Opinion statement
Estimated to burden over 300 million people and their families around the world, asthma is now considered one of the most common forms of non-communicable disease worldwide (Masoli et al. Allergy Eur J Allergy Clin Immunol 59:469–78, 2004 1). The epidemic rise in prevalence this disease has seen over recent decades (Platts-Mills J Allergy Clin Immunol 136:3–13, 2015 2) suggests that environmental factors are the primary drivers of this phenomenon. In particular, the importance of early life microbial exposure and the composition of the early life gut and lung microbiota are emerging as key determinants of asthma outcomes later in life. Borne out of epidemiological data showing associations between the composition of the early life gut microbiota and later development of asthma, interest in harnessing the human microbiome as a therapeutic tool to prevent the development of asthma is rising. As research elucidating the mechanisms, specific microbial species, and microbial products mediating this link continues, it is becoming clear that, like the disease itself, the relationships between microbes and their hosts are highly complex and heterogeneous across populations. As a result, probiotic trials aimed at the primary prevention of asthma have been largely unsuccessful thus far. Future work aiming to apply our understanding of the role of the microbiota in health and disease to the prevention of atopic asthma will likely need to take a population-specific approach and has the potential to dramatically change the face of current asthma treatment practices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although infectious and communicable diseases still represent a substantial cause of global morbidity and mortality, modern medicine and advanced technologies have meant that chronic diseases now pose the greatest threat to human health [3]. Among these, asthma is one of the most important causes of years lost to disability and affects approximately 300 million people around the world [1, 4]. Asthma is a heterogeneous disease characterized by inflammation and reversible obstruction of the conducting airways, bronchial hyperreactivity, and permanent airway remodeling [5, 6]. Allergen exposure [7], genetics [8], viral infections [9], and other environmental and immunologic factors all contribute to the development and expression of this disease. Recent work has identified clinically important subphenotypes, or endotypes, of asthma based on differences in the cellular mediators and pathological features found in the disease [10]. Bronchial wall smooth muscle hyperplasia and hypertrophy, goblet cell hyperplasia, eosinophils, neutrophils, mast cells, basophils, lymphocytes, epithelial cells, and T helper (Th)2 cytokines such as IL-4, IL-5, and IL-13 have all been implicated in the pathogenesis of asthma [5]. Classically defined allergic, or “atopic,” Th2 and IgE-mediated asthma is the most common form of asthma in children, is often associated with a family or personal history of other allergic conditions, and has been well characterized [11]. However, emerging evidence suggests that Th17 cytokines and neutrophils [12], as well as Th1 responses, can also play a central role in certain forms of this disease, particularly with respect to adult-onset and/or severe treatment-resistant forms of asthma [11, 13].
The allergy epidemic, the microbiota, and the perinatal period as a critical window for immune system priming
Recent decades have seen an alarming rise in the global prevalence of asthma [1], and affluent “westernized” countries appear to be disproportionately affected [14]. However, the “allergy epidemic” (reviewed in [2]) is now beginning to emerge as a global health concern as developing countries have gradually become more industrialized [15], suggesting that environmental factors are primarily responsible for these trends [16]. First proposed by Strachan in 1989, the “hygiene hypothesis” [17] suggests that the increased prevalence of allergic diseases observed in countries post-industrialization is a consequence of disrupted immune development resulting from improved sanitation practices leading to diminished microbial (and infectious) exposure early in life (see [18, 19] for more extensive reviews). However, as research in this area has continued, it has become clear that early life infections are not the only forms of microbial exposure capable of driving immune development.
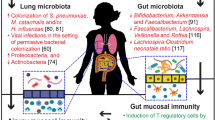
Adding to the hygiene hypothesis, Wold’s “microflora hypothesis” [20] further highlights the importance of the community of commensal and symbiotic microorganisms covering the mucosal surfaces of the human body in the normal development and expression of a healthy immune system. This community is collectively referred to as the microbiota. Congruent with this hypothesis, epidemiological studies have shown differences in the composition of the early life gut microbiota of allergic and non-allergic children [21, 22, 23•, 24–26]. Moreover, early life factors capable of altering the colonization and overall composition of the gut microbiota community, including delivery place [27] and method [28, 29], exposure to farm animals or pets [30•], birth order [29, 31], breastfeeding [29], exposure to antibiotics [32], and daycare attendance [31], have all been associated with either protection or vulnerability to asthma and/or allergy (see [33•] for more detailed review). Maternal microbiota-modifying factors such as antibiotic use [34], diet [35], and farm exposure [36] have also been shown to be important in determining their infants’ susceptibility to developing asthma and other diseases of the immune system (reviewed in [37•]).
Over thousands of years, humans and microbes have evolved together and established an intricate symbiotic relationship that we are only just beginning to understand. An inaugural community of microbes inhabiting the intestinal tract of the neonate begins at birth and is strongly influenced by delivery method [38]. A succession of bacterial communities ensues based on the environments created by the previous community and changes in host behavior or diet until an adult-like microbiota begins to become established at around age 3 [39, 40]. At this stage, the human body is colonized by at least as many bacterial cells as there are somatic cells [41].
