliably evaluate the onset and acuteness of DON, we hypothesized a relationship between the serum TSI levels and the onset of DON.
Methods
Patient data and ophthalmic assessment
At an academic referral multidisciplinary orbital center, complete data was collected regarding the presence of DON and associated clinical features in 180 consecutive and unselected patients with TED. DON of recent onset occurred early and/or within the last 6 months at the latest. Those patients had been treated with high-dose intravenous steroids and/or bony decompression surgery during this period of time. All had clinical and/or radiological evidence of DON. In patients with DON, data on visual acuity, relative afferent pupillary deficit, vision-field testing, colour desaturation testing, and visual-evoked potentials were obtained. Visual-evoked potentials (VEPs) were performed to pattern stimulation. Measurements were made of the peak-to-peak amplitude and the absolute latency of P100. VEP was considered abnormal according to the following definition: P100 amplitude reductions which were either relative (an interocular difference of >25 %) or absolute (a value <4 µV, which is the normal standard in this unit). The latency was considered abnormal if an absolute delay of P100 was measured or in case of a relative delay (interocular difference >8 ms, being beyond the upper limit of normal). Color vision was tested with Ishihara color plates. As recommended by EUGOGO [13] color vision was defined to be abnormal if more than two errors were made. Visual-field testing was performed with Goldmann or static automated perimetry using the central 30-2 full threshold program. We defined a visual field to be pathologic if a central scotoma or an inferior altitudinal defect was seen or if an enlarged blind spot, a paracentral scotoma, a nerve fiber bundle defect, or a generalized constriction were noted.
Post-DON mirrored a condition where patients were treated medically or surgically more than 6 months ago. Further ophthalmic assessments (e.g. fluorescein angiography, etc.) were performed if the “standard” investigations alone were unable to ascertain that the eye abnormality was due to DON or to other concomitant comorbidities. If both eyes had DON, the values of the more severely affected eye were chosen for analyses. In all patients, clinical assessment of TED and DON was performed according to the Consensus Statement of the European Group on Graves Orbitopathy, EUGOGO [13, 27, 45]. The clinical activity score (CAS) is based on the classical signs of inflammation and consists of seven items. One point is added for each item that is present and includes: spontaneous pain behind the globe, pain on attempted up-, side- or down-gaze, redness and/or chemosis of the eyelids, and of the caruncle. The CAS ranges from 0 to 7. Per definition, TED is called “active” if CAS is ≥3. To gain clinical information about “DON activity” the rates of active and inactive disease were compared between subjects with recent-onset and post-DON. This information was also used to assess whether the associations between TSI and the acuteness of DON were independent from clinical signs of activity or not. Regarding clinical severity, we differentiated according to EUGOGO between mild, moderate-to-severe, and sight-threatening TED or DON.
Endocrine investigation and laboratory tests
We retrospectively reviewed the medical records of all 180 patients and collected data on thyroid function and therapy that had been assessed at the endocrine outpatient clinic of the multidisciplinary JGU center. The protocol was consistent with the principles of the Declaration of Helsinki. Written informed consent from each patient for blood sampling and complete data set was obtained. Patients that had participated in previous studies of the thyroid laboratory had agreed that blood samples stored at the bio-bank of the lab were used for further research. This procedure was approved by the local Ethics Committee. None of the individual-related data were passed to third parties. Sera that had been stored anonymously at the thyroid lab were used for TSI measurements. Serum TSI activity was measured with a functional cell-based TSHR bioassay (Thyretain, Quidel, CA, USA) according to the manufacturer’s instructions. Briefly, TSI levels were measured in triplicate with the Infinite M200 micro plate reader (Tecan, Crailsheim, Germany). All measured values were corrected for the plate internal auto-luminescence by reduction of the mean value in blank wells. The results were reported as percentage of specimen-to-reference ratio (SRR %). SRR % values were calculated according to the following formula: SRR % = Average TSI specimen relative light units (RLU)/average reference standard RLU × 100 as previously described [38, 39]. Patient serum was considered positive for the presence of TSI if the resultant SRR % measured ≥140 % over the reference control. Any specimen having a coefficient of variation (CV %) >15 % was excluded from the data set and re-tested. The thyroid-binding inhibitory immunoglobulin (TBII) activity was measured with the human TSHR autoantibody radioimmunoassay (TRAK RIA, Thermo Fisher Scientific, Hennigsdorf, Germany).
To test the specificity of the TSI-bioassay, serum TSI levels were also measured in a large control group of 302 “healthy” individuals. “Healthy” was defined as a negative personal and familial history of thyroidal, metabolic, autoimmune, infectious, and tumor diseases, normal ultrasound imaging of the thyroid, as well as normal thyroid-related serum hormone levels and autoantibodies.
