Abstract
Context
Safety of intravenous (IV) steroid pulses in patients with Graves’ orbitopathy (GO) is still controversial while steroid dose and treatment application have not been finalized. Frequency, severity and characterization of adverse events (AE) were prospectively analyzed.
Setting
Academic referral orbital center with a joint thyroid-eye clinic.
Patients
Eighty consecutive and unselected patients with active and severe GO.
Methods
During an established treatment with IV methylprednisolone (cumulative dose 4.5 g) occurring AE were prospectively coded according to the standardized and recognized medical dictionary for regulatory activities (MedDRA). Outcome and severity of AE were documented. AEs judged as at least possibly related to drug treatment were graded as side effect (SE). AEs matching a seriousness criteria as defined by the ICH guideline E6 (good clinical practice) were graded as serious.
Results
A total of 38.75 % (31/80) of the treated GO patients reported at least one AE while 18 patients (22.5 %) reported at least one SE. All SE were within the safety profile of IV methylprednisolone; 31/32 SE (96.87 %) were mild-moderate and reversible and only 1/80 patient (1.25 %) stopped steroid treatment due to exacerbation of her depression. Most AE were accessory symptoms of the underlying disease and a few only were directly related to IV steroids. Most AEs (90.6 %) were graded as mild. Only six patients (7.5 %) were hospitalized, three of them due to a dysthyroid optic neuropathy.
Conclusions
Prospective and standardized evaluation with MedDRA and the ICH guideline demonstrated the good pharmacological tolerance and low morbidity of this moderate steroid regimen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The organ specific autoimmune thyroid-associated orbitopathy or Graves’ orbitopathy (GO) is the main extrathyroidal expression of Graves’ disease [1]. Management of GO should be early starting and patients should be treated in joint–thyroid–eye clinics [2–4]. Intravenous glucocorticoids are the first-line treatment for GO. However, route of administration and single as well as cumulative dose of intravenous methylprednisolone are still a matter of debate [1, 3, 4]. Adverse events (AE) of (foremost orally administered) glucocorticoids include weight gain, gastrointestinal side effects (SE), glucose intolerance, thin skin and/or osteoporosis. Within the summary of the product characteristics a list of SEs is stated but frequencies of SEs are still unknown [5]. Several cases with steroid induced hepatotoxicity [6–10] and cardiovascular complications [11, 12] have been reported. However, when searching for evaluated safety data, not a single paper has focused on careful analysis of data according to an internationally widely recognized and standardized medical dictionary abbreviated “MedDRA”. In the reported clinical trials, e.g., [13–16] the primary endpoints were efficacy parameters and SEs were poorly mentioned and not systematically evaluated. Also, when the safety parameters from clinical trials were analyzed [17] they were related to a limited study population and to a study defined drug regime. Further, in several review papers on GO, safety evaluation of the reviewed trials was only a minor part of the paper [18, 19]. Even within a systematic review on morbidity and mortality of intravenous (IV) steroid pulses in GO, the retrospectively collected data have to be seen under limitation of the trials reviewed [20]. A recent questionnaire survey among members of the European Thyroid Association (ETA) evaluated possible AE of oral and intravenous steroid therapy; however, this questionnaire was retrospective and most of the responding ETA members did not mention any AE of the steroid treatment [21]. Therefore, we aimed to prospectively record and systematically evaluate safety data using standardized tools and internationally accepted guidelines in a large number of consecutive patients with severe and active GO followed at an academic tertiary referral orbital center with a joint–thyroid–eye clinic.
Materials and methods
Patients
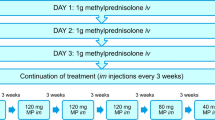
The prospective study was approved by the local Ethics Committee and the trial was performed according to the Helsinki declaration. Prospectively recorded safety data of 80 patients with active and severe GO were evaluated during a standardized and established IV methylprednisolone treatment (0.5 g, then 0.25 g, 6 weeks each; cumulative dose 4.5 g) [17, 20]. 6 and 12 weeks after starting IV steroid administration, AE were registered and blood sampling for thyroid-related hormones and autoantibodies, white blood cells, liver and kidney chemistry was done.
