Abstract
Objectives
Neighborhoods characterized by disadvantage influence multiple risk factors for chronic disease and are considered potential drivers of racial and ethnic health inequities in the USA. The objective of the present study was to examine the relationship between neighborhood disadvantage and cumulative biological risk (CBR) and the extent to which the association differs by individual income and education among a large, socioeconomically diverse sample of African American adults.
Methods
Data from the baseline examination of the Jackson Heart Study (2000–2004) were used for the analyses. The sample consisted of African American adults ages 21–85 with complete, geocoded data on CBR biomarkers and behavioral covariates (n = 4410). Neighborhood disadvantage was measured using a composite score of socioeconomic indicators from the 2000 US Census. Eight biomarkers representing cardiovascular, metabolic, inflammatory, and neuroendocrine systems were used to create a CBR score. We fit two-level linear regression models with random intercepts and included cross-level interaction terms between neighborhood disadvantage and individual socioeconomic status (SES).
Results
Living in a disadvantaged neighborhood was associated with greater CBR after covariate adjustment (B = 0.18, standard error (SE) 0.07, p < 0.05). Interactions showed a weaker association for individuals with ≤high school education but were not statistically significant.
Conclusion
Disadvantaged neighborhoods contribute to poor health among African American adults via cumulative biological risk. Policies directly addressing the socioeconomic conditions of these environments should be considered as viable options to reduce disease risk in this group and mitigate racial/ethnic health inequities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is a wealth of evidence linking the socioeconomic conditions of residential environments to health and well-being [1–5]. Specifically, disadvantaged neighborhood contexts characterized by high concentrations of poverty, high rates of unemployment, and limited material resources and services have been found to be particularly detrimental to health. These settings are viewed as important drivers of racial inequities in health in the USA because compared to Whites, African Americans are disproportionately exposed to disadvantaged neighborhoods [6, 7] and the array of contextual factors associated with these environments. For example, neighborhood disadvantage has been linked to limited access to affordable, healthy foods and deficiencies in the built environment which directly influence behavioral risk factors such as diet and physical activity [8–11]. Moreover, disadvantaged neighborhoods often have higher rates of crime, violence, and physical signs of disorder [12] which may induce stress [13], elevate blood pressure [14], promote unhealthy coping behaviors such as smoking and high alcohol use [15, 16], and may not be conducive to engaging in healthy behaviors such as physical activity [17]. Taken together, these contextual factors converge within disadvantaged neighborhoods to influence a number of biological factors across multiple physiological systems (e.g., blood lipids, adiposity, blood pressure, and cortisol), thus influencing overall, cumulative disease risk.
Cumulative disease risk has been captured by a number of multi-risk factor profiles including the Framingham Score which predicts 10-year risk of coronary heart disease [18] and the metabolic syndrome, a cluster of risk factors most directly associated with type 2 diabetes [19]. Increasingly, cumulative biological risk (CBR) has been utilized as a comprehensive measure of risk across multiple physiological systems [20]. Underpinning CBR is the notion that these systems work both additively and synergistically to influence disease pathogenesis. For example, in a sample of older adults, Seeman et al. [21] found that higher CBR was associated with an increased risk of incident cardiovascular disease (CVD) and was also associated with cognitive decline, physical functioning, and all-cause mortality [21, 22].
Previous studies have examined the extent to which CBR is influenced by neighborhood environments [23–26] with most studies to date finding that, independent of individual characteristics, declines in neighborhood socioeconomic conditions are associated with increased CBR. While there is growing evidence for an overall association, it is less clear whether these neighborhood conditions differentially influence CBR in individuals at varying levels of socioeconomic status (SES). Conceivably, individuals living in disadvantaged neighborhoods who have more economic resources and higher education may be buffered from some of the negative impacts of the neighborhood environment. Conversely, the compounded impact of individual and neighborhood disadvantage may prove detrimental to individuals who are limited in their ability to overcome deficiencies in their neighborhood environment.
Though conceivable, this remains poorly understood, particularly among African Americans who, due to residential segregation by both race and class, are disproportionately exposed to these contexts even at higher levels of income and education [7, 27]. To date, however, most studies examining the association between neighborhood socioeconomic conditions and CBR have been conducted among predominantly White populations with relatively small African American samples [23, 25, 26]. Furthermore, African Americans within these studies have tended to be less educated and have lower incomes limiting a more robust examination across the full continuum of SES for this group [24, 26]. Finally, relatively few neighborhood studies have been conducted exclusively in southern metropolitan areas, which tend to have higher proportions of African Americans, higher rates of chronic disease, and have undoubtedly been shaped by the well-documented history of institutionalized racial and economic oppression.