Data analysis and statistics
The main outcome measures of this study were the TSI serum levels in patients with DON of recent onset vs. in patients having either a post-DON status or TED without DON. The secondary outcome measure was to compare the TSI and TBII serum levels in TED patients. Regarding the TSI measures, the acquisition file templates and the parameters of data analysis were defined using the Tecan instrument control and data analysis software (Magellan Tracker version 6.6). The records of all acquired data were stored in the format of the original acquisition source files and as data calculations in exported SPSS (Statistical Package for the Social Sciences, Version 22.0, Chicago, Illinois, USA) spread sheets. T test and Chi-square test were used for univariate analyses to compare DON of recent onset with post-DON or TED with DON versus TED without DON. Spearman´s correlation coefficient was calculated for association analyses between continuous variables. Due to the small subgroups, an explorative analysis was performed regarding the differences of TSI and TBII in unilateral versus bilateral DON. One eye only from each subject was used for the analyses. For the multivariate analysis a binary logistic regression model was done. Sensitivity and specificity values with respect to discrimination between DON of recent onset and post-DON were determined for several TSI cut-points, resulting in a receiver operating characteristic (ROC) curve. As an overall measure of discrimination the area under the ROC curve (AUC) was considered. Furthermore, the ROC curve was used for determining an optimal TSI cut-point. Specifically, a pair of sensitivity and specificity was chosen to optimize the Youden index. Illustrations were made with error bars showing the mean ±95 % confidence intervals. Clinical activity of TED, thyroid function and treatment status of thyroid disease were included in the multivariate analysis to detect potential confounders.
Results
Patient characteristics
Demographic and clinical data of the 180 patients with TED are summarized in Table 1. Overall 30 (16.7 %) patients had DON of recent onset or post-DON and in 150 (83.3 %) patients the optic nerve was not affected by TED. DON of recent onset and post-DON were present in 20 of 30 (66.7 %) and in ten (33.3 %) patients, respectively. All patients had Graves’ disease and associated TED.
Clinical signs pertaining to dysthyroid optic neuropathy
Five of 20 (25 %) and 3 of 10 (33 %) patients with DON of recent onset and post-DON showed bilateral eye disease, respectively (p = 0.70). Clinical assessment of these 25 eyes with DON of recent onset and 13 eyes with post-DON revealed that optic disk swelling was present in all eyes with DON of recent onset. In post-DON optic disk swelling improved in all patients but signs of a condition after DON were detectable in all of them (paleness/minor optic disk swelling). Visual-evoked potentials were pathologic in all eyes (25 of 25; 100 %) of patients with DON of recent onset but in one of 13 (7.7 %) patients with post-DON, only (p = 0.005). A relative afferent pupillary defect was present in 21 of 25 (84 %) eyes with recent onset (unilateral or asymmetric) DON and in one of 13 (7.7 %) eyes in post-DON (p = 0.287). Color vision abnormalities were present in 30 and 10 % of patients with recent DON vs. post-DON. Vision field tests were pathologic in 73 % of affected eyes of patients with recent-onset DON and in 50 % with post-DON (p = 0.496). Visual acuity (logMAR) in the DON-affected eyes was 0.37 ± 3.28 in patients with recent-onset DON and 0.60 ± 0.99 in patients with post-DON (p = 0.449).
TSI correlated with the clinical activity score of TED (R = 0.70, p < 0.001). Mean TSI levels in active TED were 485.1 ± 132.3 vs. 277.7 ± 143.7 in inactive disease (p < 0.001). TBII was associated with clinical activity as well: it was 48.9 ± 107.6 IU/l in active vs. 16.5 ± 39.6 IU/l in inactive TED (p < 0.001).
No significant differences of TSI and TBII in unilateral or bilateral DON were found: of patients with bilateral DON of recent onset 80 % were TSI-and TBII-positive versus 100 % TSI-positivity and 86.7 % TBII-positivity in unilateral DON of recent onset. In post-DON, 60 % with a condition after bilateral optic neuropathy were both TSI and TBII positive versus 60 and 40 % being TSI- and TBII positive patients with unilateral post-DON.
TSI versus TBII to identify recent-onset dysthyroid optic neuropathy
TSI were measured in 482 individuals, 180 consecutive and unselected patients with TED with and without DON and in 302 healthy euthyroid subjects (aged 28 ± 8 years, 155 females). All controls were TSI negative with a mean SRR % (SD) 53.17 (16.12) and a median (range) of 51.5 (15–114). Differences of the TSI absolute values in patients with recent onset DON vs. in patients with post-DON or without DON are illustrated in Fig. 1. To evaluate the association between TSI and DON of recent onset, a multivariate analysis was performed (Table 2). Thyroid dysfunction and intake of antithyroid drugs were not included into the multivariate analysis, as there were no cases for these two parameters in the group of subjects with post-DON. In the binary regression analysis—independent from the potential confounders’ radioiodine treatment, thyroid surgery, or clinical activity of TED—serum positivity of TSI was associated with DON of recent onset. Given that high levels of TSI were associated with recent-onset DON, a discriminatory cut-point for TSI was determined based on a ROC analysis (reference: recent-onset DON). The ROC curve indicated reasonable discriminatory performance (area under the curve, AUC: 0.855, p = 0.002, 95 % CI 0.683–1.0; Fig. 2). A cut-point of 377 SRR % (chosen according to an optimal Youden index of 0.75) corresponded to a sensitivity and specificity of 0.95 and 0.8, respectively. Summarized in Table 3 are the rates of TSI-and TBII-positivity in clinically active versus inactive DON of recent onset and post-DON. The serum TBII values in patients with recent onset DON vs. in patients without DON and post-DON are illustrated in Fig. 3. Of patients with recent onset and post-DON 13 of 20 (65 %) and 4 of 10 (40 %) had clinically active TED, respectively. All patients but one with recent onset DON was also TSI-positive (p < 0.001), however, six of 7 (85.7 %) patients with clinically inactive TED with recent onset DON were TSI-positive. The single TSI-negative patient had thyroidectomy 10 years prior to the occurrence of DON. Of patients with post-DON and without DON, 6 (60 %) and 143 (95.3 %) were TSI-positive (p < 0.001). In contrast, TBII was positive (>1 IU/l) in 16 (84.2 %), five (50 %), and 124 (83.2 %) patients with recent onset DON, post-DON, and without DON, respectively (p = 0.031). In the subgroup of patients without DON, all 103 patients with moderate-to-severe TED but 40 of 47 (85 %) only with mild TED were TSI positive (p < 0.001).