Methods
All documented AEs were coded with the medical dictionary for regulatory affairs (MedDRA). It is a medical terminology developed by the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). The application of MedDRA is highly recommended to accurately and transparently describe the safety profile and quantify the putative AEs and SE during the administration of drug therapy like the evaluated high-dose steroid regimen [22]. The English Version 16.1 was used and the appropriated points to consider [23] were attended. Severity of all AEs was graded with the categories mild, moderate and severe classified as follows: the AE does not interfere with daily activities (mild), the AE interferes with daily activities (moderate) and the AE prevents patient daily activities (severe). A follow-up of all AEs was done and the final outcome was documented. Every AE was judged if it was related to drug intake and if it was serious according to the seriousness criteria defined in the ICH E6 guideline of the good clinical practice (GCP) [24].
Results
Demographic and clinical data
All evaluated patients were of Caucasian ethnicity. 57 of 80 (71.3 %) patients were female. The median age of the patients was 53 years (range 23–75 years), the median weight was 70 kg (48–118 kg). Further to GO, the patients had up to seven other autoimmune and non-autoimmune diseases (median 2 diseases per patient) and they were on up to eight different concomitant medications (median 2 per patient). Median clinical activity score [3, 4] at baseline was 4 (range 1–6 points) for the right eye and 4 (0–6 points) for the left eye.
Adverse events and side effects
A total of 31 patients (38.75 %) reported at least one AE while 18 (22.5 %) patients reported at least one AE graded as at least possibly IV steroid-related (SE). A total of 64 AE was documented and coded with MedDRA. Number of registered AEs according to the MedRA System organ classes (SOC) and occurred preferred terms are given in Table 1.
SOCs with a higher percentage rate than 10 % of all AEs mentioned were “psychiatric disorders”, “gastrointestinal disorders”, “infections and infestations” and “general disorders and administration site conditions”. Most often mentioned AEs were hot flush, optic neuropathy and sleep disorder (Table 1). All other AE occurred only once or twice in the study population. Several AE (e.g., cardiac arrhythmias, optic neuropathy and thyroid dysfunction) were clearly related to the underlying autoimmune thyroid disease. A total of 47 (73.4 %) of AE were recovered, 13 (20.3 %) were recovering and only one (1.6 %) AE (worsening of psoriasis) did not recover.
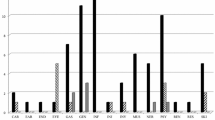
A total of 58 (90.6 %) AE were graded as mild (48 events) or moderate (8 events) (see Fig. 1). A few events, i.e., dysthyroid optic neuropathy, major depression and atrial tachyarrhythmia were graded as severe. Moderate events were hyperthyroidism, nausea, peripheral edema and/or pain in extremity and occurred in eight (10 %) patients.
Number of adverse events in specific system organ class graded by severity. AE adverse event, CAR cardiac disorders, EAR ear and labyrinth disorders, END endocrine disorders, EYE eye disorders, GAS gastrointestinal disorders, GEN general disorders and administration site conditions, INF infections and infestations, INV investigations, MUS musculoskeletal and connective tissue disorders, NER nervous system disorders, PSY psychiatric disorders, REP reproductive system and breast disorders, RES respiratory, thoracic and mediastinal disorders, SKI skin and subcutaneous tissue disorders, VAS vascular disorders
A total of 32 (50 %) of the AEs were judged as SE (see Fig. 2). All SE were distributed among the already known safety profile of IV methylprednisolone. SE occurred most in the SOCs “gastrointestinal disorders”, “vascular disorders”, “psychiatric disorders” and “skin and subcutaneous disorders”.
Number of side effects and the cumulative number of adverse events in specific system organ class. AE adverse event, SE side effect, CAR cardiac disorders, EAR ear and labyrinth disorders, END endocrine disorders, EYE eye disorders, GAS gastrointestinal disorders, GEN general disorders and administration site conditions, INF infections and infestations, INV investigations, MUS musculoskeletal and connective tissue disorders, NER nervous system disorders, PSY psychiatric disorders, REP reproductive system and breast disorders, RES respiratory, thoracic and mediastinal disorders, SKI skin and subcutaneous tissue disorders, VAS vascular disorders
Abnormal laboratory values were noted in three patients (3.75 %), only. One patient (1.25 %) experienced a reversible fivefold increase of gamma-glut amyl-transferase, one patient had a reversible twofold increased blood glucose level and the third patient experienced a reversible fourfold increased alanine aminotransferase. All three events were coded into SOC “Investigations” and had a recovered or recovering outcome.
Only six (7.5 %) patients experienced an AE requiring a hospitalization and, therefore, graded as serious AE (SAE). All SAE occurred during the first six weeks of the IV steroid treatment and showed a recovered or recovering outcome. Documented SAE were: dysthyroid optic neuropathy in three patients (3.75 %), cardiac tachyarrhythmia, lower leg edema and major depression in one patient, respectively. The patient who experienced an exacerbation of its endogenous depression was the only one who interrupted the IV steroid treatment. She recovered after steroid withdrawal.