In light of these gaps, the objectives of the present study were to (1) examine the cross-sectional association between neighborhood disadvantage and CBR independent of individual-level sociodemographic and behavioral characteristics and (2) examine whether the association differs by family income and education. A closer examination of the impact of disadvantaged neighborhoods on CBR among African Americans at varying levels of SES could provide important insights about the role these settings play in determining disease risk within this group and in contributing to racial inequities in health. We used a multi-level analytic approach to distinguish between neighborhood-level and individual-level effects of disadvantage and hypothesized that living in a disadvantaged neighborhood would be associated with higher CBR independent of individual-level sociodemographic factors. We also hypothesized that health behaviors would partially explain the relationship and thus attenuate associations between neighborhood disadvantage and CBR. Further, we hypothesized that the association would be strongest among individuals at lower levels of SES. Data from the Jackson Heart Study—the largest single-site epidemiologic study of African American adults conducted in the USA— were used in our analyses. The Jackson Heart Study (JHS) is well suited for the present investigation because it includes a socioeconomically diverse sample of African American adults who reside in both central and outlying sub-urban areas providing sufficient variability in neighborhood socioeconomic conditions.
Methods
Study Area
The JHS is based in Jackson, Mississippi, a mid-sized metropolitan area located in the southeastern USA. In 2000, when the study began, the population of the city and surrounding areas was just under 500,000 making it the largest metropolitan area in the state of Mississippi. The median household income for the Jackson metropolitan area was $39,425 and the household poverty rate was 17.6 %, comparable to the rest of the state but well above the 11.3 % for the rest of the country [28]. Moreover, like many southern cities, a relatively large portion of the population was African American (45.5 %) [29]. With regard to health, Mississippi as a whole has some of the worst chronic disease outcomes; nearly 35 % of the adult population is obese [30] and the state leads the nation in CVD mortality [31].
Study Population
The sample was drawn from the baseline wave of the JHS. The study population included adults aged 21–85 residing in the Jackson metropolitan area and was obtained using four sampling approaches: a random sample of adults drawn from a commercially available list of households with adults aged 35–84 (17 %), volunteers aged 35–84 recruited through participant referral or outreach activities (30 %), participants in the Jackson field center of the Atherosclerosis Risk in Communities (ARIC) study (31 %), and relatives of JHS participants, ≥ 21 years of age comprising the JHS Family Sub-Study (22 %). A total of 5301 participants were recruited into the JHS of whom 99 % were retrospectively geocoded to 102 census tracts [32].
Data collection for the baseline wave took place between September 2000 and March 2004 and involved a home interview, an on-site clinical examination, and 24-h follow-up data collection (for a subset of the sample) in which extensive clinical, demographic, social, cultural, and behavioral information was obtained. These methods have been described in detail elsewhere [33–36]. All data collection procedures were approved by the institutional review boards of Jackson State University, Tougaloo College, and the University of Mississippi Medical Center, and all JHS participants provided informed consent.
Analytic Sample
We initially included all geocoded participants in the baseline sample (n = 5229). We excluded participants with missing data on one or more of the biomarkers included in the assessment of CBR (n = 644) and behavioral covariates (n = 175). In order to retain sample size and statistical power, we did not exclude individuals missing data on family income level and educational attainment (n = 663) but, instead, included an indicator variable denoting missing information on these variables. The resulting analytic sample was comprised of 4410 participants residing in 102 census tracts.
Measurement
Neighborhood Disadvantage
Census tracts served as proxies for neighborhoods. Following previous studies [e.g., 1, 23, 25, 26, 37], we used exploratory factor analysis based on census tracts in the Jackson, MS, metropolitan area (i.e., Hinds, Rankin, and Madison counties) to develop a composite score of neighborhood disadvantage using area-level indicators from the 2000 US Census. Ten indicators were chosen a priori to reflect various aspects of income/wealth, education, housing, and family structure and were comparable to those outlined by Sampson et al. [12] and included in similar studies examining the effects of neighborhood disadvantage on CVD-related outcomes [1, 37]. The full set of indicators included the following: percent of households with incomes below the federally defined poverty line, percent of renter-occupied housing, percent of households receiving public assistance, percent of persons age 16 years and older in the labor force who are unemployed, percent of persons aged 25 and older with less than a HS education, percent of households with >1 person per room (crowding), percent of unoccupied housing units (vacant housing), percent of female-headed households, percent of households with no vehicle, and percent of individuals who resided in the same house in 1995 (see Table 1). Each indicator was assessed for normality, and two variables with extremely skewed distributions (i.e., skewness >1.5) [38] were transformed using the square root transformation— vacant housing units (skewness, 2.1) and households with no vehicle (skewness, 1.8).