Humans rely on microbial organisms in the intestinal tract for several functions, including the maturation of both the structural elements and various cellular arms of the innate and adaptive mucosal immune systems [42]. Correlating with the timing of the gradual establishment of a resident microbiota community by 3 years of age, the neonatal immune system undergoes a process of maturation highly susceptible to environmental influences [37•]. At birth, immune responses are skewed toward the Th2-type phenotype that dominates during the intrauterine period [43], and it is thought that early microbial exposures promoting Th1 and Treg responses are important for the dampening and prevention of persisting Th2-skewed responses characteristic of atopic diseases [16, 44]. This “critical window” of vulnerability has been demonstrated in murine models showing that the development of tolerogenic responses to allergens [45], restoration of a healthy Th1/Th2 balance [46], and normalization of gut and lung invariant natural killer T (iNKT) cell numbers [47] in germ-free mice can only be achieved if colonization occurs during the neonatal stage, but not after 5 weeks of age. Additionally, mice exposed to the broad-spectrum antibiotic vancomycin demonstrate an increased susceptibility to a murine model of asthma, with higher levels of serum IgE and reduced numbers of colonic Tregs as compared to control mice, but only if antibiotic exposure occurs early in life [48, 49••]. Taken together, these studies provide a strong argument in favor of the microflora hypothesis implicating the early life intestinal microbiota composition as a determining factor in asthma development.
Microbial dysbiosis and asthma
Disruption of the gut microbiota and asthma
Despite inconsistencies between studies, epidemiological data suggest that asthmatic children harbor an early intestinal microbiota distinct from that of healthy children (Table 1). Data linking specific microbes to asthma susceptibility is limited, but increases in Clostridium difficile levels in the feces of 1-month-old children who later develop asthmatic symptoms by age 2 [50] or asthma by age 6 to 7 [27] as compared to healthy children are among the most consistently replicated findings. Importantly, birth place and mode have been found to significantly affect early colonization by C. difficile [27], suggesting that this microbe may be implicated in the associations found between children delivered via cesarean section and their increased risk of developing asthma as compared to vaginally delivered children [28].
Differences in the overall composition and diversity of the early life gut microbiota have also been found between asthmatic and non-asthmatic children, consistent with the findings of differences in the diversity of bacterial exposures in these children [51]. Abrahamsson et al. [23•] recently followed a Swedish birth cohort from birth until 7 years of age and found that the diversity of the gut microbiota at 1 week and 1 month of age of children diagnosed with asthma by age 7 was reduced as compared to healthy children. These results are somewhat incongruent with the previous findings of Bisgaard et al. [24], who found that while reduced bacterial diversity of the gut microbiota in the first year of life was associated with IgE sensitization, early life bacterial diversity did not differ between 6-year-old asthmatic and non-asthmatic children in a Danish cohort [24]. Such discrepancies may be due in part to methodological differences but also highlight the complexities of the variability in the gut microbiota composition among populations across the globe and lend support to the idea that there is no single “healthy microbiota” composition.
The importance of a diverse early life gut microflora in the prevention of asthma has been further highlighted by studies showing that differences in early life exposures capable of altering the diversity of the microbiota are associated with differences in asthma susceptibility. Among such factors, perinatal exposure to antibiotics has been shown to induce changes in the microbiota [52] and increase the likelihood of developing wheeze [53] and asthma [33•, 35, 55••] in childhood. In a recent study, Korpela et al. [52] reported that frequent macrolide use in the first 2 years of life was associated with the development of asthma, and asthmatic children were found to have decreased levels of bacteria of the genera Rothia accompanied by increased levels of Blautia and Coprobacillus in their feces at age 2 to 7 [52], suggesting that antibiotic-induced changes in the microbiota may persist long past a single antibiotic course.
Neonatal farm and pet exposure have also been found to be protective against the development of childhood asthma by influencing the diversity of the gut microbiota [30•, 51, 55••]. It is possible that these effects are mediated by exposure to an increased bacterial load through dust particles in farming environments and households with pets. Consistent with this hypothesis, Lynch et al. [7] used microarray technology to examine the bacterial content of house dust taken from the homes of 3-month-old American children who were later determined to be allergic or non-allergic at 3 years of age. These authors found that house dust taken from the homes of children with atopic wheeze contained a reduced relative bacterial richness and diminished levels of bacteria from the Prevotellaceae, Lachnospiraceae, and Ruminococcaceae families as compared to healthy children [7].
From correlation to causation
Informed by epidemiological findings, studies using murine models have allowed researchers to begin to determine whether differences in microbial exposures or microbiota composition are a cause or consequence of asthmatic phenotypes. For instance, Arrieta et al. [56••] recently found that the feces of 3-month-old children who later developed atopic wheeze (AW) at 1 year of age contained reduced levels of bacteria from the genera Faecalibacterium, Lachnospira, Veillonella, and Rothia (FLVR). The authors then demonstrated that the addition of FLVR to an inoculum of feces from a 3-month-old AW child given to germ-free mice successfully reduced airway inflammation in their offspring in a murine model of asthma [56••].
Demonstrating a causal role for increased microbial exposure in the link between dog ownership and protection from asthma, Fujimura et al. [55••] exposed mice to dust taken from homes with or without a dog and then used a murine model of allergic asthma to determine the effects of house dust exposure on immune function. They found that mice exposed to house dust taken from a home with a dog were protected against allergic Th2 airway inflammation and pathology as compared to mice exposed to house dust taken from a home with no dog [55••]. Furthermore, these authors showed that the composition of the gut microbiota of mice exposed to house dust from the home with a dog differed from that of mice exposed to dust from a non-dog household, with levels of Clostridia and Bacilli enriched in the feces of dog-associated house dust-treated mice [55••]. Representing one of the most highly enriched taxa in the feces of mice exposed to dust from dog-associated homes, Lactobacillus johnsonii was then orally administered to mice prior to allergen sensitization and found to be protective against airway inflammation following cockroach and OVA allergen sensitization and challenge [55••]. Moreover, the lungs of L. johnsonii-treated mice following cockroach allergen challenge were found to contain fewer activated dendritic cells (DCs) as compared to control mice, suggesting a possible mechanism through which this bacterium can influence T cell maturation [55••].