Discussion
This is the first systemic analysis of the AEs and SEs of IV high-dose steroid pulse treatment according to an internationally standardized and validated medical dictionary as well as based on internationally accepted guidelines. This prospectively performed study demonstrated the low IV steroid-related morbidity and practically an inexistent mortality directly related to the moderate cumulative dose used. The analysis was performed in a representative group of 80 consecutive patients; foremost females suffering from active and moderate-to-severe GO.
Thirty-one patients (38.7 %) experienced at least one AE. Most other published clinical trials state the number of events only and not the number of patients experiencing an AE. A recent systemic review [20] analyzing 14 studies encompassing 1,045 patients reported that 68 (6.5 %) of evaluated patients experienced at least one AE. Due to the fact that in most of the evaluated clinical trials [13, 16, 25], the number of AEs and not the number of affected patients were mentioned and that minor events were often not reported, the frequency of 6.5 % is most probably underestimated. In contrast, in this paper, all events, regardless if mild, moderate or severe were carefully recorded, judged with either possible causality to drug intake or not and, therefore, the registered number of AE was much higher.
In a recently published questionnaire prepared by the European Group of Graves’ Orbitopathy (EUGOGO) and filled by clinically active members of the European Thyroid Association (ETA) [21], only 32 of 83 ETA members (39 %) using IV steroids reported events. Detailed data were reported for 39 patients, most of them receiving very high doses of IV glucocorticoids. Due to missing number of treated patients the percentage rates of AEs are unfortunately not evaluable in this joint ETA/EUGOGO survey. Thus, a direct comparison of the results from our prospective standardized registration and this survey is not feasible. As only severe AEs were reported in the questionnaires of the ETA survey, underreporting of mild and moderate AEs is highly probable.
In contrast, carefully documented and useful data are available from a large randomized trial of EUGOGO. When comparing three different doses of IV methylprednisolone, Bartalena et al. [15] observed in 18 of 54 (33.3 %) patients at least one AE with the lower cumulative dose of 4.98 g methylprednisolone which fits with the slightly higher rate of 38.7 %, registered in this study. Based on these data, at least 50 % of patients treated with a cumulative dose of 4.5 g methylprednisolone will not experience any AE.
In our prospective study no IV steroid-related mortality was registered and only six GO patients were admitted to the hospital because of AEs. Only one had an SAE with a probable relationship to IV methylprednisolone. This is in line with the published literature. The EUGOGO/ETA Survey [21] described seven of 39 AEs (18 %) with a fatal outcome. With one exception, all of them occurred in patients receiving high cumulative dose (>8 g) of steroids, a high single or daily dose above 0.75 g and most of them were administered daily doses instead of intermittent drug administration. The recently published large systemic analysis reported a mortality rate of 0.6 % [20]. Fatal events occurred with one single exception in patients receiving daily doses higher than 0.5 g. The conclusions drawn from the systemic review are confirmed by the results of the presented standardized prospective analysis and clearly demonstrate that considering the already published exclusion criteria, i.e., active hepatitis, severe hypertension, severe coronary heart disease, very instable diabetes, no fatal AE or SE (iv steroid-related AE) are to be expected during a 12-week-treatment scheme with a cumulative dose of 4.5 g of IV methylprednisolone.
An often discussed topic concerning safety of IV steroids is hepatotoxicity. In the literature several case reports [6, 7] and a small study with 30 cases are reported [26]. The four cases of acute liver failure with fatal outcome described in the EUGOGO/ETA Survey [21] were treated with a much higher cumulative dose than 4.5 g methylprednisolone. In comparison, not a single patient monitored at our institution experienced severe liver injury. Only two patients experienced elevated liver parameters (increased gamma-glutamyl-transferase; increased alanine aminotransferase) six weeks after starting treatment. These SE were self-limiting and patients recovered during the following six weeks receiving a weekly dose of 250 mg. In a study comparing two regimens of IV steroids [27] the authors came to the conclusion that acute liver failure could be a dose-depending SE. According to these authors and as strongly recommended in the Consensus statement of EUGOGO [3, 4] it is advised to regularly monitor liver function parameters of patients prior as well as during IV steroid treatment.
