Abstract
Background
Impaired production of adipocytokines is a major factor incriminated in the occurrence of lipodystrophy (LD).
Objective
To evaluate LD prevalence and subtypes in HIV treatment-multiexperienced patients, and to determine the correlations between adipocytokines and LD subtypes.
Methods
Cross-sectional study in a Romanian tertiary care hospital, between 2008 and 2010, in HIV-positive patients, undergoing cART for ≥6 months. LD diagnosis, based on clinical and anthropometric data, was classified into lipoatrophy (LA), lipohypertrophy (LH) and mixed fat redistribution (MFR). Blood samples were collected for leptin, adiponectin and resistin assessments.
Results
We included 100 patients, 44 % with LD, among which LA had 63 %. LA patients had sex ratio, median age, treatment duration and median number of ARV regimens of 1, 20, 93 and 3.5 compared to non-LD patients: 1.65, 31, 44 and 1. LH and MFR patients were older and had higher total and LDL cholesterol versus non-LD patients. For both overall group and female group, LA was associated in univariate and multivariate analysis with increased resistin (p = 0.02 and 0.04) and number of ARV regimens (p < 0.001). Determination coefficient (Nagelkerke R 2) of increased resistin and the number of ARV combinations in the presence of LA was 33 % in overall group and 47 % in female patients.
Conclusions
In our young HIV-positive population, LD had high prevalence with predominance of LA subtype. LA was associated with high resistin levels and greater number of ARV regimens in overall group and female subgroup. Resistin could be used as a marker of peripheral adipose tissue loss and might be used as a target for new anti-LD therapeutic strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The introduction of combined antiretroviral therapy (cART), in 1996, was a milestone in the course of HIV infection, extending life expectancy of HIV-positive patients. But the long-term use of these drugs has a large spectrum of adverse effects, one of the most important being the lipodystrophy (LD) syndrome [1, 2].
The term LD was first used 2 years after the advent of cART to describe morphological and metabolic changes that are side effects of this treatment [2, 3]. It is difficult to establish the prevalence of LD, due to the absence of a consensus definition of this syndrome; however, it relies mainly on clinical examination [4, 5]. The prevalence ranged widely between 2 and 84 % in different studies, depending on definition criteria of LD, type and duration of cART [2, 6, 7].
The mechanisms of these complex metabolic modifications are not completely understood, but it is accepted that several dysfunctions are the underlying causes of LD. One of these is impaired production of adipose tissue-derived cytokines [1, 3, 8, 9]. Adipose tissue is widely acknowledged as an active organ involved in the synthesis of many mediators, such as leptin, adiponectin and resistin. These adipocytokines participate in various metabolic pathways, influencing peripheral insulin sensitivity and the risk of developing diabetes mellitus, atherosclerosis and cardiovascular diseases [8, 9].
Our objective was to assess the prevalence of LD syndrome and its subtypes in Romanian treatment-multiexperienced HIV patients and to determine the correlation between adipocytokine levels and the presence of LD subtypes in this population.
Methods
We conducted a cross-sectional study in a tertiary care hospital, the Prof. Dr. Matei Bals National Institute of Infectious Diseases, Bucharest, between June 2008 and June 2010, on HIV treatment-experienced patients. The study was approved by the local ethics committee and all patients have signed an informed consent form before enrollment.
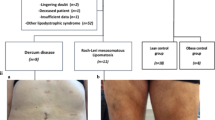
Study questionnaire included demographic data, medical history and physical findings. Also, height, weight, body mass index (BMI) and waist circumference were measured. Adipose tissue changes were classified into three types of lipodystrophy: lipoatrophy (LA), lipohypertrophy (LH) and mixed fat redistribution (MFR). LA was defined as the presence of one or more of the following: loss of peripheral subcutaneous fat from the face, arms, legs and buttocks and pronounced blood vessels in the arms and legs. LH was defined as the presence of one or more of the following: fat accumulation on the back of the neck and on the breasts and increased abdominal circumference. MFR was defined as the presence of at least one feature of LA and one of LH [1, 8–10]. BMI values between 18.50 and 24.99 kg/m2 were considered normal. Patients were classified as underweight for BMI values <18.50 kg/m2 and overweight for BMI values ≥25 kg/m2 [11]. The waist circumference was considered normal for women <88 cm and for men <102 cm [12].
Blood samples were collected and analyzed for CD4 count, HIV viral load, fasting triglycerides, total cholesterol, and high-density lipoprotein (HDL) cholesterol. Low-density lipoprotein (LDL) cholesterol was calculated using the formula: total cholesterol − triglycerides/5 − HDL cholesterol. Blood was also collected for leptin, adiponectin and resistin plasma levels assessment.