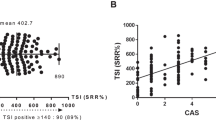
Thyroid stimulating immunoglobulins, TSI [in SRR %] in patients with recent-onset dysthyroid optic neuropathy (DON) versus in patients with a condition post-DON versus in subjects with thyroid eye disease without DON. Plotted are the means ±95 % confidence intervals. Mean TSI (±standard deviation) was SRR % 478.3 ± 98.0 in patients with DON of recent onset versus 397.8 ± 174.4 in post-DON (p < 0.001) versus 408.5 ± 170.0 in patients without DON (p = 0.074). p values according to t test
Receiver operating characteristing (ROC)-curve: the true positive rate (sensitivity) is plotted in function of the false positive rate (100-Specificity) for different cut-off points. Each point on the ROC curve represents a sensitivity/specificity pair corresponding to a particular decision threshold (here: dysthyroid optic neuropathy of recent onset). Given that high levels of TSI were associated with recent-onset DON, a discriminatory cut-point for TSI was determined based on a ROC analysis (reference: recent-onset DON). The ROC curve indicated reasonable discriminatory performance (area under the curve, AUC: 0.855, p = 0.002, 95 % CI 0.683–1.0; Fig. 2). A cut-point of 377 SRR % (chosen according to an optimal Youden index of 0.75) corresponded to a sensitivity and specificity of 0.95 and 0.8, respectively
Thyroid binding inhibitory immunoglobulins, TBII [in IU/l] in patients with recent-onset dysthyroid optic neuropathy (DON) versus in patients with a condition post-DON versus in subjects with thyroid eye disease without DON. Plotted are the means ±95 % confidence intervals. Mean TBII (±standard deviation) was 25.0 ± 30.3 IU/l in patients with recent onset DON versus 38.3 ± 94.5 IU/l in post-DON (p = 0.280) versus 40.1 ± 97.1 IU/l in patients without DON (p = 0.504). p values according to t test
Association with therapy status of DON
All patients had initially been treated with high-dose intravenous steroids (500 mg methylprednisolone 2–3 times per week for a maximum of 2 weeks. At time of inclusion 8 of 20 (40 %) with recent-onset DON and no patient with post-DON had intravenous steroids in the past 6 weeks (p = 0.020). Steroid therapy did not interfere with the rates of TSI-positivity in the present cohort: of patients having had intravenous steroids recently all were TSI-positive. If response to steroids was insufficient bony decompression surgery was performed as recommended by EUGOGO [13]. At time of inclusion 7 of 25 eyes of patients with recent onset DON and 11 of 13 eyes with post-DON had been decompressed (p = 0.165).
Thyroid function and serological parameters
Twenty-eight of 30 (93 %) patients with DON/post-DON were euthyroid, while two were mildly hyperthyroid. In patients without DON, euthyroidism and thyroid dysfunction were present in 87 (58 %) and 63 (42 %) cases, respectively. Median time since onset of thyroid disease was 36 (range 0–216) months in patients with recent onset DON and 24 (0–96) in patients with post-DON (p = 0.450). Median time since onset of TED was 12 months both in patients with recent onset and in post-DON (ranges 0–216 and 0–72), respectively (p = 0.821). Graves’ hyperthyroidism was treated with antithyroid drugs in 103 (68.7 %) patients without DON and in five (25 %) patients with recent-onset DON (p < 0.001). Radioiodine had been administered in three (2 %) and 15 (50 %) patients without vs. with DON (p < 0.001). Similar data are noted for thyroid surgery: three (2 %) and 15 (50 %) patients without vs. with DON had thyroidectomy.
