Abstract
Purpose
Metformin and pioglitazone are believed to exert their long-term benefits by means of amelioration of chronic low-grade inflammation, a key event in development of diabetes and its long-term complications. The present trial was designed to investigate the comparative efficacy of the two anti-diabetes medications on serum concentrations of YKL-40, a novel marker of inflammation.
Methods
In a parallel-group, open-label, randomized trial setting (ClinicalTrials.gov Identifier No. NCT01521624), 84 newly diagnosed, medication-naïve type 2 diabetes patients were assigned to metformin 1,000 mg daily (n = 42) or pioglitazone 30 mg daily (n = 42). Serum concentrations of YKL-40, along with highly sensitive C-reactive protein, indices of glycemic control and lipid profile were measured at baseline and after 3 months.
Results
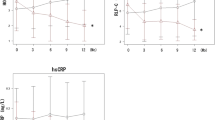
In the analyzed sample (metformin = 40, pioglitazone = 42), both medications were equally effective with regard to control of hyperglycemia, and hsCRP reduction (p > 0.05). However, metformin caused a significant decline in weight (p = 0.005), BMI (p = 0.004), and total cholesterol levels (p = 0.028) of the patients. Metformin also significantly reduced YKL-40 concentrations after 3 months (1.90 ± 17 vs. 1.66 ± 0.15 µg/L, p = 0.019). The amount of change in the pioglitazone arm did not reach statistical significance (2.18 ± 0.14 vs. 2.25 ± 0.16 µg/L, p = 0.687). When compared, metformin was significantly more effective than pioglitazone with respect to YKL-40 reduction in both univariate (p = 0.020, effect size = 6.7 %) and multivariate models (p = 0.047, effect size = 5.7 %).
Conclusions
Metformin is more effective in reduction of YKL-40 concentration in short term and the effect seems to be independent of degree of glycemic control, or hsCRP reduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with type 2 diabetes are at an increased risk for developing atherosclerosis, even long before a clinical diagnosis is made [1–3]. Compared with the general population, mortality rate in patients with diabetes is increased by more than two folds; 69.5 % of which is due to cardiovascular events [4]. Experimental and epidemiological studies in the past two decades have made great strides toward understanding the etiopathogenesis of atherosclerosis, and have often highlighted the key role of chronic low-grade inflammation in thrombotic plaque initiation and progression [5–7]. According to the “common soil” hypothesis, diabetes and atherosclerosis both stem from the shared root of chronic low-grade inflammation [8]. Sustained subclinical inflammation is orchestrated by a wide array of pro-inflammatory mediators among which C-reactive protein (CRP) has been most often cited [8, 9]. Available evidence shows that elevated serum concentrations of CRP antecede the onset of type 2 diabetes and atherosclerosis [5, 10, 11].
Despite substantive evidence depicting the influential effects of CRP in the development of insulin resistance and atherogenesis, it is clear that the complex phenomenon of innate immune system activation is elicited by various pro-inflammatory cytokines, some yet to be discovered. YKL-40, a novel surrogate for acute and chronic inflammatory states, has been implicated to have a putative role in this regard [12]. Patients with angiography-proven coronary artery disease (CAD) have elevated YKL-40 concentrations, which directly corresponds to the severity of involvement, defined by the number of vessels involved [13]. Moreover, YKL-40 concentrations increase in acute myocardial infarction, and decrease following resolution of the symptoms [14].
Given the shared pathway of insulin resistance and atherosclerosis, it is conceivable that anti-diabetes medications are able to modify successive CAD risk via direct and indirect amelioration of inflammation. Available studies suggest that metformin and pioglitazone, two commonly prescribed anti-diabetes agents mitigate inflammatory processes by significant reduction in CRP levels [15, 16]. It is not clear however, whether these medications exert similar effects on YKL-40; and if so, whether a preponderance exists with respect to efficacy. The present clinical trial was thus launched to examine the comparative effects of metformin and pioglitazone on YKL-40 concentrations in medication-naïve type 2 diabetes patients.