Similarly providing mechanistic insights into epidemiological data, Schuijs et al. [57••] demonstrated that chronic low-dose exposure to endotoxin or farm dust is protective against airway inflammation and hyperresponsiveness in a murine model of asthma. This effect likely occurred through interactions with toll-like receptor (TLR)-4 ligands on the surfaces of the epithelial cells and was mediated by the induction of the enzyme A20 in lung epithelial cells [57••]. A20 was in turn found to be responsible for preventing the secretion of pro-inflammatory cytokines and chemokines, including granulocyte-macrophage colony-stimulating factor (GM-CSF), a molecule that promotes DC-induced Th2 cell responses [57••]. Further confirming their results, these authors also showed that a point mutation in the gene encoding A20 was associated with an increased risk of asthma in a European birth cohort, and that a gene-by-environment effect existed between this mutation, farming exposure, and asthma [57••]. Interestingly, farm exposure conferred greater protection against asthma in children with the defective gene as compared to those with the normal gene variant [57••]. These data suggest that as work investigating the relationship between the microbiota and asthma continues, it will be important to consider the genetic backgrounds of the specific populations being studied.
The use of murine models in asthma research as stand-alone studies has further allowed researchers to determine both the immune cell types affected by general early life microbial exposure (Table 2) and the means by which particular microbes affect these cell types.
Immune mechanisms
A growing body of evidence suggests that the microbiota plays a key role in promoting the development and maturation of mucosal Treg cells (summarized in Table 2), known to be critical in the induction of tolerance and prevention of allergic responses [61, 62]. Germ-free mice [63] and mice with antibiotic-induced shifts in the perinatal gut microbiota exhibit enhanced asthma susceptibility in murine models of asthma associated with elevated serum IgE levels and reduced colonic CD4+CD25+FoxP3+ Tregs as compared to control mice [63]. Tregs have also been implicated in the mechanism by which pathogenic Helicobacter pylori [64] and commensal species from Clostridium clusters IV and XIVa [63] confer protection against asthma and allergic sensitization, suggesting that these cells may represent a common pathway through which early life exposures to both pathogenic and commensal bacteria influence host immune responses [64]. IL-10-producing Treg cell abundance has also been independently shown to alter the composition of the gut microbiota, highlighting the bidirectional nature of host-microbe interactions [62].
Further mechanistic insights into the influence of the gut microbiota on asthma-specific immunology suggest that IgE-promoting intrinsic MyD88 B cell signaling and basophil hematopoiesis are susceptible to microbial modulation through signaling by PRRs [65]. Peyer’s patch-derived B cells have also been implicated in tolerance induction and the reduction of airway inflammation through the induction of Treg cells in a murine model of allergic asthma [66], possibly suggesting that MyD88 signaling represents a common pathway by which B cells modulate various aspects of the allergic response [67]. Finally, microbe-mediated modulation of iNKT cell activity has also been implicated in the pathological features of asthma, with mice harboring a conventionalized microbiota showing blunted asthmatic phenotypes and reduced iNKT cell recruitment to colon and lung tissue as compared to germ-free mice [47].
Microbial structures and metabolic products
Further honing in on the particular means through which bacteria alter host immune responses, bacterial metabolites and fermentation products such as the short chain fatty acids (SCFAs) butyrate, propionate, and acetate are emerging as important mediators of microbiota-associated asthma susceptibility. SCFAs are natural histone deacetylase inhibitors, therefore acting not only as an important source of nutrients for the human host but also to regulate gene expression through epigenetic modifications, modulate the microbial environment around them, and as important signaling molecules in directing the host immune system [68].
In mouse studies, it has been shown that levels of microbiota-derived SCFAs differ according to diet [69] and are reduced in the feces of both germ-free mice and mice treated with antibiotics as compared to control mice [70•]. Moreover, diminished colonic Treg cell populations associated with vancomycin treatment can be restored if SCFAs are given concurrently [70•]. While both propionate and acetate have been shown to promote Treg cell accumulation in the colon [69, 70•], butyrate and propionate have been shown to enhance de novo extrathymic CD4+Foxp3+IL-10+ Treg cell differentiation through direct actions on T cells as well as indirect interactions through DCs [68, 69, 70•]. Butyrate can directly stimulate colonic Treg differentiation when administered locally [71] or in combination with dietary starch [69, 71], and has also recently been shown by Kelly et al. [72] to enhance the integrity of the epithelial barrier of the gut. Interestingly, Arrieta et al. [56••] found that butyrate levels were reduced in the feces of mice harboring a microbiota derived from a child with AW, suggesting that SCFA production may be a mechanism through which the key microbes identified in that study act to moderate asthma susceptibility. The provision of SCFAs, either alone or in combination with dietary starch, to children as a means to reduce allergic susceptibility thus represents a potentially high-yield, but yet unexplored, area of investigation.
Epigenetics
More recent work suggests that epigenetic DNA modifications may represent the mechanistic link between maternal exposures and childhood allergic disease [73]. Epigenetic modifications are known to be involved in the maturation and regulation of the development of the various branches of the immune system, with acetylation typically promoting transcription and methylation typically having silencing effects on transcription [74].
In a murine model of experimental asthma, airway inflammation and hyperresponsiveness were prevented via epigenetic mechanisms in the offspring of mothers intranasally exposed to the non-pathogenic cowshed-derived bacterium Acinetobacter lwoffi F78 [59, 60] (Fig. 1). This asthma-protective effect was dependent on bacterium-induced maternal TLR signaling and associated with counter-balanced changes in maternal lung and placental TLR expression [60]. Asthma protection was further shown to be mediated in the offspring by increased IFN-γ expression acting to suppress the Th2-type response seen in control mice following allergen sensitization/challenge, as the offspring of A. lwoffi 78-treated mothers were protected from the decreased acetylation of histone H4 in the IFNG promoter region and other epigenetic modifications found to occur following OVA sensitization in splenic CD4 +CD25− T cells of control mice [59]. Taken together, these findings may at least partially explain how maternal exposure to farming environments during pregnancy protects against atopic sensitization and is associated with increased TLR-2, TLR-4, and CD14 expression in their infants [36]. Further investigations in this area may also provide insights into the factors mediating the increased Treg activity and reduced Th2 cytokine levels found in the cord blood of farm-exposed mothers as compared to mothers with no farming exposures [75].