We used VARIMAX orthogonal factor rotation for factor extraction. Individual indicators were retained if they had a factor loading (i.e., standardized regression coefficient) ≥0.6 (see Table 1). One primary factor emerged from the analysis which we identified as “neighborhood disadvantage.” This factor explained 83 % of the variability between neighborhoods. Two factors were excluded from the final set of indicators based on their factor loadings: percent of renter-occupied housing units (factor loading, 0.55) and percent of individuals residing in the same house in 1995 (factor loading, 0.23).
The final neighborhood disadvantage score was developed by summing the standardized z-scores for the remaining eight indicators (Cronbach’s alpha = 0.93) with higher scores denoting higher levels of disadvantage (range, −10.3 to 20.2; median, −2.3). The score was then dichotomized at the median value such that neighborhoods with scores above the median were classified as the “most disadvantaged” and neighborhoods with scores at or below the median were classified as the “least disadvantaged.” Consistent with previous work in this study population [37], these two categories demonstrated excellent convergent validity with neighborhood characteristics that have been found to be associated poor neighborhood socioeconomic conditions [12]. For example, the most disadvantaged neighborhoods had a much higher African American population (76.5 vs. 22.6 %) and had a lower median household income ($25,413 vs. $53,436).
Cumulative Biological Risk
CBR was assessed at baseline using eight biomarkers representing four physiological systems: cardiovascular (systolic blood pressure, mmHg; diastolic blood pressure, mmHg; resting heart rate, beats per 30 s), metabolic (glycosylated hemoglobin A1c, %; fasting total cholesterol–HDL cholesterol ratio; waist circumference, cm), inflammatory (high sensitivity C-reactive protein, mg/dL), and neuroendocrine (serum cortisol, μg/mL). Inclusion of biomarkers was based on availability within the JHS dataset as well as use in similar studies [e.g., 23, 25, 26]. All biomarkers utilized in the score were assessed using standard laboratory and clinical procedures which have been published elsewhere [34].
The CBR score used in the analyses was developed using a standardized z-score approach [22] which has been used in prior work in the JHS [39]. While count-based approaches exist which assign individuals a point for each biomarker that exceeds a clinically relevant cutoff or other threshold, we chose to use the z-score approach to retain information on the full range of values for each biomarker and in recognition that even when clinically relevant cutoffs are not reached, moderate elevations in risk factors across multiple systems can result in increased risk of disease [20, 40]. Skewed distributions (i.e., C-reactive protein) were transformed using the square root transformation. Standardized z-scores for each biomarker were averaged within each system to create sub-index scores; these scores were then summed to create the overall CBR score with higher scores denoting higher CBR.
Covariates
Sociodemographic variables included age, gender, family income, and educational attainment. Self-reported validated questionnaires [35] administered by trained African American interviewers during the home induction interview were used to obtain data on these variables. Response options for family income included 11 categories ranging from <$5000 to >$100,000 that were collapsed into four categories that accounted for family size and poverty level: low, lower-middle, upper-middle, and high (reference group). For example, participants were categorized as having a “low” family income if they lived in a family of four that had a combined income of ≤$15,000 and a “high” family income if they lived in a family of four that had a combined income >$75,000. Educational attainment responses were classified into three categories: less than or equal to high school, some college, and college or more (reference group).
We also included health behaviors in the analysis, as they may confound or partially mediate the association between neighborhood disadvantage and CBR. These included physical activity, percentage of calories from dietary fat, cigarette smoking status, and alcohol consumption. Leisure time physical activity was assessed during the clinic examination via a 30-item self-report questionnaire modified from the Baecke physical activity questionnaire and validated against accelerometer [41]. Information from the questionnaire was then used to create an Active Living Index, a summary score of the frequency and duration of watching television, walking and/or biking to work, school or errands, and physical exercise that ranged from 1 (low leisure time physical activity) to 5 (high leisure time physical activity) [41]. Consumption of dietary fat was assessed during the clinic examination using items from a 158-item validated self-report food-frequency questionnaire (FFQ) [42] and calculated as the percentage of calories from fat consumed per day. Cigarette smoking status was assessed via self-report during the home interview and categorized as current versus former or never. Finally, alcohol consumption (grams per day) was estimated from the frequency and portion sizes of beer, wine, and liquor reported in the FFQ and included as a continuous variable.
Statistical Analysis
We assessed the distribution of the exposure, individual biomarkers, CBR sub-indices, the CBR score, and covariates overall and by categories of neighborhood disadvantage. Means and standard deviations were calculated for continuous variables, and frequency distributions were calculated for categorical variables. We also calculated unadjusted mean CBR scores for cross-classified categories of neighborhood disadvantage and individual SES (family income and educational attainment).