Materials and methods
Trial design and participants
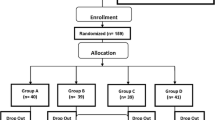
In the present study, data collected in an open-label, parallel-arm, randomized clinical trial were used to investigate the comparative efficacy of metformin and pioglitazone on serum concentrations of YKL-40. The trial is registered with ClinicalTrials.gov (Reg. No. NCT01963663). Between November 2012 and February 2013, 84 newly diagnosed, medication-naïve type 2 diabetes patients visited in the diabetes clinic of the Vali-Asr Hospital (a teaching hospital affiliated with the Tehran University of Medical Sciences, Tehran, Iran) were recruited; with the aid of a randomization software, by the process of simple randomization, patients were allocated to metformin (n = 42) or pioglitazone (n = 42) arms of the trial. Patients were considered eligible for participation in the trial if the following inclusion criteria were met: (1) recent diagnosis of type 2 diabetes mellitus in the absence of the use of medications known to cause insulin resistance; (2) negative history for taking anti-diabetes medications of any class in the past; (3) negative history for taking over-the-counter vitamin or anti-oxidant supplements; and (4) absence of clinically significant chronic illnesses of the heart, lungs, or kidneys. Diabetes was diagnosed according to the American Diabetes Association (ADA) diagnostic criteria [17].
Patients allocated to the metformin arm received 1,000 mg metformin (in divided doses, 500 mg tablets two times daily), whereas 30 mg pioglitazone (in divided doses, 15 mg tablets two times daily) was prescribed to patients in the other arm. Patients were educated regarding the main side effects of the prescribed medication, and were educated to schedule an appointment in case a major side effect was experienced. No dose adjustment was made during the trial. For each patient, a second visit 3 months after the baseline visit was scheduled.
All procedures concerning human subjects were conducted in accordance with the standards and guidelines laid down in the sixth revision of the Helsinki Declaration (2008). Local ethics committee of the university also approved the trial protocol. Written informed consent forms were signed by all participating patients prior to enrollment.
Baseline and follow-up assessments
A similar assessment protocol was used to evaluate patients at baseline and follow-up visits. At the time of enrollment age, sex, medical and drug history of patients were recorded using a pre-designed questionnaire. Once patient’s information was collected, a thorough physical examination was performed by the interviewing physician. Weight was measured with the patient wearing only light clothing, and was recorded to the nearest 0.1 kg. Height was measured with a standard stadiometer, and was recorded to the nearest 0.1 cm. Accordingly, body mass index (BMI) was determined as weight in kilograms divided by height in squared meters. Using an inflexible measurement tape, waist circumference was measured at the mid-point between the inferior costal margin and the anterior superior iliac crest, and was reported to the nearest 0.1 cm. After sitting for at least 10 min, two blood pressure readings were obtained from each patient, 5 min apart, from the right arm, using a standard mercury sphygmomanometer (Riester, Big Ben adults, Germany). The average of the two readings was calculated.
Laboratory evaluations
At each visit, patients were instructed to go on an overnight fasting for 12 h. Ten milliliters of venous blood was taken the next morning, and was sent to the hospital laboratory for biochemical measurements. Serum concentrations of fasting plasma glucose (FPG) were determined using the glucose oxidize method (GOD). Fasting insulin levels were measured using radioimmunoassay techniques (Immunotech, Prague, Czech Republic). Accordingly, homeostasis model assessment of insulin resistance was calculated as FPG in mmol/L multiplied by insulin in IU divided by 22.5. Percentage of glycated hemoglobin A1c (HbA1c) was determined using the high performance liquid chromatography (HPLC) method. Equivalent IFCC (International Federation of Clinical Chemistry) values in mmol/mol were calculated using the following equation: IFCC A1c = (10.93*A1c in %) − 23.50. Serum lipid concentrations were measured with enzymatic methods (Pars Azmun commercial kits, Karaj, Iran). Highly sensitive C-reactive protein (hsCRP) was quantitatively assessed by available commercial kits (Diagnotics Biochem Canada Inc, Ontario, Canada) using the enzyme-linked immunosorbent assay (ELISA) method. Serum concentrations of YKL-40 were determined using the ELISA method (Cusabio, Wuhan, China); inter- and intra-assay coefficients of variation were <10 %.