Proposed epigenetic mechanisms underlying the protective effects of maternal exposure to farming environments against asthma in childhood, based on mouse and human data [59, 60, 75]. Chronic exposure to the cowshed-derived bacterium Acinetobacter lwoffi F78 results in a low-grade inflammatory state in the maternal lung and increased serum IL-6 levels. In response to these stimuli, maternal lung TLR-2, TLR-6, and TLR-7 are upregulated while TLR-5 is downregulated. Chronic bacterial exposure is further accompanied by downregulation of placental TLRs, especially TLR-6 and TLR-7. Maternal A. lwoffi F78 exposure prevents the splenic CD4+CD25− T cell IFNG promoter histone 4 acetylation (H4ac) reduction that occurs in mice born to A. lwoffi F78 naive mothers following antigen sensitization/challenge. Reduced H4ac levels at the IL-4 promoter and increased methylation of CpG sites in the promoter region of the Th2 regulatory region conserved noncoding sequence 1 (CNS1) are also observed in these splenic cells following prenatal A. lwoffi F78 exposure and antigen sensitization/challenge. Finally, cord blood from mothers exposed to farming environments and farm milk contains increased levels of demethylation at the FOXP3 locus. Arrows represent the direction of change in gene expression or regulatory region enhancer function resulting from the indicated epigenetic modification.
Maternal lipopolysaccharide (LPS) exposure may also have a protective effect against childhood asthma through epigenetic mechanisms. Mice born to mothers exposed to LPS during pregnancy are born with a Th1-skewed cytokine milieu as compared to mice born to mothers not exposed to LPS [76]. Moreover, perinatal LPS exposure was found to be protective against Th2-type airway inflammation in a murine model of allergic asthma and associated with increased expression of TLR-2 and TLR-4 in lung tissues [76]. Based on the findings of Brand et al. [59] described above, it would be interesting to see if epigenetic changes to Th1/Th2 genes are involved in the effects of perinatal exposure to LPS on asthma susceptibility.
Epigenetic changes mediated by SCFAs have also recently been linked to the influence of maternal diet on offspring’s susceptibility to asthma [58••]. Thorburn et al. [58••] found that maternal serum acetate levels during pregnancy were negatively associated with their infant’s number of visits to a doctor for cough or wheeze [58••]. These authors further showed that the progeny of mice fed a high-fiber diet or a diet supplemented with acetate were protected against airway inflammation in a murine model of allergic asthma, and this effect was maintained even when pups were delivered by cesarean section [58••]. The allergy-protective effects of the high-fiber diet/acetate were shown to be mediated by Treg cells and hypothesized to be a result of acetate-associated HDAC9 inhibition with resulting changes in lung tissue gene expression, including Foxp3 expression [58••]. These data, combined with findings that diet-induced losses in gut microbiota diversity are transmittable over generations [35], suggest that dietary fiber is likely an important modulator of host immunity through its effects on the gut microbiota.
Looking beyond the gut microbiota
While most studies to date have found that asthmatic children are either deficient in particular microbes or harbor a less diverse microbiota as compared to healthy children in early life, exposures to certain early life viral [9] and bacterial infections have also been found to exacerbate or increase the likelihood of developing asthma later in life [77]. These findings have been found especially with respect to neutrophilic asthma [78, 79•, 80]. Adding to this complexity, certain members of the microbiota have been shown to influence susceptibility to certain viral infections [81], while the presence of pathogenic bacteria in the airways has been associated with more severe viral infections [82]. Thus, it is clear that early life exposures to microorganisms are complex and that different microbes act on the host immune system to maintain a precarious balance between pro- and anti-inflammatory signals (for a discussion of the microbial species and mechanisms mediating the relationship between particular infectious microbes and asthma, see Holt [83•]).
As interest in the area of the microbiome and asthma has burgeoned, scientists have primarily focused on the influence of the gut microbiota on the development of mucosal immunity and asthma under the assumption that mucosal tissues somehow together form a single system-wide organ [67, 84–86]. However, recent technological advances in sequencing techniques have allowed for the identification and characterization of microbial communities previously inaccessible for study or thought to be sterile [87]. Among these, the lung [88–92] and nasopharyngeal [93, 94] microbiomes are emerging as potentially important drivers of airway diseases such as asthma [95••]. These investigations are as yet in their infancy but will likely lead to important developments in our understanding of the pathogenesis and heterogeneity of this disease.
Therapeutic applications
Asthma is now considered the most common chronic childhood condition in affluent countries [96–98], and no curative treatment options currently exist for the disease. Disease management is currently focused on symptom reduction and typically consists of life-long β2-broncholidlator and/or inhaled corticosteroid therapy [99]. Although asthma is difficult to diagnose in children under the age of six, early identification of at-risk children is critical. Studies have shown that children under the age of nine with asthma are more likely than older patients to require visits to the emergency room [100], and early interventions have the greatest potential to prevent irreversible airway remodeling not addressed by current treatments options [6]. Moreover, a primary prevention therapy for asthma would save healthcare systems billions of dollars each year and significantly improve the quality of life of patients and families affected by the disease.