Serum TSI levels were higher in patients with Graves’ hyperthyroidism vs. in euthyroidism (SRR % 457.4 ± 143.4 vs. 378.2 ± 177.0; p < 0.001) and in patients still requiring antithyroid drugs vs. in those being euthyroid in remission (459.8 ± 151.8 vs. 327.2 ± 164.7; p < 0.001). In patients with TED, thyroidectomy led to a slight decrease of TSI serum levels only (409.7 ± 168.3 vs. 380.0 ± 183.3; p = 0.487). To assess the relationship between radioiodine administration and thyroid surgery with DON, the time lag between the occurrence of DON and these specific thyroid treatments was assessed: in five of 11 (45 %) and in five of 25 (25 %) patients, respectively, radioiodine therapy and surgery were performed within 1-year prior to the onset of DON.
Discussion
To the best of our knowledge, this is the first study to demonstrate within a group of consecutive and unselected patients with TED that high serum TSI levels are associated with the presence of DON of recent onset. Despite the strong association between clinical activity and the levels of TSI [39], the vast majority of patients with inactive TED and DON of recent onset were TSI-positive. This study is the first to indicate that serum TSI levels may be a useful diagnostic tool to identify patients with early onset DON who require urgent specific therapy—even if signs for activity are missing.
Since the vast majority of the patients with DON and/or post-DON (93 %) was euthyroid, thyroid dysfunction i.e. Graves’ hyperthyroidism had no relevant impact on this condition. The multivariate analysis showed a significant correlation and a very high odds ratio between high serum levels of TSI and the occurrence/presence of DON of recent onset.
As previously shown [27–29, 32], clinical features in patients with suspected DON were noted to be highly heterogeneous. In the DON-patients assessed within this study, based on clinical findings alone and without any information regarding medical history it would have been difficult to decide whether DON had developed recently and warranted urgent therapy or not. Due to irreversible nerve fiber loss clinical signs of DON and decreased visual acuity may persist subsequently to medical or surgical decompression of the optic nerve (post-DON). In the present study, TSI usefully differentiated between three subgroups: DON of recent onset, post-DON and no DON. Except in one case in which total thyroidectomy was performed 10 years prior to the onset of DON all patients with recent onset DON were highly TSI-positive. In contrast, there was a wide overlap of the serum TBII values between the three study groups. Furthermore, the specificity of the novel cell-based bioassay was confirmed by the analysis of serum TSI-levels in a large healthy control group as all of these individuals were TSI-negative.
Since it has been shown that TSI is associated with both clinical activity of TED [39, 46–48] as well as with the treatment status of thyroid disease [38, 49–53], these variables were also included in the multivariate analysis. Interestingly, the association between TSI and DON of recent onset were independent from the clinical activity of TED. The comparison of TSI-positivity in clinically active versus inactive DON of recent onset and post-DON revealed the highest positivity rates in both clinically active and inactive DON of recent onset. In contrast, only one quarter of patients with clinically active post-DON was TSI-positive. This is in line with the previously published EUGOGO-survey on DON which emphasized that both soft tissue signs and signs of clinical activity did not help confirm or refute the diagnosis of DON, as such features were absent in several patients [27]. TSI could therefore be used as a biomarker to identify patients with acute DON even in the absence of signs of acute inflammation.
In the present study, no significant differences of TSI and TBII in unilateral or bilateral DON were found. This emphasizes DON as being part of a systemic disease. Nevertheless, DON usually occurs bilaterally in the majority of patients. Nevertheless, in the present cohort we had a relatively high proportion of unilateral DON. This might be because only eyes with definite DON (diagnosis was considered certain) were included. Eyes with equivocal DON (diagnosis likely but not certain) were not included into analysis. This might have led to an underestimation of the proportion of bilateral DON. The fact that only eyes with definite DON were included into analysis is also the explanation for the frequencies of the clinical signs of DON: for example, optic disk swelling was found in all patients with recent onset DON in the present study, whereas it is missing in up to 50 % of cases in daily routine [25]. This would have not been like this if we had included eyes with a suspicion of DON also. This might have led to an underestimation of the total prevalence of DON.
In this study, there were patients that had received radioiodine treatment in the year prior to the onset of DON. Hence, potentially, radioiodine could have increased the risk for DON at least in some of these patients [54, 55]. In another study a post-radioiodine therapy induced increase of TSHR autoantibodies in 50 % of the patients with Graves’ disease [56]. Further, a prospective controlled trial in Europe showed a dramatic surge of the TSHR autoantibodies serum levels post-radioiodine therapy while the high serum levels were sustained 5 years after radioiodine treatment [57]. However, multivariate analysis revealed that the association of serum TSI-positivity with recent onset DON was independent of radioiodine therapy.
The following limitations of the present study have to be addressed. First, this study compared the clinical and laboratory data of patients with different stages of TED with and without DON only at one time point. As this study was retrospective, we were not able to collect data of “very acute DON” but only a subgroup of patients that had been treated during the past 6 months. A prospective study should systematically measure the serum values of TSI and TBII prior to the occurrence, within the active phase of the disease, during and after specific therapy. Second, the collective of patients with DON was relatively small. Therefore, the results need to be confirmed or disproved in larger series. Furthermore, the method used for TSI measurement is not widely available and therefore the conclusion of this study cannot be widely used in clinical practice. Luckily, a careful clinical assessment and imaging is able to identify patients requiring urgent therapy in the vast majority of cases. Despite these limitations, this study is the first to provide assess TSI as a biomarker for DON. Optimized diagnostics of DON are necessary to ensure that patients requiring sight-saving treatment receive it in a timely manner, whereas those who do not need it spared the unnecessary risk of adverse events. Diagnostic tools such as measuring serum TSI levels seem to be useful especially in patients with atypical presentation and when clinical signs of activity are missing. High-quality prospective studies are warranted to assess whether measuring TSI may be used for the development and evaluation of novel therapies for patients with sight-threatening TED.