Statistical analysis
Statistical analyses were performed using the statistical software for social sciences (SPSS®) version 20 (IBM® corp., NY, United States). Categorical variables are presented as proportions. Continuous variables are presented as mean ± standard error of mean (SEM). Continuous variables with non-normal distribution were logarithmically transformed. Distribution of categorical variables between the trial arms was compared using the Chi-square test. For comparison of continuous variables, independent t test was employed. Within-group comparison of dependent variables between baseline and follow-up visits was done using paired t test. Comparative efficacy of anti-diabetes medications between the two trial arms was investigated using the analysis of covariance (ANCOVA) procedure. The effect size of the between-group difference was estimated using partial eta squared. According to Cohen’s recommendations [18], an effect size of around 1 % indicates small effect, while values around 6 % and above 13.8 % correspond to medium and large effect sizes, respectively. In all analyses, a p value of less than 0.05 was considered statistically significant.
Results
A total of 107 patients were initially assessed of whom 84 received treatment. Two patients in the metformin arm did not return for a follow-up visit and upon inquiry over the phone, receiving care from the private sector was described as the main reason for discontinuation of visits. The final analyses were thus performed on 82 patients (metformin n = 40, pioglitazone n = 42). Baseline characteristics of trial participants are summarized in Table 1. Age and sex of the patients did not significantly differ between the two arms of the trial. Level of glycemic control (FPG, insulin, HOMA-IR, and HbA1c) was also comparable between the two groups. Although patients in the metformin arm were on average 3 kg heavier than their counterparts in the pioglitazone arm, the difference did not reach statistical significance (p = 0.232). Moreover, BMI and waist circumference in both groups were similar. No significant differences were identifiable with respect to baseline concentrations of hsCRP, total cholesterol, low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), and also blood pressure in the trial arms. Higher concentrations of serum YKL-40 were observed in the pioglitazone arm, however, the difference was not statistically meaningful (p = 0.218).
Table 2 describes the within-group effects of metformin and pioglitazone on glycemic control, anthropometric parameters, lipid profile and hsCRP; also, the results for between-group comparison are presented in Table 3. Although both medications significantly improved glycemic control after 3 months, reduction in the metformin arm was greater for HOMA-IR; conversely, pioglitazone caused a larger decline in HbA1c concentrations. However, the difference did not reach statistical significance in both instances (p = 0.058 and 0.108, respectively). While weight and BMI of the patients in the metformin arm remained relatively unchanged, pioglitazone resulted in increases in both measurements (p = 0.011 and 0.011, respectively) and the between-group difference was also significant for both measures (p = 0.005 and 0.004, respectively). Waist circumference did not differ after 3 months. A larger reduction in serum concentrations of total cholesterol (p = 0.028) and LDL-c (p = 0.072) was evident in the metformin group. The same was not observed for HDL-c (p = 0.901). Amelioration of hsCRP concentrations was also comparable between the two drugs (p = 0.929).
Comparison of the efficacy of metformin and pioglitazone on YKL-40 concentrations in baseline and adjusted models is presented in Table 4. In the baseline ANCOVA model, metformin resulted in a larger reduction of YKL-40 concentrations compared with pioglitazone (p = 0.020). After step-by-step adjustment for possible confounding variables of age, HOMA-IR, HbA1c, BMI, total cholesterol, HDL-c and log-hsCRP, metformin was still significantly more effective in YKL-40 reduction, albeit the effect was of small to moderate size (effect size = 5.7 %, p = 0.047).
Discussion
YKL-40, also known as human cartilage glycoprotein 39, is a 40 kDa glycoprotein that belongs to the chitinase family, and has the tyrosine-lysine-leucine sequence at its N-terminal [19]. The encoding gene for human YKL-40, CHI3L-1, is located on chromosome 1q31-q32 [20]. According to in vitro and in vivo studies, YKL-40 is secreted by a variety of cells including chondrocytes, synoviocytes, as well as macrophages and vascular smooth cell muscles [12, 21].
YKL-40 exerts pro-inflammatory properties; thus it has been implicated to play an important role in a number of pathologic conditions associated with acute and chronic inflammation [12]. A breadth of evidence has linked elevated YKL-40 concentrations to insulin resistance, T2DM and its microvascular complications, as well as to stable and unstable CAD [13, 14, 22–26]. In one of the earliest studies on an experimental design, Boot and colleagues [27] (1999) provided convincing evidence that YKL-40 is linked to the atherosclerotic plaque. Based on their observations, cultured macrophages extracted from atherosclerotic specimens of human artery show the distinct pattern of significantly enhanced YKL-40 mRNA expression [27]. Collectively, these observations provide a strong basis for the hypothesis that YKL-40 plays an influential role in the pathogenesis of diabetes and its corollary micro- and macrovascular complications. Subsequently, reduction in YKL-40 by means of pharmacologic intervention might be effective in diminishing the prevalence of complications.