Probiotics, Prebiotics, and Microbial Products
A logical progression from the growing body of epidemiological and mouse model data supporting the hypothesis that the early life gut microbiota is associated with later asthma development is the development of effective probiotics, defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” [101], and prebiotics, non-digestible food products that favor the growth of probiotic bacteria [78, 102], aimed at preventing asthma. However, despite some success in murine models [103–105], probiotic trials in humans have been largely unsuccessful in the prevention of asthma thus far [106, 107••]. As a result, the World Allergy Organization does not currently recommend their use for the prevention of childhood asthma [107••, 108]. However, studies to date have failed to use probiotic strains shown to be of clinical relevance in human epidemiological studies with respect to asthma and thus may not be representative of the true potential of their use in the prevention of this disease.
The heterogeneity and failure of most studies to find an effect of probiotic treatment on airway disease is likely due to differences in the types of bacteria used, the timing of probiotic administration, host factors, and differences in other environmental factors capable of altering the gut microbiota [109]. In particular, the success of probiotics appears to depend on both maternal and neonatal exposure [110]. Thus, as interest in the use of probiotics in asthma prevention rises, future work will need to focus on further characterizing the nature, dosage regimen, and contexts in which probiotics can be applied as a means to alter asthma susceptibility.
Prebiotic trials thus far are limited, but non-digestible oligosaccharides that promote the growth of particular bacteria, antioxidants, and various minerals and diets have all been shown to have promising protective effects against the development of asthma [79•]. However, more research is needed in this area to determine the long-term efficacy and mechanisms through which particular prebiotic formulations can protect against asthma.
Finally, the use of microbial products or components to effectively prevent allergic airway disease should be further explored. Based on the findings of increased TLR-2 expression and protection from asthma in children raised in a farming environment, Stiehm et al. [111] synthesized a lipopeptide derived from a germination lipoprotein of Bacillus cereus and found that pretreatment with this construct protected mice from airway inflammation in a murine model of asthma through the induction of tolerogenic DCs [111]. Whether or not these findings translate to humans has yet to be determined.
It is likely that different microbe-host interactions play different roles in the expression of different subtypes of asthma [79•]. This notion is supported by the findings of differences in both the specific gut microbiota species found to be altered in asthmatic children in different birth cohorts and the dominant asthma phenotype seen among different countries [112]. Therefore, the success of probiotics, prebiotics, and bacterial products/structures in preventing asthma may be highly specific to the populations being studied, and it is likely that no potential “one-size-fits-all” therapy exists.
Future directions
In order to develop effective probiotics or other products that take advantage of our understanding of the microbiome and asthma, the mechanisms underlying microbial influences on the immune system will need to be further characterized. Moreover, the identification of early life biomarkers that predict the later development of asthma will be essential to the effective and efficient implementation of any sort of prebiotic-, probiotic-, diet-, or lifestyle- based primary prevention intervention. Such biomarkers would ultimately allow physicians to identify at-risk children through an early life screen as a means to reduce the disease burden through primary prevention. The current cost of fecal sample nucleic acid sequencing likely renders the implementation of widespread sequencing as a screening tool unfeasible. However, microbiota-associated changes in urine and fecal metabolites are detectable within the first 100 days of life [56••] and thus have the potential to be studied as surrogate markers of microbial dysbiosis.
Furthermore, although the significance of these results are not yet known, a recent study by Hevia et al. [113] found that subtle differences in the gut microbiota of asthmatic and non-asthmatic patients also exist in adulthood. These data suggest that it may be too early to discount the potential for probiotic or prebiotic-type products to ameliorate asthma or allergy later in life. Adult-onset asthma has been found to differ phenotypically from allergic asthma [11], therefore rendering it possible that different microbes differentially influence host immune responses at different developmental stages. This line of inquiry has potential implications for the treatment of those already affected by the disease and could serve to inform secondary and tertiary treatment options.
Conclusions
In support of the microflora hypothesis, epidemiological and murine model data suggest that early life microbial exposure and colonization of the intestinal tract with symbiotic bacteria impact allergic asthma susceptibility later in life. Recent research has begun to identify the particular mechanisms through which the gut microbiota can exert immunomodulatory effects in the host to manipulate both host immune responses and the composition of the gut microbiota itself. Tregs, NKT cells, TLR signaling, SCFAs, and epigenetic changes have all been identified as important mediators of asthma protection conferred by microbial exposure early in life. Asthma is currently an incurable disease, and treatment options are primarily symptom-based. The inability of these treatment options to fully prevent disease progression means that there is a need for the development of treatment options aimed at primary prevention. Research into the relationship between the early life gut microbiota and asthma holds promise in this regard. However, the lack of success of probiotic trials in preventing asthma thus far indicates the complexities of this relationship deserve further attention and characterization.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy Eur J Allergy Clin Immunol. 2004;59:469–78.
Platts-Mills TAE. The allergy epidemics: 1870-2010. J. Allergy Clin. Immunol. 2015;136:3–13.
WHO. Global status report on noncommunicable diseases 2014. World Health. 2014;176.
Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, Bolliger I, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800.
Busse WW, Lemansk RFJR. Asthma. N Engl J Med. 2001;344:350–62.
Hirota N, Martin JG. Mechanisms of airway remodeling. Chest. 2013;144:1026–32.
Lynch SV, Wood RA, Boushey H, Bacharier LB, Bloomberg GR, Kattan M, et al. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J. Allergy Clin. Immunol. 2014;134:593–601.
Ober C, Hoffjan S. Asthma genetics 2006: the long and winding road to gene discovery. Genes Immun. 2006;7:95–100.
Beigelman A, Bacharier LB. Early-life respiratory infections and asthma development. Curr Opin Allergy Clin Immunol. 2016;16:172–8.
Lin T-Y, Poon AH, Hamid Q. Asthma phenotypes and endotypes. Curr Opin Pulm Med. 2013;19:18–23.
Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16:45–56.