Given the nested data structure (individuals nested within neighborhoods) and statistically significant variability in the outcome across neighborhoods (intra-class correlation = 0.02, p < 0.05), we fit two-level linear regression models with random intercepts to assess the relationship between neighborhood disadvantage and CBR. The outcome, CBR, and covariates were included in the model at level 1. Neighborhood disadvantage was included in the model at level 2 as a two-category predictor variable.
We first examined the association between living in a disadvantaged neighborhood and CBR, adjusting for sociodemographic variables (model 1) and further adjusting for health behaviors (model 2). We then tested for differences in the association based on individual SES by including cross-level interaction terms between family income levels (low, lower-middle, and upper-middle) and neighborhood disadvantage (models 3 and 5) and educational attainment (≤high school and some college) and neighborhood disadvantage (models 4 and 6). Since the interaction between neighborhood disadvantage and gender was not statistically significant (p = 0.22), analyses were pooled and controlled for gender. In secondary analyses, we examined the overall association between neighborhood disadvantage and each sub-index of CBR following the same modeling procedure.
Given that use of anti-hypertensive and lipid-lowering medications can reduce readings on blood pressure and cholesterol measures, we conducted sensitivity analyses further adjusting for these variables. We used the PROC Mixed procedure in SAS to fit all two-level models [43]. All analyses were carried out using SAS® software, Version 9.3 (SAS® Institute, Inc., Cary, NC).
Results
Neighborhood Characteristics
Neighborhood characteristics differed considerably for the most disadvantaged neighborhoods compared to the least disadvantaged neighborhoods in the Jackson Metropolitan Area. For example, the average median household income was $25,413 in the most disadvantaged neighborhoods compared to $53,437 in the least disadvantaged neighborhoods. Moreover, the percentage of households in poverty was on average 28.1 in the most disadvantaged neighborhoods and 7.2 in the least disadvantaged neighborhoods (Table 2).
Sample Characteristics
The sample included 2801 women and 1609 men with an average age of 54.5 years. Individuals missing data on biomarkers and covariates were slightly older, more likely to have never smoked, and more likely to have less than a high school education. The individual SES of participants was slightly higher than that of the underlying population of Jackson with over 30 % of the sample reporting a completed college education and over half reporting upper-middle or high family incomes [33]. Even still, nearly three quarters of the sample (73 %) resided in the most disadvantaged neighborhoods. With regard to health behaviors, the majority of the sample had never smoked (68.5 %) and had an average Active Living Score of 2.1, and on average dietary fat comprised 35 % of their daily caloric intake. Generally, SES, health behavior, and risk factor profiles were worse in the most disadvantage neighborhoods compared to those in the least disadvantaged neighborhoods (Table 3).
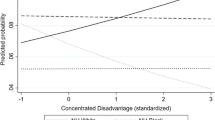
Unadjusted mean CBR scores stratified by neighborhood disadvantage and educational attainment (Fig. 1) showed that CBR was highest for individuals who had less than high school education and resided in the most disadvantaged neighborhoods (0.21, 95 % confidence interval (CI) −0.05–0.48) and lowest for individuals with college education or more residing in the least disadvantaged neighborhood (−0.50, 95 % CI −0.64–0.35). Similarly, mean CBR scores stratified by neighborhood disadvantage and family income were highest for individuals who reported low family incomes and resided in the most disadvantaged neighborhoods (0.27, 95 % CI 0.10–0.46) and lowest for individuals who reported high family incomes and resided in the least disadvantaged neighborhoods (−0.46, 95 % CI −0.60–0.31). The average difference in CBR between individuals residing in the most disadvantaged neighborhood and those residing in the least disadvantaged neighborhood was smallest among individuals who had a high school education or less and greatest among those who reported some college (see Fig. 1).
Regression Results
Two-level linear regression models assessing the association between neighborhood disadvantage and CBR are shown in Table 4. After controlling for age, gender, and individual SES, we found a positive association between living in the most disadvantaged neighborhoods and CBR (B = 0.22, standard error (SE) 0.07, p < 0.05). This supports our original hypothesis that living in a disadvantaged neighborhood would be associated with higher levels of CBR. Further adjustment for health behaviors attenuated the association, as expected, but it remained statistically significant (B = 0.18, SE 0.07, p < 0.01). Similar patterns were found for CBR sub-indices in fully adjusted models and were strongest for the neuroendocrine (B = 0.09, SE = 0.05, p < 0.05) and metabolic (B = 0.05, SE = 0.02, p <0.05) components (Table 5). Associations for the cardiovascular and inflammatory components were not statistically significant (Table 5).