HIV viral load was determined using Roche LightCycler® 480 Real-Time PCR System, with a cutoff of 50 copies/mL. CD4 cell count was assessed using BD FACSCanto II flow cytometer.
Adipocytokine serum levels were assessed using the following techniques: BioSource EASIA (Enzyme Amplified Sensitivity Immunoassay) KAP2281, Belgium, for leptin, BioSource ELISA (Enzyme-linked immunosorbent assay) KAPME09, Belgium, for adiponectin and BioSource ELISA KAPME 50, Belgium, for resistin. Normal values of the adipocytokines, according to the manufacturer, are presented in Table 1.
Statistical Package for the Social Sciences (SPSS) (version 17, USA) was used for statistical analysis. In descriptive analysis, non-parametric variables were expressed as median and interquartile range (IQR). We used Chi-square tests to evaluate the associations between LA and adipocytokines in univariate analysis. Comparisons between groups were performed by means of Mann–Whitney U test for continuous variables. We used multiple multivariate regression to investigate the independent effect of adipocytokines (as categorical variables) on LA as the dependent variable. Statistical significance was defined as p < 0.05.
Results
We enrolled 100 HIV-positive patients, under cART for at least 6 months, consecutively as they presented for routine check-up in our hospital. The patients had a median age of 31 (20–42) years and approximately even sex distribution (Table 2). Fat redistribution defining the LD syndrome was observed in 44 % of the patients, among which 63 % (N = 28) of patients had LA, 18.5 % (N = 8) LH and 18.5 % (N = 8) MFR. The demographic characteristics and studied parameters are presented in Table 2.
We found a statistically significant difference in age between LA and non-LD groups (p = 0.042) and between MRF and non-LD groups (p = 0.021) (Table 2).
The patients from studied subgroups had a good immunological status, with CD4 cell count >400/mm3 and more than 70 % of cases had undetectable HIV viral load, except in LH patients in whom 37.5 % of cases were undetectable. There were no significant differences between subgroups (Table 2).
The LA patients received ARV therapy for longer duration (median time of cART 93 months) and higher number of cART regimens (3.5), compared with non-LD group (median time 44 month and 1 combination), p < 0.001(Table 2).
Regarding dyslipidemia, LDL and total cholesterol plasma levels were higher among MFR patients compared with non-LD patients, with very high statistical significance (p = 0.001 and 0.005, respectively). In LH patients, only LDL cholesterol showed significant high values compared with non-LD patients (p = 0.038).
We assessed the adipocytokine patterns in patients with LA compared with non-LD patients, as the most significantly results were obtained for this subtype of patients.
All tested plasma adipocytokine levels were higher in LA patients group than in the group of non-LD patients; however, we observed a significant difference only for resistin values: 39 % of LA patients versus 15 % of non-LD patients had increased resistin levels. This difference reached statistical significance, with p value of 0.0029 (Table 3). When we analyzed the adipocytokine values separately in males and females, we observed that resistin levels were higher in female patients with LA than in those without fat redistribution (Table 4).
We performed a multivariate regression model, using as dichotomic dependent variable the presence of LA and as independent variable increased resistin values and the number of ARV combinations, variables that were correlated in the univariate analysis with the presence of LA. The model showed that these two parameters were significantly associated with LA and the determination coefficient was 33 % (Nagelkerke R 2 = 0.33) (Table 5). We divided the LA group according to the sex and we applied the multivariate regression model in these two subgroups. We noticed that increased values of resistin and the number of ARV combinations were significantly correlated with the LA presence in female patients (p value 0.003 and 0.001, respectively), with a determination coefficient of 47 % (Nagelkerke R 2 = 0.470) (Table 6), whereas in male patients there was no correlation between resistin levels and the presence of LA (p value 0.122 and Nagelkerke R 2 = 0.18).
Discussions
Our study showed that around half of the HIV treatment-multiexperienced patients were clinically diagnosed with LD syndrome. Among these subjects, two-thirds had peripheral adipose tissue loss—defining the LA subtype. LA presence was further correlated with increased resistin values and with greater number of previous cART regimens, especially in women. According to PubMed, this is the first study showing a sex difference in resistin levels in HIV multiexperienced patients with lipoatrophy syndrome.
The morphological changes are of great concern for patients and physicians, because they can be a stigma for and could disclose the HIV-positive status. Also they could induce low self-esteem, especially for adolescents and young adults, leading finally to a decrease of therapy adherence and virological failure.