Mckinley L, Alcorn JF, Peterson A, Dupont B, Kapadia S, Logar A, et al. Th17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;6:4089–97.
Nakagome K, Nagata M. Pathogenesis of airway inflammation in bronchial asthma. Auris Nasus Larynx. 2011;38:555–63.
Mallol J, Crane J, von Mutius E, Odhiambo J, Keil U, Stewart A. The International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three: a global synthesis. Allergol Immunopathol (Madr). 2013;41:73–85.
Pearce N, Aït-Khaled N, Beasley R, Mallol J, Keil U, Mitchell E, et al. Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax. 2007;62:758–66.
Noverr MC, Huffnagle GB. The “microflora hypothesis” of allergic diseases. Clin Exp Allergy. 2005;35:1511–20.
Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–60.
Kondrashova A, Seiskari T, Ilonen J, Knip M, Hyöty H. The “hygiene hypothesis” and the sharp gradient in the incidence of autoimmune and allergic diseases between Russian Karelia and Finland. APMIS. 2013;121:478–93.
Brown EM, Arrieta M-C, Finlay BB. A fresh look at the hygiene hypothesis: how intestinal microbial exposure drives immune effector responses in atopic disease. Semin Immunol. 2013;25:378–87.
Wold AE. The hygiene hypothesis revised : is the frequency of allergy d ue to changes in the intestinal flora? Allergy. 1998;53:20–5.
Björkstén B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J. Allergy Clin. Immunol. 2001;108:516–20.
Kalliomäki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J. Allergy Clin. Immunol. 2001;107:129–34.
Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. 2014;44:842–50. Using 16s rDNA 454 pyrosequencing, these authors demonstrate using data from a long-term follow-up study in a Swedish birth cohort that children who develop asthma by age seven harbor a less diverse microbiota at one week and one month of age as compared to healthy children. These differences do not persist into the first year of life
Bisgaard H, Li N, Bonnelykke K, Chawes BLK, Skov T, Paludan-Müller G, et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J. Allergy Clin. Immunol. 2011;128:646–52.
Sjögren YM, Jenmalm MC, Böttcher MF, Björkstén B, Sverremark-Ekström E. Altered early infant gut microbiota in children developing allergy up to 5 years of age. Clin Exp Allergy. 2009;39:518–26.
Björkstén B, Naaber P, Sepp E, Mikelsaar M. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy. 1999;29:342–6.
Van Nimwegen FA, Penders J, Stobberingh EE, Postma DS, Koppelman GH, Kerkhof M, et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J. Allergy Clin. Immunol. 2011;128:948–55.
Tollånes MC, Moster D, Daltveit AK, Irgens LM. Cesarean section and risk of severe childhood asthma: a population-based cohort study. J Pediatr. 2008;153:112–6.
Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–21.
Fall, T, Lundholm, C, Örtqvist, AK, Fall, K, Fang, F, Hedhammar, Å, et al. Early Exposure to Dogs and Farm Animals and the Risk of Childhood Asthma. JAMA Pediatr. 2015;169. In the biggest nationwide cohort study to date, these authors found that dog ownership and farm animal exposure during the first year of life are associated with protection against asthma at age six. The study enrolment of over one million subjects, stringent asthma outcome criteria, comprehensive assessment and correction for potential confounds, and long-term follow up period all contribute to the validity of these findings.
Ball TM, Castro-Rodriguez JA, Griffith KA, Holberg CJ, Martinez FD, Wright AL. Siblings, day-care attendance, and the risk of asthma and wheezing during childhood. N Engl J Med. 2009;343:538–43.
Murk W, Risnes KR, Bracken MB. Prenatal or early-life exposure to antibiotics and risk of childhood asthma: a systematic review. Pediatrics. 2011;127:1125–38.
Meropol SB, Edwards A. Development of the infant intestinal microbiome: a bird’s eye view of a complex process. Birth Defects Res Part C - Embryo Today Rev. 2015;105:228–39. Up-to-date review of the process of colonization of the infant gut by the gut microbiota, the factors influencing this process, and studies showing associations between microbial dysbiosis and the development of pathologies of the immune system, metabolism, and brain
Stensballe LG, Simonsen J, Jensen SM, Bønnelykke K, Bisgaard H. Use of antibiotics during pregnancy increases the risk of asthma in early childhood. J Pediatr. 2013;162:832–8.
Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529:212–5.
Ege MJ, Bieli C, Frei R, van Strien RT, Riedler J, Üblagger E, et al. Prenatal farm exposure is related to the expression of receptors of the innate immunity and to atopic sensitization in school-age children. J. Allergy Clin. Immunol. 2006;117:817–23.
Gollwitzer ES, Marsland BJ. Impact of early-life exposures on immune maturation and susceptibility to disease. Trends Immunol. 2015;36:684–96 .Timely review covering the nature of and mechanisms by which early life environmental factors influence the development of the immune system during a critical period of maturation, including an in-depth discussion of the role of the microbiota in this process
Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–5.
Eggesbø M, Moen B, Peddada S, Baird D, Rugtveit J, Midtvedt T, et al. Development of gut microbiota in infants not exposed to medical interventions. APMIS. 2011;119:17–35.
Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2009;457:222–7.
Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164:337–40.
Renz H, Brandtzaeg P, Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat Rev Immunol. 2011;12:9–23.
Prescott SL, Macaubas C, Holt BJ, Troy B, Loh R, Sly PD, et al. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T cell responses toward the Th2 cytokine profile. J Immunol. 1998;160.
Prescott SL, Macaubas C, Smallacombe T, Holt BJ, Sly PD, Holt PG. Development of allergen-specific T-cell memory in atopic and normal children. Lancet. 1999;353:196–200.