IV steroid therapy may also affect the cardiovascular system [11, 12, 28]. In this study, eight events within the SOC “cardiac disorders” or “vascular disorders” occurred (frequency of 12.5 %). These AE (hot flush, palpitations, tachyarrhythmia, disorder circulatory system, hypertension) are well-known SE of methylprednisolone [5]. Tachyarrhythmia and disorder of the circulatory system were graded as not related due to possible alternative causes. None of the patients observed had to stop IV steroid treatment or reduce the single dose due to a cardiovascular event. According to the registered data IV steroid-related cardiovascular SE cannot be totally excluded. If any, these SE were mild to moderate and were well tolerated.
A limitation of the study is the relatively low number of patients included. Although using standardized and internationally accepted tools, rare cardiovascular and hepatic SE could have been missed despite the relatively high documentation rate (22 %) of mild to moderate SE. Therefore, the prospective study is further extended to additional patients. Further in this paper documented AE were not graded in either minor or major since there is no internationally accepted written definition of “minor” and “major” AE.
In conclusion, the standardized, prospectively evaluated AE and IV steroid-related SE documents the safety and good tolerance of an IV steroid scheme with a moderate cumulative dose.
References
Bartalena L, Fatourechi V (2014) Extrathyroidal manifestations of Graves’ disease: a 2014 update. J Endocrinol Invest 37:691–700
Piantanida E, Tanda ML, Lai A, Sassi L, Bartalena L (2013) Prevalence and natural history of Graves’ orbitopathy in the XXI century. J Endocrinol Invest 36:444–449
Bartalena L, Baldeschi L, Dickinson AJ, Eckstein A, Kendall-Taylor P, Marcocci C, Mourits MP, Perros P, Boboridis K, Boschi A, Curro N, Daumerie C, Kahaly GJ, Krassas G, Lane CM, Lazarus JH, Marino M, Nardi M, Neoh C, Orgiazzi J, Pearce S, Pinchera A, Pitz S, Salvi M, Sivelli P, Stahl M, von Arx G, Wiersinga WM (2008) Consensus statement of the European group on Graves’ orbitopathy (EUGOGO) on management of Graves’ orbitopathy. Thyroid 18:333–346
Bartalena L, Baldeschi L, Dickinson A, Eckstein A, Kendall-Taylor P, Marcocci C, Mourits M, Perros P, Boboridis K, Boschi A, Curro N, Daumerie C, Kahaly GJ, Krassas GE, Lane CM, Lazarus JH, Marino M, Nardi M, Neoh C, Orgiazzi J, Pearce S, Pinchera A, Pitz S, Salvi M, Sivelli P, Stahl M, von Arx G, Wiersinga WM, European Group on Graves O (2008) Consensus statement of the European Group on Graves’ orbitopathy (EUGOGO) on management of GO. Eur J Endocrinol 158:273–285
Methylprednisolone 1000 mg powder and solvent for solution for injection/infusion (2014) Summary of product characteristic
Melamud B, Lurie Y, Goldin E, Levi I, Esayag Y (2014) Methylprednisolone-induced liver injury: a diagnostic challenge. Isr Med Assoc J 16:180–181
Marino M, Morabito E, Brunetto MR, Bartalena L, Pinchera A, Marocci C (2004) Acute and severe liver damage associated with intravenous glucocorticoid pulse therapy in patients with Graves’ ophthalmopathy. Thyroid 14:403–406
Weissel M, Hauff W (2000) Fatal liver failure after high-dose glucocorticoid pulse therapy in a patient with severe thyroid eye disease. Thyroid 10:521
Salvi M, Vannucchi G, Sbrozzi F, Del Castello AB, Carnevali A, Fargion S, Beck-Peccoz P (2004) Onset of autoimmune hepatitis during intravenous steroid therapy for thyroid-associated ophthalmopathy in a patient with Hashimoto’s thyroiditis: case report. Thyroid 14:631–634
Marino M, Morabito E, Altea MA, Ambrogini E, Oliveri F, Brunetto MR, Pollina LE, Campani D, Vitti P, Bartalena L, Pincheral A, Marcocci C (2005) Autoimmune hepatitis during intravenous glucocorticoid pulse therapy for Graves’ ophthalmopathy treated successfully with glucocorticoids themselves. J Endocrinol Invest 28:280–284
Owecki M, Sowinski J (2006) Acute myocardial infarction during high-dose methylprednisolone therapy for Graves’ ophthalmopathy. Pharm World Sci 28:73–75