The patients with LA were young adults (median age 20 years) with an equally balanced sex distribution. These are characteristic features for the HIV infection epidemiology in Romania, which differs from the western European countries. In Romania, until 2002, the incidence of HIV cases was significantly higher in children than in adults who were most likely parenterally infected with subtype F strain in late 1980s [13–16] while in western European countries the HIV population consists, especially of men having sex with men and intravenous drug users. These patients are slow progressors and multiexperienced to treatment (median number of cART regimens is 3.5), which could explain the high prevalence of LA subtype. The fact the most of these young multiexperienced patients have good immunological status (median CD4 cell count 528 c/mL) and undetectable HIV viral load in 71 % cases shows that they have a good adherence to therapy, making them prone to long-term antiretroviral adverse events, like LD syndrome.
LH patients were older than non-LD patients; most of them were males, had a median period of ARV therapy of 47.5 months and had highest LDL cholesterol levels. MFR patients were older than non-LD patients and with a long ARV therapy period (99 months), but normal levels for plasma LDL cholesterol. Since these two groups have similar median age, but different clinical aspects of adipose tissue redistribution and different periods of ARV treatment, we suppose that treatment had better correlation with LA, whereas LDL cholesterol levels do not correlate with loss of adipose tissue in studied patients. In our study, the longer duration of therapy was associated with adipose tissue loss since the patients with shorter duration of cART had no LA. In the same time, longer duration of cART history is associated also with more toxicity in older drugs and a short history of treatment can mean that these patients received newer drugs which are less toxic, especially on lipidic metabolism.
Furthermore, we investigated the possible correlation between adipocytokines and the presence of LA, because the patients from LA group were better represented in our study group. In the same time, this type of patients is of great interest for us since the majority of HIV-infected patients monitored in Romania are young adults with a long HIV and antiretroviral history.
We observed both in the univariate analysis and in multivariate regression that the LA syndrome presence was statistically associated with increased resistin levels. Resistin is an adipocyte-secreted protein, which was first discovered in association with obesity and diabetes mellitus [8]. Several studies that evaluated the correlation between resistin values and the presence of metabolic changes have reported controversial results, both in HIV negative population and in HIV-positive patients. Degawa-Yamauchi et al. [17] reported elevated plasma levels of resistin in obese patients, Youn et al. [18] showed that resistin is increased in patients with type II diabetes mellitus, but in both studies an association between increased resistin levels and insulin-resistance could not be demonstrated [1, 8]. This lack of correlation between increased resistin values and insulin-resistance was also seen in HIV patients with and without LD [8, 19]. In one recent cohort study published in 2011 on 299 HIV-positive patients, resistin levels were correlated with the presence of insulin resistance and resistin levels were lower in patients with LD compared with those without LD [10]. Another study conducted in 27 HIV-positive children showed that increased levels of resistin were present in children with ultrasonographically quantified fat redistribution [20].
Our results showed that increased resistin values were significantly associated with the presence of LA and we were also able to observe that resistin levels were correlated with the presence of LA in female and not in male subjects. We did not find in the literature any study in HIV-positive patients showing any sex-related differences in resistin levels. The pattern of this particular adipocytokine is not yet well defined in HIV-infected patients and needs further studies to clarify these conflicting data. The differences that we observed according to the sex may be explained by a different mechanism of fat redistribution in male and female probably genetically linked. There is more evidence that a genetic background plays a role in the metabolic disturbances secondary to cART, as suggested by the presence of single-nucleotide polymorphism in the resistin gene in HIV-experienced patients with LD [8, 10, 21]. Recent studies showed that these pathological levels of resistin could be a target for new therapeutical approaches for the LD, such as the use of thiazolidinediones [19, 21].
Leptin and adiponectin are two other adipocyte-secreted proteins, which are involved in fat disturbances in HIV-infected patients undergoing cART. Hypoleptinemia is associated with the development of obesity and insulin-resistance, but some studies showed increased levels of leptin in obese patients, suggesting that leptin-resistance could be involved in this process [22, 23].
Some studies showed that adiponectin levels are correlated with body fat composition, with decreased adiponectin being associated with peripheral fat loss and accumulation of visceral fat [22, 24]. In our study, one-third of the patients had decreased leptin levels and 10.7 % had increased leptin levels, while none of the patients had low adiponectin levels. We found no correlation between these levels and the presence of LA. A recent study on 27 vertically infected children also found no correlation between the levels of these two adipocytokines and LD. The authors suggested that normal secretion of adiponectin compensated the negative effect of pathological levels of leptin at the beginning of cART [25]. Our results suggest that this hypothesis may be true, even after a few years of cART.