In the present clinical trial, effects of metformin and pioglitazone on amelioration of YKL-40 concentrations were compared during a 3-months trial. Herein, we observed that metformin is superior to pioglitazone in terms of decreasing YKL-40 concentrations. On the other hand, both medications resulted in significant and comparable reductions in hsCRP concentrations, suggesting that the changes in YKL-40 are not dependent upon changes in hsCRP. In concert with our findings, previous studies have also suggested that YKL-40 is only weakly correlated with hsCRP in populations with diabetes [22, 28, 29]. In a study of 639 elderly patients without known cardiovascular disease at baseline, serum YKL-40 was a significant predictor of cardiovascular mortality, independent of hsCRP levels [29]. Moreover, changes in HbA1c and HOMA-IR were not statistically different between the trial arms. It suggests that the effect of metformin on YKL-40 concentrations may be independent of its glucose-lowering and insulin-sensitizing effects. It might be possible that metformin directly modulates YKL-40 expression in metabolic tissues including adipose tissue and muscles. However, since our study was not designed to delve into possible mechanisms of action of YKL-40, or identifying the molecular pathways by which metformin causes a reduction in YKL-40, future studies are needed to propel these preliminary findings.
Differential effects of metformin and pioglitazone on YKL-40 could be explained in part by the distinctive effects that these medications exert on bodyweight. While metformin is considered to be a weight-neutral medication when used long term, pioglitazone results in moderate weight gains of 3–5 kg due to peripheral edema and increase in subcutaneous fat tissue [30–32]. In the same line, in the present study, no significant change in bodyweight was observed in the metformin group, whereas a mean gain of 1.4 kg over 3 months was noted in the pioglitazone arm. Obese patients have elevated levels of YKL-40 which tends to decline after weight loss due to either hypocaloric diet or bariatric surgery [33, 34]. Hence, it is possible that the weight gain observed with pioglitazone has negated its possible beneficial effect on YKL-40. Contrariwise, Nielsen et al. [35] (2008) argue that YKL-40 is not associated with obesity in patients with type 2 diabetes. In their sample of 199 patients with type 2 diabetes, obese patients (BMI level above 30 kg/m2) were not significantly different from lean/overweight subjects with respect to plasma YKL-40 concentrations and YKL-40 mRNA expression in the adipose tissue [35].
YKL-40 has also been associated with abnormal cholesterol and triglyceride levels. In a population-based study of 2,656 Danish adults, subjects in the highest YKL-40 quartile had a 1.4-fold increased risk for hypercholesterolemia compared with subjects in the lowest quartile [36]. Herein, we demonstrated that although both medications were able to decrease total cholesterol level to a moderate extent, the observed reduction with metformin was slightly greater than with pioglitazone. Similar observations could not be made with regard to either HDL-c, or LDL-c. Again, it is possible that the observed disparities in changes of total cholesterol level between the two trial arms lead to the difference in serum YKL-40 reduction. However, since our clinical study was not explicitly designed to elucidate the causal relationships between the dependent variables, these interpretations remain speculative at best.
The result of the present trial revealed the short-term effects of metformin on reduction of YKL-40 concentrations; however, future studies with extended durations of follow-up are needed to show the long-term effects and also to investigate the exact implications of YKL-40 decrease on microvascular and macrovascular complications of diabetes.
To the best of our knowledge, this is the first clinical trial to examine the comparative efficacy of two commonly anti-diabetes medications belonging to biguanide and thiazolidinedione classes in patients with diabetes. We herein demonstrated that despite similar glycemic control and hsCRP reduction achieved by both medications, metformin is more effective in terms of reducing YKL-40 concentrations, and these effects are not dependent upon changes in hsCRP concentrations or glycemic control. These preliminary findings propel our understanding of the pathogenesis of type 2 diabetes, atherosclerosis, and their shared inflammatory underpinning forward. Future studies are warranted to address whether the superior effect of metformin on YKL-40 reduction is sustained over long term. In addition, randomized clinical trials with long-term follow-ups are paramount to elucidate whether the beneficial effects in biochemical profile of patients with diabetes are translated into reduction of adverse clinical outcomes.