The point estimate for the interaction between neighborhood disadvantage and less than or equal to high school was −0.27 (SE 0.17, p = 0.10) suggesting that the association between living in a disadvantaged neighborhood and CBR was weaker for individuals with a high school education or less compared to those who have a college education. Moreover, the point estimate for the interaction between neighborhood disadvantage and some college was 0.24 (SE 0.15, p = 0.12) suggesting a stronger association for those who have some college compared to those with a college education. The direction of these point estimates was counter to our original hypothesis; however, they were not statistically significant. Findings were less consistent for the interaction between neighborhood disadvantage and family income (Table 4).
Finally, sensitivity analysis further adjusting for anti-hypertensive and lipid-lowering medication did not substantially alter our results (data not shown).
Discussion
In a large socioeconomically diverse sample of African American adults residing in a southern metropolitan area, we found that living in a disadvantaged neighborhood was associated with higher levels of cumulative biological risk after adjustment for sociodemographic characteristics. This finding supports our primary hypothesis that disadvantaged neighborhood contexts increase overall, cumulative risk of disease and that these settings impact multiple physiological systems simultaneously. Additionally, we found that adjustment for health behaviors attenuated the association between neighborhood disadvantage and cumulative biological risk suggesting that behavioral risk factors may be one pathway through which neighborhood disadvantage influences cumulative risk of disease. We also found a positive association between neighborhood disadvantage and CBR sub-indices; however, only estimates for the metabolic and neuroendocrine components remained statistically significant in fully adjusted models. This finding suggests that the cumulative impact of neighborhood disadvantage on multiple systems may be more important than the impact on any single system and supports a more integrated approach to understanding the influence of neighborhood disadvantage on cumulative risk of disease. Furthermore, in addition to the metabolic and cardiovascular components of cumulative risk that have traditionally been the focus of neighborhood research, neuroendocrine markers such as cortisol may also play an important role in contributing to disease risk and subsequent disease onset. This is particularly true in disadvantaged neighborhoods where the confluence of economic deprivation and poor social conditions such as crime, violence, and physical signs of disorder may be sources of psychosocial stress within these settings. Finally, we found no evidence that the association between neighborhood disadvantage and cumulative biological risk differed based on family income or educational attainment.
Our main finding is consistent with previous studies that have examined the cross-sectional association between neighborhood socioeconomic conditions and CBR [23, 24, 26]. For example, a sub-group analysis of African Americans using NHANES data found increasing mean cumulative risk across quintiles of neighborhood disadvantage after adjustment for sociodemographic variables (p for trend = 0.0001) [26]. Only one study found no statistically significant relationship between neighborhood disadvantage and CBR [25]; however, this study had a modest sample size (n = 549) which may have limited statistical power to detect an association.
Findings from studies that have examined the extent to which the association between neighborhood socioeconomic conditions and cumulative risk profiles differs based on individual SES have been less consistent. For example, a study examining neighborhood SES and CBR among a nationally representative sample of US adults reported no evidence of differences based on individual SES [23] with others reporting similar findings [24]. However, at least two studies among African Americans have found stronger associations for individuals with higher levels of SES [44, 45]. In a study of young adults (ages 18–30), Diez-Roux et al. [44] found a stronger association between low neighborhood SES and insulin resistance among African American men at higher levels of SES [44] which is similar to the pattern we found, but counter to conventional wisdom and descriptive evidence [1] that would predict a stronger association for individuals with lower levels of SES. Though few in number, these counterintuitive findings among African Americans warrant further investigation.
Our study has important strengths. To our knowledge, this is the first study to examine the influence of neighborhood disadvantage on cumulative biological risk and the extent to which the relationship differs based on individual SES among a large community-based sample of African American adults residing in a southern metropolitan area. This is particularly relevant given the high prevalence of chronic disease conditions among this racial group and in this region of the country and the need to consider larger contextual factors that influence disease risk in this population. Moreover, participants in our sample varied with respect to educational attainment and family income and resided in a range of neighborhoods that provided sufficient variation in neighborhood socioeconomic conditions. This variation gave us a unique opportunity to examine disease risk among African American adults across a much wider continuum of individual and neighborhood SES than has previously been investigated. In addition, we improved upon the assessment of cumulative biological risk utilized in past neighborhood studies [23–26] by including a measure of the neuroendocrine system (i.e., cortisol) in an effort to better differentiate it from other cumulative risk profiles (e.g., metabolic syndrome) and to capture more fully the accumulation of risk across multiple physiological systems. Furthermore, all biomarkers were assessed objectively in a controlled clinical setting using standard procedures, thereby reducing the potential for measurement error. Finally, we utilized a multi-level analytic approach to distinguish between individual and contextual effects and controlled for a number of sociodemographic confounders in an effort to reduce the potential for spurious associations.