Abbreviations
- TED:
-
Thyroid eye disease
- TSI:
-
Thyroid stimulating immunoglobulins
- DON:
-
Dysthyroid optic neuropathy
- TSH:
-
Thyroid stimulating hormone
- TSHR:
-
TSH-receptor
- EUGOGO:
-
European Group on Graves Orbitopathy
- CAS:
-
Clinical activity score
- TBII:
-
Thyroid binding inhibitory immunoglobulin
- SRR %:
-
Percentage of specimen-to-reference ratio
- RLU:
-
Relative light units
- CV %:
-
Coefficient of variation
References
Bartalena L, Fatourechi V (2014) Extrathyroidal manifestations of Graves’ disease: a 2014 update. J Endocrinol Invest 37(8):691–700. doi:10.1007/s40618-014-0097-2
Tanda ML, Piantanida E, Liparulo L, Veronesi G, Lai A, Sassi L, Pariani N, Gallo D, Azzolini C, Ferrario M, Bartalena L (2013) Prevalence and natural history of Graves’ orbitopathy in a large series of patients with newly diagnosed graves’ hyperthyroidism seen at a single center. J Clin Endocrinol Metab 98(4):1443–1449. doi:10.1210/jc.2012-3873
Marcocci C, Kahaly GJ, Krassas GE, Bartalena L, Prummel M, Stahl M, Altea MA, Nardi M, Pitz S, Boboridis K, Sivelli P, von Arx G, Mourits MP, Baldeschi L, Bencivelli W, Wiersinga W (2011) Selenium and the course of mild Graves’ orbitopathy. N Engl J Med 364(20):1920–1931. doi:10.1056/NEJMoa1012985
Piantanida E, Tanda ML, Lai A, Sassi L, Bartalena L (2013) Prevalence and natural history of Graves’ orbitopathy in the XXI century. J Endocrinol Invest 36(6):444–449. doi:10.3275/8937
Bartley GB (1994) The epidemiologic characteristics and clinical course of ophthalmopathy associated with autoimmune thyroid disease in Olmsted County, Minnesota. Trans Am Ophthalmol Soc 92:477–588
Ebner R (2002) Dysthyroid optic neuropathy (DON). Semin Ophthalmol 17(1):18–21. doi:10.1076/soph.17.1.18.10289
Neigel JM, Rootman J, Belkin RI, Nugent RA, Drance SM, Beattie CW, Spinelli JA (1988) Dysthyroid optic neuropathy. The crowded orbital apex syndrome. Ophthalmology 95(11):1515–1521
Du Y, Ye H, Li K, Xiao X, Chen R, He JF, Yang H (2014) Vision-related quality of life tends to be more severely impaired in patients with dysthyroid optic neuropathy. Curr Eye Res 39(5):532–536. doi:10.3109/02713683.2013.848901
Ponto KA, Hommel G, Pitz S, Elflein H, Pfeiffer N, Kahaly GJ (2011) Quality of life in a german graves orbitopathy population. Am J Ophthalmol 152(3):483–490. doi:10.1016/j.ajo.2011.02.018 (e481)
Ponto KA, Merkesdal S, Hommel G, Pitz S, Pfeiffer N, Kahaly GJ (2013) Public health relevance of Graves’ orbitopathy. J Clin Endocrinol Metab 98(1):145–152. doi:10.1210/jc.2012-3119
Zang S, Ponto KA, Pitz S, Kahaly GJ (2011) Dose of intravenous steroids and therapy outcome in Graves’ orbitopathy. J Endocrinol Invest 34(11):876–880
Bartalena L, Krassas GE, Wiersinga W, Marcocci C, Salvi M, Daumerie C, Bournaud C, Stahl M, Sassi L, Veronesi G, Azzolini C, Boboridis KG, Mourits MP, Soeters MR, Baldeschi L, Nardi M, Curro N, Boschi A, Bernard M, von Arx G (2012) Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves’ orbitopathy. J Clin Endocrinol Metab 97(12):4454–4463. doi:10.1210/jc.2012-2389
Bartalena L, Baldeschi L, Dickinson AJ, Eckstein A, Kendall-Taylor P, Marcocci C, Mourits MP, Perros P, Boboridis K, Boschi A, Curro N, Daumerie C, Kahaly GJ, Krassas G, Lane CM, Lazarus JH, Marino M, Nardi M, Neoh C, Orgiazzi J, Pearce S, Pinchera A, Pitz S, Salvi M, Sivelli P, Stahl M, von Arx G, Wiersinga WM (2008) Consensus statement of the European group on Graves’ orbitopathy (EUGOGO) on management of Graves’ orbitopathy. Thyroid Off J Am Thyroid Assoc 18(3):333–346. doi:10.1089/thy.2007.0315
Choe CH, Cho RI, Elner VM (2011) Comparison of lateral and medial orbital decompression for the treatment of compressive optic neuropathy in thyroid eye disease. Ophthalmic Plast Reconstr Surg 27(1):4–11. doi:10.1097/IOP.0b013e3181df6a87