References
Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M (1998) Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. New Eng J Med 339:229–234. doi:10.1056/NEJM199807233390404
Pyorala K, Laakso M, Uusitupa M (1987) Diabetes and atherosclerosis: an epidemiologic view. Diabetes Metab Rev 3:463–524
Hu FB, Stampfer MJ, Haffner SM, Solomon CG, Willett WC, Manson JE (2002) Elevated risk of cardiovascular disease prior to clinical diagnosis of type 2 diabetes. Diabetes Care 25:1129–1134. doi:10.2337/diacare.25.7.1129
Gu K, Cowie CC, Harris MI (1998) Mortality in adults with and without diabetes in a national cohort of the US population, 1971–1993. Diabetes Care 21:1138–1145. doi:10.2337/diacare.21.7.1138
Libby P, Ridker PM, Maseri A (2002) Inflammation and atherosclerosis. Circulation 105:1135–1143. doi:10.1161/hc0902.104353
Hansson GK (2005) Inflammation, atherosclerosis, and coronary artery disease. New Eng J Med 352:1685–1695. doi:10.1056/NEJMra043430
Gustafson B (2010) Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb 17:332–341
Stern MP (1995) Diabetes and cardiovascular disease. The “common soil” hypothesis. Diabetes 44:369–374
Pradhan AD, Ridker PM (2002) Do atherosclerosis and type 2 diabetes share a common inflammatory basis? Eur Heart J 23:831–834. doi:10.1053/euhj.2001.3052
Festa A, D’Agostino R Jr, Tracy RP, Haffner SM (2002) Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes 51:1131–1137
Pickup JC (2004) Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 27:813–823
Rathcke C, Vestergaard H (2006) YKL-40, a new inflammatory marker with relation to insulin resistance and with a role in endothelial dysfunction and atherosclerosis. Inflamm Res 55:221–227
Kucur M, Isman FK, Karadag B, Vural VA, Tavsanoglu S (2007) Serum YKL-40 levels in patients with coronary artery disease. Coron Artery Dis 18(391–396):3. doi:10.1097/MCA.1090b1013e328241d328991
Nøjgaard C, Høst NB, Christensen IJ, Poulsen SH, Egstrup K, Price PA, Johansen JS (2008) Serum levels of YKL-40 increases in patients with acute myocardial infarction. Coron Artery Dis 19:257–263
Chu NV, Kong APS, Kim DD, Armstrong D, Baxi S, Deutsch R, Caulfield M, Mudaliar SR, Reitz R, Henry RR, Reaven PD (2002) Differential effects of metformin and troglitazone on cardiovascular risk factors in patients with type 2 diabetes. Diabetes Care 25:542–549. doi:10.2337/diacare.25.3.542
Satoh N, Ogawa Y, Usui T, Tagami T, Kono S, Uesugi H, Sugiyama H, Sugawara A, Yamada K, Shimatsu A, Kuzuya H, Nakao K (2003) Antiatherogenic effect of pioglitazone in type 2 diabetic patients irrespective of the responsiveness to its antidiabetic effect. Diabetes Care 26:2493–2499. doi:10.2337/diacare.26.9.2493
American Diabetes Association (2013) Diagnosis and classification of diabetes mellitus. Diabetes Care 36:S67–S74. doi:10.2337/dc13-S067
Cohen J (1988) Statistical power analysis for the behavioral sciences. L. Erlbaum Associates, Hillsdale
Volck B, Price PA, Johansen JS, Sørensen O, Benfield TL, Nielsen HJ, Calafat J, Borregaard N (1998) YKL-40, a mammalian member of the chitinase family, is a matrix protein of specific granules in human neutrophils. Proc Assoc Am Phys 110:351
Rehli M, Krause S, Andreesen R (1997) Molecular characterization of the gene for human cartilage gp-39 (CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics 43:221–225
Recklies AD, White C, Ling H (2002) The chitinase 3-like protein human cartilage glycoprotein 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase- and protein kinase B-mediated signalling pathways. Biochem J 365:119–126. doi:10.1042/bj20020075
Rathcke CN, Johansen JS, Vestergaard H (2006) YKL-40, a biomarker of inflammation, is elevated in patients with type 2 diabetes and is related to insulin resistance. Inflamm Res 55:53–59. doi:10.1007/s00011-005-0010-8