Protease inhibitors such as lopinavir, nelfinavir, ritonavir, saquinavir are the most incriminated in producing fat disturbances and metabolic changes [1, 2, 8]. The nucleoside reverse transcriptase inhibitors such as stavudine, zalcitabine, zidovudine and didanosine are responsible for peripheral fat loss, and combined use of these drugs acts synergistically [1]. Different studies concluded that fat redistribution occurs shortly after cART initiation, and the prevalence of these modifications increases with therapy duration [6]. Our findings were consistent with these statements: 44 % of our study population was diagnosed with LD syndrome and two-third of them had LA; the median period of time of antiretroviral therapy was significantly higher in those with LA compared with non-LD patients.
We applied a multivariate regression model to see how increased resistin levels and the number of ARV combinations are influencing the presence of LA and we observed a coefficient of determination of 33 %. A similar model as described above was created for female patients with LA and we obtained a determination coefficient of 47 %. A possible explanation for the implication of total number of ARV combinations is that at the beginning most of our patients were treated with at least one of the drugs known to induce lipoatrophy (zidovudine, stavudine, didanosine, nelfinavir). Subsequently, these patients received combinations less prone to induce LD, but body fat redistributions were not reversible.
One limitation of our study was the qualitative diagnosis of LD rather than quantitative; however, the patient’s classification into LD categories was correct, the clinical changes being more than typical. Other limitations were the heterogeneous therapy consisting of a wide range of therapy duration (from 6 months to more than 10 years) and multiple drug combinations (11 % of patients being treated with more than 5 ARV combinations), but our study group reflects the real features of HIV-positive patients in Romania, a young and treatment-multiexperienced population, whereof there are not enough data in the literature.
In conclusion, in our young adult population, the prevalence of LD syndrome was 44 %, with the predominance of LA subtype. The presence of LA was associated with increased values of resistin and the number of ARV regimens, this correlation being valid for the female subpopulation of the study too. These pathological levels of resistin, consequence of many years of cART, may be the target of new therapeutic strategies for LD syndrome in young multiexperienced HIV patients.
Abbreviations
- cART:
-
Combined antiretroviral therapy
- ARV:
-
Antiretroviral therapy
- BMI:
-
Body mass index
- ELISA:
-
Enzyme-linked immunosorbent assay
- EASIA:
-
Enzyme Amplified Sensitivity Immunoassay
- HDL:
-
High-density lipoprotein
- HIV:
-
Human immunodeficiency virus
- IQR:
-
Interquartile range
- LA:
-
Lipoatrophy
- LD:
-
Lipodystrophy
- LDL:
-
Low-density lipoprotein
- LH:
-
Lipohipertrophy
- MFR:
-
Mixed fat redistribution
- SPSS:
-
Statistical Package for the Social Sciences
References
Mallewa JE, Wilkins E, Vilar J et al (2008) HIV-associated lipodystrophy: a review of underlying mechanisms and therapeutic options. J Antimicrob Chemother 62:648–660
Omolayo O, Sealy PL (2008) HIV lipodystrophy syndrome. Hosp Physician 44(5):7–14
Jianu DM (2012) A new scientific era in biomedicine—regenerative surgery based on lipotransplant. New therapeutic horizons for HIV-positive patients. GERMS 2(4):135–136
Georg Behrens, Reinhold E (2011) Schmidt. Lipodystrophy syndrome. Chapter 8 HIV In: Christian Hoffmann and Jürgen K. Rockstroh (ed) Medizin Fokus Verlag, Berlin
Galescu O, Bhangoo A, Ten A (2013) Insulin resistance, lipodystrophy and cardiometabolic syndrome in HIV/AIDS. Rev Endocr Metab Disord 14(2):133–140
Carr A, Emery S, Law M et al (2003) An objective case definition of lipodystrophy in HIV-infected adults: a case-control study. Lancet 361:726–735
Barbaro G (2006) Metabolic and cardiovascular complications of highly active antiretroviral therapy for HIV infection. Curr HIV Res 4:79–85
Sweeney LL, Brennan AM, Mantzoros CS (2007) The role of adipokines in relation to HIV lipodystrophy. AIDS 21:895–904