Guy JR, Fagien S, Donovan JP, Rubin ML (1989) Methylprednisolone pulse therapy in severe dysthyroid optic neuropathy. Ophthalmology 96(7):1048–1052 (discussion 1052–1043)
Kazim M, Trokel S, Moore S (1991) Treatment of acute Graves orbitopathy. Ophthalmology 98(9):1443–1448
Leone CR Jr, Bajandas FJ (1981) Inferior orbital decompression for dysthyroid optic neuropathy. Ophthalmology 88(6):525–532
Panzo GJ, Tomsak RL (1983) A retrospective review of 26 cases of dysthyroid optic neuropathy. Am J Ophthalmol 96(2):190–194
Zang S, Ponto KA, Kahaly GJ (2011) Clinical review: Intravenous glucocorticoids for Graves’ orbitopathy: efficacy and morbidity. J Clin Endocrinol Metab 96(2):320–332. doi:10.1210/jc.2010-1962
Tanda ML, Piantanida E, Bartalena L (2012) Treating Graves’ orbitopathy: where are we? Endocrine 41(2):167–168. doi:10.1007/s12020-012-9607-1
Bartalena L, Lai A, Compri E, Marcocci C, Tanda ML (2008) Novel immunomodulating agents for Graves orbitopathy. Ophthalmic Plast Reconstr Surg 24(4):251–256. doi:10.1097/IOP.0b013e318179f8a5
Bartalena L, Lai A, Sassi L, Lombardi V, Dalle Mule I, Gandolfo M, Liparulo L, Azzolini C, Piantanida E, Tanda ML (2010) Novel treatment modalities for Graves’ orbitopathy. Pediatr Endocrinol Rev PER 7(Suppl 2):210–216
Bartalena L, Tanda ML (2006) Immunotherapy for Graves’ orbitopathy: easy enthusiasm, but let’s keep trying. J Endocrinol Invest 29(11):1012–1016
Mourits MP, Bijl H, Altea MA, Baldeschi L, Boboridis K, Curro N, Dickinson AJ, Eckstein A, Freidel M, Guastella C, Kahaly GJ, Kalmann R, Krassas GE, Lane CM, Lareida J, Marcocci C, Marino M, Nardi M, Mohr C, Neoh C, Pinchera A, Orgiazzi J, Pitz S, Saeed P, Salvi M, Sellari-Franceschini S, Stahl M, von Arx G, Wiersinga WM (2009) Outcome of orbital decompression for disfiguring proptosis in patients with Graves’ orbitopathy using various surgical procedures. Br J Ophthalmol 93(11):1518–1523. doi:10.1136/bjo.2008.149302
Dayan CM, Dayan MR (2007) Dysthyroid optic neuropathy: a clinical diagnosis or a definable entity? Br J Ophthalmol 91(4):409–410. doi:10.1136/bjo.2006.110932
da Silva FL, de Lourdes Veronese Rodrigues M, Akaishi PM, Cruz AA (2009) Graves’ orbitopathy: frequency of ocular hypertension and glaucoma. Eye (London, England) 23(4):957–959. doi:10.1038/eye.2008.155
McKeag D, Lane C, Lazarus JH, Baldeschi L, Boboridis K, Dickinson AJ, Hullo AI, Kahaly G, Krassas G, Marcocci C, Marino M, Mourits MP, Nardi M, Neoh C, Orgiazzi J, Perros P, Pinchera A, Pitz S, Prummel MF, Sartini MS, Wiersinga WM (2007) Clinical features of dysthyroid optic neuropathy: a European Group on Graves’ Orbitopathy (EUGOGO) survey. Br J Ophthalmol 91(4):455–458. doi:10.1136/bjo.2006.094607
Mensah A, Vignal-Clermont C, Mehanna C, Morel X, Galatoire O, Jacomet PV, Morax S (2009) Dysthyroid optic neuropathy: atypical initial presentation and persistent visual loss. Orbit (Amsterdam, Netherlands) 28(6):354–362. doi:10.3109/01676830903104728
Bartley GB, Gorman CA (1995) Diagnostic criteria for Graves’ ophthalmopathy. Am J Ophthalmol 119(6):792–795
Lipski A, Eckstein A, Esser J, Loesch C, Mann K, Mohr C, Jurklies B (2011) Course of pattern-reversed visual evoked cortical potentials in 30 eyes after bony orbital decompression in dysthyroid optic neuropathy. Br J Ophthalmol 95(2):222–226. doi:10.1136/bjo.2009.173658
Perez-Rico C, Rodriguez-Gonzalez N, Arevalo-Serrano J, Blanco R (2012) Evaluation of multifocal visual evoked potentials in patients with Graves’ orbitopathy and subclinical optic nerve involvement. Doc Ophthalmol Adv Ophthalmol 125(1):11–19. doi:10.1007/s10633-012-9325-2
Weis E, Heran MK, Jhamb A, Chan AK, Chiu JP, Hurley MC, Rootman J (2011) Clinical and soft-tissue computed tomographic predictors of dysthyroid optic neuropathy: refinement of the constellation of findings at presentation. Arch Ophthalmol 129(10):1332–1336. doi:10.1001/archophthalmol.2011.276