Our study is not without limitations. First, because this is a cross-sectional study, we are unable to determine the temporal sequence of the exposure, outcome, and covariates which limits our ability to make causal claims about the observed associations, including claims about possible mediating mechanisms. Additionally, although our study is one of the first neighborhood studies to include cortisol in the assessment of CBR, our measure was a one-time, serum measure which has been found to be susceptible to factors associated with the timing (e.g., circadian rhythm fluctuations) and context of assessment (e.g., acute stress responses) [46]. Although variability in cortisol due to circadian rhythm may have been mitigated in our study because blood draws for most study participants were taken in the morning, we cannot rule out the potential influence of other factors. We also had a considerable amount of missing data on individual SES (n = 633) which could result in biased point estimates. However, the indicator variables for missing information on education and family income were not statistically significant (p = 0.62 and p = 0.21, respectively) providing some evidence that the presence of missing data would not substantially alter our results. Finally, although census tracts are routinely used as proxies for neighborhoods in empirical studies, they may not fully capture the organic residential environments of the study population.
Our study has important implications. The coupling of economic disadvantage and racial residential segregation in the USA has resulted in the disproportionate exposure of African Americans to deleterious residential environments that influence multiple risk factors for chronic disease. This disproportionate exposure is true for both poor and middle-class African Americans implicating these contexts as important drivers of racial inequities in disease outcomes. In The Truly Disadvantaged, William Julius Wilson [47] points out that poor African Americans in urban settings are more likely to reside in areas of concentrated disadvantage than their poor White counterparts. However, others have noted [7, 48] that middle-class African Americans are also more likely to be exposed to disadvantaged neighborhood environments [48, 49]. Unlike middle-class Whites who tend to occupy neighborhoods that are more aligned with their own individual socioeconomic status, middle-class African Americans are exposed to a much wider range of neighborhood socioeconomic conditions [27]. Not only that, the range of neighborhood environments in which middle-class African Americans reside overlap very little with Whites and include thresholds of concentrated disadvantage (e.g., >20 % poverty) that have been found to be particularly detrimental to health and well-being [26, 27]. This phenomenon among African Americans was reflected in our study sample; of the individuals who reported having a college education or more, nearly 60 % resided in the most disadvantaged neighborhoods. Although some would argue that this differential exposure is due to preferences on the part of African Americans who choose to live in more “racially homogenous” communities even when they have the economic means to live elsewhere [50], structural barriers such as the historical practices of redlining and contemporary discriminatory lending practices within housing markets [51] cannot be ignored as potential drivers of limited residential opportunities [7]. Consequently, the gains in health status that middle-class African Americans might experience as their individual socioeconomic conditions improve may be stymied by structural barriers that limit their mobility out of disadvantaged neighborhoods [7, 52–54]. This in turn exposes them to the array of contextual factors that increase disease risk and, as our findings suggest, may not be entirely buffered by their own socioeconomic resources. Thus, racial differences in cumulative risk of disease and related health conditions linked to disadvantaged residential environments may not only be a reflection of the disparate health status of poor African Americans but also of middle-class African Americans that reside in these settings.
Conclusion
Our findings add to the growing body of evidence that shows that beyond individual dimensions of SES, neighborhood context matters and disadvantaged neighborhoods influence multiple physiological systems simultaneously resulting in an accumulation of disease risk. The implications of these findings are particularly important for African Americans, given their disproportionate exposure to these contexts even at higher levels of income and education. Thus, policies and interventions that directly address contextual features that contribute to disease risk and address the underlying economic and social conditions of these settings should be considered as viable options to reduce disease risk and mitigate inequities in this group.
References
Diez-Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345(2):99–106.
Kawachi I, Subramanian SV. Neighbourhood influences on health. J Epidemiol Community Health. 2007;61(1):3–4.
Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter?: the public health disparities geocoding project. Am J Epidemiol. 2002;156(5):471–82.
Leal C, Chaix B. The influence of geographic life environments on cardiometabolic risk factors: a systematic review, a methodological assessment and a research agenda. Obes Rev. 2011;12(3):217–30.
Pickett KE, Pearl M. Multilevel analyses of neighbourhood socioeconomic context and health outcomes: a critical review. J Epidemiol Community Health. 2001;55(2):111–22.
Jargowsky P. Stunning progress, hidden problems: the dramatic decline of concentrated poverty in the 1990s: The Brookings Institution; 2003.
Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116(5):404–16.
Diez-Roux AV. Rev Epidemiol. 2007;55(1):13–21.
Lovasi GS, Hutson MA, Guerra M, Neckerman KM. Built environments and obesity in disadvantaged populations. Epidemiol Rev. 2009;31:7–20.
Morland K, Wing S, Diez Roux A, Poole C. Neighborhood characteristics associated with the location of food stores and food service places. Am J Prev Med. 2002;22(1):23–9.