Gursoy A, Cesur M, Erdogan MF, Corapcioglu D, Kamel N (2006) New-onset acute heart failure after intravenous glucocorticoid pulse therapy in a patient with Graves’ ophthalmopathy. Endocrine 29:513–516
Macchia PE, Bagattini M, Lupoli G, Vitale M, Vitale G, Fenzi G (2001) High-dose intravenous corticosteroid therapy for Graves’ ophthalmopathy. J Endocrinol Invest 24:152–158
Marcocci C, Bartalena L, Tanda ML, Manetti L, Dell’Unto E, Rocchi R, Barbesino G, Mazzi B, Bartolomei MP, Lepri P, Cartei F, Nardi M, Pinchera A (2001) Comparison of the effectiveness and tolerability of intravenous or oral glucocorticoids associated with orbital radiotherapy in the management of severe Graves’ ophthalmopathy: results of a prospective, single-blind, randomized study. J Clin Endocrinol Metab 86:3562–3567
Bartalena L, Krassas GE, Wiersinga W, Marcocci C, Salvi M, Daumerie C, Bournaud C, Stahl M, Sassi L, Veronesi G, Azzolini C, Boboridis KG, Mourits MP, Soeters MR, Baldeschi L, Nardi M, Curro N, Boschi A, Bernard M, von Arx G, European Group on Graves O (2012) Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves’ orbitopathy. J Clin Endocrinol Metab 97:4454–4463
van Geest RJ, Sasim IV, Koppeschaar HP, Kalmann R, Stravers SN, Bijlsma WR, Mourits MP (2008) Methylprednisolone pulse therapy for patients with moderately severe Graves’ orbitopathy: a prospective, randomized, placebo-controlled study. Eur J Endocrinol 158:229–237
Kahaly GJ, Pitz S, Hommel G, Dittmar M (2005) Randomized, single blind trial of intravenous versus oral steroid monotherapy in Graves’ orbitopathy. J Clin Endocrinol Metab 90:5234–5240
Stiebel-Kalish H, Robenshtok E, Hasanreisoglu M, Ezrachi D, Shimon I, Leibovici L (2009) Treatment modalities for Graves’ ophthalmopathy: systematic review and metaanalysis. J Clin Endocrinol Metab 94:2708–2716
Zang S, Ponto KA, Pitz S, Kahaly GJ (2011) Dose of intravenous steroids and therapy outcome in Graves’ orbitopathy. J Endocrinol Invest 34:876–880
Zang S, Ponto KA, Kahaly GJ (2011) Clinical review: Intravenous glucocorticoids for Graves’ orbitopathy: efficacy and morbidity. J Clin Endocrinol Metab 96:320–332
Marcocci C, Watt T, Altea MA, Rasmussen AK, Feldt-Rasmussen U, Orgiazzi J, Bartalena L, European Group of Graves O (2012) Fatal and non-fatal adverse events of glucocorticoid therapy for Graves’ orbitopathy: a questionnaire survey among members of the European Thyroid Association. Eur J Endocrinol 166:247–253
ICH (2001) Data elements for transmission of individual case safety reports E2B. In: International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use
MSSO (2013) MedDRA Term selection: points to consider Release 4.6 Based on MedDRA Version 16.1
ICH (1996) Guideline for good clinical practice E6(R1). In: International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use
Menconi F, Marino M, Pinchera A, Rocchi R, Mazzi B, Nardi M, Bartalena L, Marcocci C (2007) Effects of total thyroid ablation versus near-total thyroidectomy alone on mild to moderate Graves’ orbitopathy treated with intravenous glucocorticoids. J Clin Endocrinol Metab 92:1653–1658
Wichary H, Gasinska T (2012) Methylprednisolone and hepatotoxicity in Graves’ ophthalmopathy. Thyroid 22:64–69
Le Moli R, Baldeschi L, Saeed P, Regensburg N, Mourits MP, Wiersinga WM (2007) Determinants of liver damage associated with intravenous methylprednisolone pulse therapy in Graves’ ophthalmopathy. Thyroid 17:357–362
Lendorf ME, Rasmussen AK, Fledelius HC, Feldt-Rasmussen U (2009) Cardiovascular and cerebrovascular events in temporal relationship to intravenous glucocorticoid pulse therapy in patients with severe endocrine ophthalmopathy. Thyroid 19:1431–1432
Conflict of interest
The authors have nothing to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Riedl, M., Kolbe, E., Kampmann, E. et al. Prospectively recorded and MedDRA-coded safety data of intravenous methylprednisolone therapy in Graves’ orbitopathy. J Endocrinol Invest 38, 177–182 (2015). https://doi.org/10.1007/s40618-014-0227-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-014-0227-x