Sudo N, Sawamura S, Tanaka K, Aiba Y, Kubo C, Koga Y. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol. 1997;159:1739–45.
Herbst T, Sichelstiel A, Schär C, Yadava K, Bürki K, Cahenzli J, et al. Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am J Respir Crit Care Med. 2011;184:198–205.
Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–93.
Russell SL, Gold MJ, Hartmann M, Willing BP, Thorson L, Wlodarska M, et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 2012;13:440–7.
Russell SL, Gold MJ, Willing BP, Thorson L, Mcnagny KM, Finlay BB. Perinatal antibiotic treatment affects murine microbiota, immune responses and allergic asthma. Gut Microbes. 2013;4:158–64. In an addendum to their previous work, these authors use a murine model of asthma to show that the exacerbated allergic asthma phenotype observed in mice treated perinatally with vancomycin occurs only if antibiotic exposure occurs between birth and three weeks of age, and is associated with increased circulating IgE levels and reduced colonic CD4+CD25+Foxp3+ Tregs. Prenatal antibiotic exposure alone was not sufficient to increase allergic asthma susceptibility compared to controls
Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, et al. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA birth cohort study. Gut. 2007;56:661–7.
Ege MJ, Mayer M, Normand A-C, Genuneit J, Cookson WOCM, Braun-Fahrlander C, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364:701–9.
Korpela K, Salonen A, Virta LJ, Kekkonen RA, Forslund K, Bork P, et al. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat Commun. 2016;7:1–8.
Kummeling I, Stelma FF, Dagnelie PC, Snijders BEP, Penders J, Huber M, et al. Early life exposure to antibiotics and the subsequent development of eczema, wheeze, and allergic sensitization in the first 2 years of life: the KOALA Birth Cohort Study. Pediatrics. 2007;119.
Risnes KR, Belanger K, Murk W, Bracken MB. Antibiotic exposure by 6 months and asthma and aAllergy at 6 Years: findings in a cohort of 1,401 US children. Am J Epidemiol. 2010;173:310–8.
Fujimura KE, Demoor T, Rauch M, Faruqi AA, Jang S, Johnson CC, et al. House dust exposure mediates gut microbiome Lactobacillus enrichment and airway immune defense against allergens and virus infection. Proc Natl Acad Sci U S A. 2014;111:805–10. Using two different murine models of asthma, these authors show that early life exposure to house dust from a home containing a dog is associated with protection against the development of Th2-type asthma pathology and with increased levels of fecal microbes from the genera Clostridia and Bacilli as compared to mice exposed to dust from a non-dog house. Representing one of the most highly enriched groups, these authors further show that oral administration of L. johnsonii to wild-type animals alone is sufficient to protect against both asthmatic airway inflammation and infection by respiratory syncytial virus
Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7:307ra152. In the first study to show a causal link between early life microbial dysbiosis and the later development of asthma, these authors found that children at high risk of developing asthma exhibit reduced levels of bacteria from the genera Lachnospira, Veillonella, Faecalibacterium, and Rothia (FLVR) in their feces as well as differences in fecal and urine metabolite concentrations at three months of age relative to healthy children. They further show in a murine model of asthma that the addition of FLVR organisms to an inoculum of feces from a three month old child at high risk of developing asthma given to germ-free mice reduces airway inflammation observed in their progeny as compared to the progeny of mice inoculated with the non-supplemented feces
Schuijs MJ, Willart MA, Vergote K, Gras D, Deswarte K, Ege MJ, et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science. 2015;349:1106–10. This is the first study to show a mechanistic link between farm dust exposure and protection against allergic asthma. These authors used a murine model of asthma to show that mice chronically exposed intranasally to low-dose endotoxin or farm dust are protected against the airway inflammation observed in control animals. This effect was found to be mediated by reduced expression of the A20 protein in lung epithelial tissue leading to diminished DC infiltration to the lungs and reduced DC-induced Th2 cell maturation following allergen sensitization and challenge. Further confirming the clinical relevance of their findings, the authors also showed that humans with a mutation in the gene encoding A20 are at increased risk of developing asthma, especially when they are raised in a farming environment
Thorburn AN, McKenzie CI, Shen S, Stanley D, Macia L, Mason LJ, et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun. 2015;6:7320. In a potentially landmark study, these authors show that mice fed a high-fiber diet harbor a gut microbiota that is compositionally distinct from that of mice fed a control or no-fiber diet. This microbiota was associated with higher levels of fecal acetate and protection against allergic airway inflammation in a murine model of asthma. Moreover, mice born to mothers who consumed a high-fiber diet or acetate were protected against airway inflammation in the same model. This effect was maintained even if mice were delivered by caesarean section and thought to be mediated by an increase in the numbers and activation of Treg cells resulting from epigenetically-induced changes in gene expression in the lungs of these mice
Brand S, Teich R, Dicke T, Harb H, Yildirim AO, Tost J, et al. Epigenetic regulation in murine offspring as a novel mechanism for transmaternal asthma protection induced by microbes. J. Allergy Clin. Immunol. 2011;128:618–25.
Conrad ML, Ferstl R, Teich R, Brand S, Blümer N, Yildirim AO, et al. Maternal TLR signaling is required for prenatal asthma protection by the nonpathogenic microbe Acinetobacter lwoffii F78. J Exp Med. 2009;206:2869–77.
Braga M, Schiavone C, Di Gioacchino G, De Angelis I, Cavallucci E, Lazzarin F, et al. Environment and T regulatory cells in allergy. Sci. Total Environ. Elsevier B.V.; 2012;423:193–201.
Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, et al. Extrathymically generated regulatory T cells control mucosal Th2 inflammation. Nature. 2012;482:395–9.
Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–41.
Arnold IC, Dehzad N, Reuter S, Martin H, Becher B, Taube C, et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest. 2011;121:3088–93.