Powell LM, Slater S, Mirtcheva D, Bao Y, Chaloupka FJ. Food store availability and neighborhood characteristics in the United States. Prev Med. 2007;44(3):189–95.
Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and violent crime: a multilevel study of collective efficacy. Science. 1997;277(5328):918–24.
Ross CE, Mirowsky J. Neighborhood disadvantage, disorder, and health. J Health Soc Behav. 2001;42(3):258–76.
Mujahid MS, Diez Roux AV, Morenoff JD, Raghunathan TE, Cooper RS, Ni H, et al. Neighborhood characteristics and hypertension. Epidemiology. 2008;19(4):590–8.
Kuipers MA, van Poppel MN, van den Brink W, Wingen M, Kunst AE. The association between neighborhood disorder, social cohesion and hazardous alcohol use: a national multilevel study. Drug Alcohol Depend. 2012;126(1–2):27–34.
Slopen N, Dutra LM, Williams DR, Mujahid MS, Lewis TT, Bennett GG, et al. Psychosocial stressors and cigarette smoking among African American adults in midlife. Nicotine Tob Res. 2012;14(10):1161–9.
Cleland V, Ball K, Hume C, Timperio A, King AC, Crawford D. Individual, social and environmental correlates of physical activity among women living in socioeconomically disadvantaged neighbourhoods. Soc Sci Med. 2010;70(12):2011–8.
Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–47.
Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120(16):1640–5.
Crimmins EM, Seeman TE. Integrating biology into the study of health disparities. Popul Dev Rev. 2004;30:89–107.
Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci U S A. 2001;98(8):4770–5.
Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35(1):2–16.
Bird CE, Seeman T, Escarce JJ, Basurto-Dávila R, Finch BK, Dubowitz T, et al. Neighbourhood socioeconomic status and biological 'wear and tear' in a nationally representative sample of US adults. J Epidemiol Community Health. 2010;64(10):860–5.
Schulz AJ, Mentz G, Lachance L, Johnson J, Gaines C, Israel BA. Associations between socioeconomic status and allostatic load: effects of neighborhood poverty and tests of mediating pathways. Am J Public Health. 2012;102(9):1706–14.
King KE, Morenoff JD, House JS. Neighborhood context and social disparities in cumulative biological risk factors. Psychosom Med. 2011;73(7):572–9.
Merkin SS, Basurto-Dávila R, Karlamangla A, Bird CE, Lurie N, Escarce J, et al. Neighborhoods and cumulative biological risk profiles by race/ethnicity in a national sample of U.S. adults: NHANES III. Ann Epidemiol. 2009;19(3):194–201.
Osypuk TL, Galea S, McArdle N, Acevedo-Garcia D. Quantifying separate and unequal: racial-ethnic distributions of neighborhood poverty in metropolitan America. Urban Aff Rev Thousand Oaks Calif. 2009;45(1):25–65.
U.S. Census Bureau. Current Population Reports, P60-213, Money Income in the United States: 2000, U.S. Government Printing Office, Washington, DC, 2001.
U.S. Census Bureau. 2010 Census Special Reports, Patterns of Metropolitan and Micropolitan Population Change: 2000 to 2010, C2010SR-01, U.S. Government Printing Office, Washington, DC, 2012.
Centers for Disease Control. Adult Obesity Facts; 2013. Retrieved February 9, 2014, from http://www.cdc.gov/obesity/data/adult.html.
National Institutes of Health. Morbidity and mortality: 2012 chart book on cardiovascular, lung and blood diseases; 2012.
Robinson JC, Wyatt SB, Hickson D, Gwinn D, Faruque F, Sims M, et al. Methods for retrospective geocoding in population studies: the Jackson heart study. J Urban Health. 2010;87(1):136–50.
Fuqua SR, Wyatt SB, Andrew ME, Sarpong DF, Henderson FR, Cunningham MF, et al. Recruiting African-american research participation in the Jackson heart study: methods, response rates, and sample description. Ethn Dis. 2005;15(4 Suppl 6):S6-18–29.
Carpenter MA, Crow R, Steffes M, Rock W, Heilbraun J, Evans G, et al. Laboratory, reading center, and coordinating center data management methods in the Jackson heart study. Am J Med Sci. 2004;328(3):131–44.
Payne TJ, Wyatt SB, Mosley TH, Dubbert PM, Guiterrez-Mohammed ML, Calvin RL, et al. Sociocultural methods in the Jackson heart study: conceptual and descriptive overview. Ethn Dis. 2005;15(4 Suppl 6):S6-38–48.
Taylor HA, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, et al. Toward resolution of cardiovascular health disparities in African americans: design and methods of the Jackson heart study. Ethn Dis. 2005;15(4 Suppl 6):S6. -4-17.