Samaras K (2008) Metabolic consequences and therapeutic options in highly active antiretroviral therapy in human immunodeficiency virus-1 infection. J Antimicrob Chemother 61:238–245
Escote X, Miranda M, Veloso S et al (2011) Lipodystrophy and insulin resistance in combination Antiretroviral treated HIV-1–infected patients: implication of resistin. J Acquir Immune Defic Syndr 57(1):16–23
World Health Organization (2000) Obesity: preventing and managing the global epidemic. Technical Report Series No. 894. WHO, Geneva
The IDF consensus worldwide definition of the metabolic syndrome. International Diabetes Federation 2006. http://www.idf.org/webdata/docs/IDF_Meta_def_final.pdf. Accessed Sept 2012
HIV/AIDS Monitoring and Evaluation Department of the National AIDS Comission for Romania—http://www.cnlas.ro/images/doc/epidemiology_sept2011.pdf. Accessed Sept 2011
Country Progress Report on AIDS January 2010–December 2011. http://www.unaids.org/en/dataanalysis/monitoringcountryprogress/progressreports/2012countries/ce_RO_Narrative_Report.pdf. Accessed Sept 2012
Paraschiv S, Foley B, Otelea D (2011) Diversity of HIV-1 subtype C strains isolated in Romania. Infection, genetics and evolution. J Mol Epidemiol Evol Genet Infect Dis 11(2):270–275
Arama V, Tiliscan C, Streinu-Cercel A et al (2013) Insulin resistance and adipokines serum levels in a Caucasian cohort of HIV-positive patients undergoing antiretroviral therapy: a cross sectional study. BMC Endocr Disord 13:4
Degawa-Yamauchi M, Bovenkerk JE, Juliar BE et al (2003) Serum resistin (FIZZ3) protein is increased in obese humans. J Clin Endocrinol Metab 88(11):5452–5455
Youn BS, Yu KY, Park HJE et al (2004) Plasma resistin concentrations measured by enzyme-linked immunosorbent assay using a newly developed monoclonal antibody are elevated in individuals with type 2 diabetes mellitus. J Clin Endocrinol Metab 89(1):150–156
Barb D, Wadhwa SG, Kratzsch J et al (2005) Circulating resistin levels are not associated with fat redistribution, insulin resistance, or metabolic profile in patients with the highly active antiretroviral therapy-induced metabolic syndrome. J Clin Endocrinol Metab 90(9):5324–5328
Spagnuolo MI, Caiazzo MA, Franzese A et al (2010) Side effects of highly active antiretroviral therapy in children with AIDS: phase angle alpha, serum resistin levels, and ultrasound as predictors of malnutrition. Nutr Ther Metab 28(4):193–197
Fiorenza CG, Chou SH, Mantzoros CS (2011) Lipodystrophy: pathophysiology and advances in treatment. Nat Rev Endocrinol 7:137–150
Tsiodras S, Mantzoros C (2006) Leptin and adiponectin in the HIV associated metabolic syndrome: physiologic and therapeutic implications. Am J Infect Dis 2(3):141–152
Kelesidis T, Kelesidis I, Chou S, Mantzoros CS (2010) Narrative review: the role of Leptine in human physiology: emerging clinical applications. Ann Intern Med 152(2):93–100
Kosmiski L, Kuritzkes D, Lichtenstein K, Eckel R (2003) Adipocyte-derived hormone levels in HIV lipodystrophy. Antivir Ther 8:9–15
Resino S, Micheloud D, Lorente R, Bellon JM, Navarro ML, Munoz-Fernandez MA (2011) Adipokine profiles and lipodystrophy in HIV-infected children during the first 4 years on highly active antiretroviral therapy. HIV Med 12:54–60
Acknowledgments
The authors thank all patients that agreed to participate in this study and all the study team for their contribution to the research, as well as all the medical staff involved in this study, especially: Ioana Diana Olaru, Cristina Loredana Benea, Anca Streinu Cercel, Mihaela Radulescu for their implication in recruiting and in the follow-up of the patients, and Viorica Leoveanu (deceased) for performing the assessments for the adipocytokines. The study was funded by the National Authority for Scientific Research (Grant No. 62077/2008, 2008–2011). Coordinating center: Prof. Dr. Matei Bals National Institute of Infectious Diseases, Bucharest, Romania. Project Manager: Prof. Adrian Streinu Cercel. Scientific coordinator: Assoc. Prof. Victoria Arama.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arama, V., Munteanu, D.I., Streinu Cercel, A. et al. Lipodystrophy syndrome in HIV treatment-multiexperienced patients: implication of resistin. J Endocrinol Invest 37, 533–539 (2014). https://doi.org/10.1007/s40618-014-0057-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-014-0057-x