Brix J-M, Höllerl F, Koppensteiner R, Schernthaner G, Schernthaner G-H (2011) YKL-40 in type 2 diabetic patients with different levels of albuminuria. Eur J Clin Inv 41:589–596. doi:10.1111/j.1365-2362.2010.02446.x
Rathcke CN, Persson F, Tarnow L, Rossing P, Vestergaard H (2009) YKL-40, a marker of inflammation and endothelial dysfunction, is elevated in patients with type 1 diabetes and increases with levels of albuminuria. Diabetes Care 32:323–328. doi:10.2337/dc08-1144
Wang Y, Ripa RS, Johansen JS, Gabrielsen A, Steinbrüchel DA, Friis T, Bindslev L, Haack-Sørensen M, Jørgensen E, Kastrup J (2008) YKL-40 a new biomarker in patients with acute coronary syndrome or stable coronary artery disease. Scand Cardiovasc J 42:295–302
Kastrup J, Johansen JS, Winkel P, Hansen JF, Hildebrandt P, Jensen GB, Jespersen CM, Kjøller E, Kolmos HJ, Lind I (2009) High serum YKL-40 concentration is associated with cardiovascular and all-cause mortality in patients with stable coronary artery disease. Eur Heart J 30:1066–1072
Boot RG, van Achterberg TAE, van Aken BE, Renkema GH, Jacobs MJHM, Aerts JMFG, de Vries CJM (1999) Strong induction of members of the chitinase family of proteins in atherosclerosis: chitotriosidase and human cartilage gp-39 expressed in lesion macrophages. Arterioscler Thromb Vasc Biol 19:687–694. doi:10.1161/01.atv.19.3.687
Rathcke CN, Vestergaard H (2009) YKL-40–an emerging biomarker in cardiovascular disease and diabetes. Cardiovasc Diabetol 8:61. doi:10.1186/1475-2840-8-61
Rathcke CN, Raymond I, Kistorp C, Hildebrandt P, Faber J, Vestergaard H (2010) Low grade inflammation as measured by levels of YKL-40: Association with an increased overall and cardiovascular mortality rate in an elderly population. Int J Cardiol 143:35–42. doi:10.1016/j.ijcard.2009.01.043
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR (2012) Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the american diabetes association (ADA) and the european association for the study of diabetes (EASD). Diabetes Care 35:1364–1379. doi:10.2337/dc12-0413
Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Koranyi L, Laakso M, Mokan M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J (2005) Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366:1279–1289. doi:10.1016/s0140-6736(05)67528-9
Belcher G, Matthews D (2000) Safety and tolerability of pioglitazone. Exp Clin Endocr Diabetes 108:S267–S273
Hempen M, Kopp HP, Elhenicky M, Hobaus C, Brix JM, Koppensteiner R, Schernthaner G, Schernthaner GH (2009) YKL-40 is elevated in morbidly obese patients and declines after weight loss. Obes Surg 19:1557–1563. doi:10.1007/s11695-009-9917-4
Catalan V, Gomez-Ambrosi J, Rodriguez A, Ramirez B, Rotellar F, Valenti V, Silva C, Gil MJ, Salvador J, Fruhbeck G (2011) Increased circulating and visceral adipose tissue expression levels of YKL-40 in obesity-associated type 2 diabetes are related to inflammation: impact of conventional weight loss and gastric bypass. J Clin Endocr Metab 96:200–209. doi:10.1210/jc.2010-0994
Nielsen AR, Erikstrup C, Johansen JS, Fischer CP, Plomgaard P, Krogh-Madsen R, Taudorf S, Lindegaard B, Pedersen BK (2008) Plasma YKL-40: a BMI-independent marker of type 2 diabetes. Diabetes 57:3078–3082. doi:10.2337/db08-0182
Thomsen SB, Rathcke CN, Skaaby T, Linneberg A, Vestergaard H (2012) The association between genetic variations of CHI3L1, levels of the encoded glycoprotein YKL-40 and the lipid profile in a Danish population. PLoS One 7:e47094. doi:10.1371/journal.pone.0047094
Acknowledgments
This study was funded by the Tehran University of Medical Sciences.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Esteghamati, A., Rezvani, S., Khajeh, E. et al. Comparative effects of metformin and pioglitazone on YKL-40 in type 2 diabetes: a randomized clinical trial. J Endocrinol Invest 37, 1211–1218 (2014). https://doi.org/10.1007/s40618-014-0154-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-014-0154-x