Clark CR, Ommerborn MJ, Hickson DA, Grooms KN, Sims M, Taylor HA, et al. Neighborhood disadvantage, neighborhood safety and cardiometabolic risk factors in African americans: biosocial associations in the Jackson heart study. PLoS One. 2013;8(5):1–10.
Diez-Roux AV, Kiefe CI, Jacobs Jr DR, Haan M, Jackson SA, Nieto FJ, et al. Area characteristics and individual-level socioeconomic position indicators in three population-based epidemiologic studies. Ann Epidemiol. 2001;11(6):395–405.
Hickson DA, Diez Roux AV, Gebreab SY, Wyatt SB, Dubbert PM, Sarpong DF, et al. Social patterning of cumulative biological risk by education and income among African americans. Am J Public Health. 2012;102(7):1362–9.
Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS. Socio-economic differentials in peripheral biology: cumulative allostatic load. Ann N Y Acad Sci. 2010;1186:223–39.
Smitherman TA, Dubbert PM, Grothe KB, Sung JH, Kendzor DE, Reis JP, et al. Validation of the Jackson heart study physical activity survey in African americans. J Phys Act Health. 2009;6(1):S124–32.
Carithers TC, Talegawkar SA, Rowser ML, Henry OR, Dubbert PM, Bogle ML, et al. Validity and calibration of food frequency questionnaires used with African-american adults in the Jackson heart study. J Am Diet Assoc. 2009;109(7):1184–93.
Singer J, Using SAS. PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat. 1998;24(4):323–55.
Diez-Roux AV, Jacobs DR, Kiefe CI, Study CARDiYAC. Neighborhood characteristics and components of the insulin resistance syndrome in young adults: the coronary artery risk development in young adults (CARDIA) study. Diabetes Care. 2002;25(11):1976–82.
Diez-Roux AV, Nieto FJ, Muntaner C, Tyroler HA, Comstock GW, Shahar E, et al. Neighborhood environments and coronary heart disease: a multilevel analysis. Am J Epidemiol. 1997;146(1):48–63.
Stalder T, Kirschbaum C. Analysis of cortisol in hair--state of the art and future directions. Brain Behav Immun. 2012;26(7):1019–29.
Wilson J. The Truly Disadvantage: the inner city, the underclass and social policy: University of Chicago Press; 1987.
Pattillo M. Black middle-class neighborhoods. Annu Rev Sociol. 2005;31:305–29.
Jackson PB, Williams DR. The intersection of race, gender, and SES, health paradoxes. In: Mullings' Sa, editor. Gender, race, class, and health: Intersectional Approaches: Jossey-Bass; 2006. p. 131–162.
Charles CZ. The dynamics of racial residential segregation. Annu Rev Sociol. 2003;29:167–207.
Turner MA, Ross SL, Galster GC, Yinger J. Discrimination in metropoltian housing markets: phase I. Washington: US Department of Housing and Urban Development; 2002.
Briggs X, Benjamin KJ. Has exposure to poor neighborhoods changed in America? Race, risk and housing locations in two decades. Urban Stud. 2009;46:429–53.
Crowder K, South S. Race. class, and changing patterns of migration between poor and nonpoor neighborhoods. Am J Sociol. 2005;110(6):1751–63.
South S, Pais J, Crowder K. Metropolitan influences on migration to poor and nonpoor neighborhoods. Soc Sci Res. 2011;40(3):950–64.
Acknowledgments
This research was supported by funding from the National Institutes of Health National Research Service Award training grant, CVD Epidemiology Training Program in Behavior, the Environment and Global Health (T32 HL 098048–02), and the NIH Initiative to Maximize Student Diversity grant funding. Special thanks to the JHS research staff and study participants.
Compliance with Ethical Standards
This research was supported by funding from the National Institutes of Health National Research Service Award training grant, CVD Epidemiology Training Program in Behavior, the Environment and Global Health (T32 HL 098048–02), and the NIH Initiative to Maximize Student Diversity grant funding.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all participants included in the JHS, and the study protocol was approved by the Institutional Review Boards of Tougaloo College and the University of Mississippi Medical Center. Secondary data analyses conducted in this study was approved by the Harvard School of Public Health Institutional Review Board.
Conflict of Interest
Dr. Barber, Dr. Hickson, Dr. Kawachi, Dr. Subramanian, and Dr. Earls declare that they have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barber, S., Hickson, D.A., Kawachi, I. et al. Neighborhood Disadvantage and Cumulative Biological Risk Among a Socioeconomically Diverse Sample of African American Adults: An Examination in the Jackson Heart Study. J. Racial and Ethnic Health Disparities 3, 444–456 (2016). https://doi.org/10.1007/s40615-015-0157-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40615-015-0157-0