Weis E, Heran MK, Jhamb A, Chan AK, Chiu JP, Hurley MC, Rootman J (2012) Quantitative computed tomographic predictors of compressive optic neuropathy in patients with thyroid orbitopathy: a volumetric analysis. Ophthalmology 119(10):2174–2178. doi:10.1016/j.ophtha.2012.04.021
Dragan LR, Seiff SR, Lee DC (2006) Longitudinal correlation of thyroid-stimulating immunoglobulin with clinical activity of disease in thyroid-associated orbitopathy. Ophthalmic Plast Reconstr Surg 22(1):13–19
Eckstein A, Esser J, Mann K, Schott M (2010) Clinical value of TSH receptor antibodies measurement in patients with Graves’ orbitopathy. Pediatr Endocrinol Rev PER 7(Suppl 2):198–203
Kahaly G, Grubl M, Moncayo R, Schilling S, Weber P, Beyer J, Krause U (1989) Thyroid-stimulating and eye muscle antibodies in Graves’ disease and Graves’ orbitopathy. Dev Ophthalmol 20:68–78
Kahaly G, Moncayo R, Stover C, Beyer J (1991) Relationship of eye muscle antibodies with HLA phenotypes and thyroid-stimulating immunoglobulins in endocrine orbitopathy. Res Exp Med Zeitschrift fur die gesamte experimentelle Medizin einschliesslich experimenteller Chirurgie 191(2):137–144
Lytton SD, Ponto KA, Kanitz M, Matheis N, Kohn LD, Kahaly GJ (2010) A novel thyroid stimulating immunoglobulin bioassay is a functional indicator of activity and severity of Graves’ orbitopathy. J Clin Endocrinol Metab 95(5):2123–2131. doi:10.1210/jc.2009-2470
Ponto KA, Kanitz M, Olivo PD, Pitz S, Pfeiffer N, Kahaly GJ (2011) Clinical relevance of thyroid-stimulating immunoglobulins in graves’ ophthalmopathy. Ophthalmology 118(11):2279–2285. doi:10.1016/j.ophtha.2011.03.030
Diana T, Brown RS, Bossowski A, Segni M, Niedziela M, Konig J, Bossowska A, Ziora K, Hale A, Smith J, Pitz S, Kanitz M, Kahaly GJ (2014) Clinical relevance of thyroid-stimulating autoantibodies in pediatric graves’ disease-a multicenter study. J Clin Endocrinol Metab 99(5):1648–1655. doi:10.1210/jc.2013-4026
Diana T, Kanitz M, Lehmann M, Li Y, Olivo PD, Kahaly GJ (2014) Standardization of a bioassay for thyrotropin receptor stimulating autoantibodies. Thyroid Off J Am Thyroid Assoc. doi:10.1089/thy.2014.0346
Kamijo K, Murayama H, Uzu T, Togashi K, Kahaly GJ (2010) A novel bioreporter assay for thyrotropin receptor antibodies using a chimeric thyrotropin receptor (mc4) is more useful in differentiation of Graves’ disease from painless thyroiditis than conventional thyrotropin-stimulating antibody assay using porcine thyroid cells. Thyroid Off J Am Thyroid Assoc 20(8):851–856. doi:10.1089/thy.2010.0059
Kamijo K, Murayama H, Uzu T, Togashi K, Olivo PD, Kahaly GJ (2011) Similar clinical performance of a novel chimeric thyroid-stimulating hormone receptor bioassay and an automated thyroid-stimulating hormone receptor binding assay in Graves’ disease. Thyroid Off J Am Thyroid Assoc 21(12):1295–1299. doi:10.1089/thy.2011.0056
Leschik JJ, Diana T, Olivo PD, Konig J, Krahn U, Li Y, Kanitz M, Kahaly GJ (2013) Analytical performance and clinical utility of a bioassay for thyroid-stimulating immunoglobulins. Am J Clin Pathol 139(2):192–200. doi:10.1309/ajcpzut7cnueu7op
Wiersinga WM, Perros P, Kahaly GJ, Mourits MP, Baldeschi L, Boboridis K, Boschi A, Dickinson AJ, Kendall-Taylor P, Krassas GE, Lane CM, Lazarus JH, Marcocci C, Marino M, Nardi M, Neoh C, Orgiazzi J, Pinchera A, Pitz S, Prummel MF, Sartini MS, Stahl M, von Arx G (2006) Clinical assessment of patients with Graves’ orbitopathy: the European Group on Graves’ Orbitopathy recommendations to generalists, specialists and clinical researchers. Eur J Endocrinol/European Fed Endocr Soc 155(3):387–389. doi:10.1530/eje.1.02230
Eckstein AK, Plicht M, Lax H, Hirche H, Quadbeck B, Mann K, Steuhl KP, Esser J, Morgenthaler NG (2004) Clinical results of anti-inflammatory therapy in Graves’ ophthalmopathy and association with thyroidal autoantibodies. Clin Endocrinol 61(5):612–618. doi:10.1111/j.1365-2265.2004.02143.x
Gerding MN, van der Meer JW, Broenink M, Bakker O, Wiersinga WM, Prummel MF (2000) Association of thyrotrophin receptor antibodies with the clinical features of Graves’ ophthalmopathy. Clin Endocrinol 52(3):267–271
Subekti I, Boedisantoso A, Moeloek ND, Waspadji S, Mansyur M (2012) Association of TSH receptor antibody, thyroid stimulating antibody, and thyroid blocking antibody with clinical activity score and degree of severity of Graves ophthalmopathy. Acta medica Indonesiana 44(2):114–121
Bai Y, Dai WX, Sun ML, Guo ZS, Jin X, Chi ZS (1987) Changes of serum TSI in hyperthyroidism with long-term antithyroid therapy. Proc Chin Acad Med Sci Peking Union Med Coll = Chung-kuo i hsueh k’o hsueh yuan, Chung-kuo hsieh ho i k’o ta hsueh hsueh pao 2(3):172–174
Kautbally S, Alexopoulou O, Daumerie C, Jamar F, Mourad M, Maiter D (2012) Greater efficacy of total thyroidectomy versus radioiodine therapy on hyperthyroidism and thyroid-stimulating immunoglobulin levels in patients with Graves’ disease previously treated with antithyroid drugs. Eur Thyroid J 1(2):122–128. doi:10.1159/000339473
Prentice MG, Rayman GA, Alaghband-Zadeh J, Wise PH (1987) Thyroid stimulating immunoglobulin bioactivity during carbimazole therapy as measured by the cytochemical bioassay. J Endocrinol Invest 10(5):483–489
Rittmaster RS, Zwicker H, Abbott EC, Douglas R, Givner ML, Gupta MK, Lehmann L, Reddy S, Salisbury SR, Shlossberg AH, Tan MH, York SE (1996) Effect of methimazole with or without exogenous L-thyroxine on serum concentrations of thyrotropin (TSH) receptor antibodies in patients with Graves’ disease. J Clin Endocrinol Metab 81(9):3283–3288. doi:10.1210/jcem.81.9.8784084
Szabo J, Leovey A, Karanyi Z, Varvolgyi C, Forizs E, Herceg L (1989) Detectability of thyroid anti-microsomal antibodies, changes in thyroid-stimulating immunoglobulins (TSI) and thyrotropin-binding-inhibiting immunoglobulins (TBII) during methimazole treatment of Graves’ disease patients. Acta Med Hung 46(1):23–30
Ponto KA, Zang S, Kahaly GJ (2010) The tale of radioiodine and Graves’ orbitopathy. Thyroid Off J Am Thyroid Assoc 20(7):785–793. doi:10.1089/thy.2010.1640
Vannucchi G, Campi I, Covelli D, Dazzi D, Curro N, Simonetta S, Ratiglia R, Beck-Peccoz P, Salvi M (2009) Graves’ orbitopathy activation after radioactive iodine therapy with and without steroid prophylaxis. J Clin Endocrinol Metab 94(9):3381–3386. doi:10.1210/jc.2009-0506
Michelangeli VP, Poon C, Topliss DJ, Colman PG (1995) Specific effects of radioiodine treatment on TSAb and TBAb levels in patients with Graves’ disease. Thyroid Off J Am Thyroid Assoc 5(3):171–176
Laurberg P, Wallin G, Tallstedt L, Abraham-Nordling M, Lundell G, Torring O (2008) TSH-receptor autoimmunity in Graves’ disease after therapy with anti-thyroid drugs, surgery, or radioiodine: a 5-year prospective randomized study. Eur J Endocrinol/Eur Fed Endocr Soc 158(1):69–75. doi:10.1530/eje-07-0450
Acknowledgments
Katharina A Ponto is funded by the Federal Ministry of Education and Research (BMBF 01EO1003). The authors are grateful to Paul D. Olivo, MD, PhD and to Jeff Houtz for the critical evaluation of the manuscript.
Conflict of interest
Katharina A. Ponto, Tanja Diana, Harald Binder, Nina Matheis, Susanne Pitz, and Norbert Pfeiffer have nothing to disclose; George J. Kahaly consults for and has received research funding from Quidel, CA, USA. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ponto, K.A., Diana, T., Binder, H. et al. Thyroid-stimulating immunoglobulins indicate the onset of dysthyroid optic neuropathy. J Endocrinol Invest 38, 769–777 (2015). https://doi.org/10.1007/s40618-015-0254-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-015-0254-2