Hill DA, Siracusa MC, Abt MC, Kim BS, Kobuley D, Kubo M, et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nat Med. 2012;18:538–46.
Chu KH, Chiang BL. Regulatory T cells induced by mucosal B cells alleviate allergic airway hypersensitivity. Am J Respir Cell Mol Biol. 2012;46:651–9.
Russell SL, Finlay BB. The impact of gut microbes in allergic diseases. Curr Opin Gastroenterol. 2012;28:563–9.
Steinmeyer S, Lee K, Jayaraman A, Alaniz RC. Microbiota metabolite regulation of host immune homeostasis: a mechanistic missing link. Curr Allergy Asthma Rep. 2015;15:24.
Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–50.
Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–73. One of the first studies to show that bacteria-derived SCFAs are important in the regulation of colonic Foxp3+IL-10–producing Treg cell activity and proliferation. The authors used the SCFA propionate to further show that these SCFA-mediated effects depend on normal Ffar2 expression
Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, DeRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–5.
Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17:662–71.
Vuillermin PJ, Ponsonby AL, Saffery R, Tang ML, Ellis JA, Sly P, et al. Microbial exposure, interferon gamma gene demethylation in naïve T-cells, and the risk of allergic disease. Allergy Eur J Allergy Clin Immunol. 2009;64:348–53.
Suarez-Alvarez B, Rodriguez RM, Fraga MF, López-Larrea C. DNA methylation: a promising landscape for immune system-related diseases. Trends Genet. 2012;28:506–14.
Schaub B, Liu J, Höppler S, Schleich I, Huehn J, Olek S, et al. Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J Allergy Clin Immunol. 2009;123:774–82.
Gerhold K, Avagyan A, Seib C, Frei R, Steinle J, Ahrens B, et al. Prenatal initiation of endotoxin airway exposure prevents subsequent allergen-induced sensitization and airway inflammation in mice. J. Allergy Clin. Immunol. 2006;118:666–73.
Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bonnelykke K, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357:1487–95.
Korppi M. Bacterial infections and pediatric asthma. Immunol Allergy Clin N Am. 2010;30:565–74.
Earl CS, An S, Ryan RP. The changing face of asthma and its relation with microbes. Trends Microbiol. 2015;23:408–18. Recent review focusing primarily on the influences of airway microorganisms on the development of specific subtypes of asthma. The authors also include a nice summary table of the findings of studies since 2011 looking at the effects of pre- and probiotics as well as other supplements on asthma and asthma-related symptoms
Green BJ, Wiriyachaiporn S, Grainge C, Rogers GB, Kehagia V, Lau R, et al. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS One. 2014;9:4–10.
Tomosada Y, Chiba E, Zelaya H, Takahashi T, Tsukida K, Kitazawa H, et al. Nasally administered Lactobacillus rhamnosus strains differentially modulate respiratory antiviral immune responses and induce protection against respiratory syncytial virus infection. BMC Immunol. 2013;14:40.
Kloepfer KM, Lee WM, Pappas TE, Kang TJ, Vrtis RF, Evans MD, et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J. Allergy Clin. Immunol. 2014;133:1301–7.
Holt PG. The mechanism or mechanisms driving atopic asthma initiation: the infant respiratory microbiome moves to center stage. J Allergy Clin Immunol. 2015;136:15–22. Detailed review of the role of early life viral respiratory tract infections in the development of the pathological features of asthma, and how this relationship is further complicated by the influences of pathogenic bacteria in the airway and the airway microbiome
Forsythe P. Probiotics and lung diseases. Chest. 2011;139:901–8.
Gill N, Wlodarska M, Finlay BB. The future of mucosal immunology: studying an integrated system-wide organ. Nat Immunol. 2010;11:558–60.
Noverr MC, Huffnagle GB. Does the microbiota regulate immune responses outside the gut? Trends Microbiol. 2004;12:562–8.
Arrieta M-C, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. The intestinal microbiome in early life: health and disease. Front Immunol. 2014;5:1–18.
Gollwitzer ES, Saglani S, Trompette A, Yadava K, Sherburn R, McCoy KD, et al. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat Med. 2014;20:642–7.
Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5.
Huang YJ, Nelson CE, Brodie EL, Desantis TZ, Baek MS, Liu J, et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J. Allergy Clin. Immunol. 2011;127:372–81.
Park H, Shin JW, Park S-G, Kim W. Microbial communities in the upper respiratory tract of patients with asthma and chronic obstructive pulmonary disease. PLoS One. 2014;9.
Marri PR, Stern DA, Wright AL, Billheimer D, Martinez FD. Asthma-associated differences in microbial composition of induced sputum. J Allergy Clin Immunol. 2013;131:346–52.
Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704–15.
Wilson MT, Hamilos DL. The nasal and sinus microbiome in health and disease. Curr Allergy Asthma Rep. 2014;14:485.
Fujimura KE, Lynch SV. Microbiota in allergy and asthma and the emerging relationship with the gut microbiome. Cell Host Microbe. 2015;17:592–602. Extensive and detailed review of the literature to date implicating the gut and lung microbiota in the development of asthma and other allergic diseases. The authors discuss the mechanisms involved, as well as the factors capable of influencing these relationships
Asher I, Pearce N. Global burden of asthma among children. Int J Tuberc Lung Dis. 2014;18:1269–78.
Jackson KD, Howie LD, Akinbami LJ. Trends in allergic conditions among children : United States, 1997–2011. NCHS Br. 2013;1–8.
Royce D. Knowledge translation opportunities in allergic disease and asthma. Allergy, asthma. Clin Immunol. 2010;6:A2.
Lougheed DM, Lemiere C, Dell SD, Ducharme FM, FitzGerald MJ, Leigh R, et al. Canadian Thoracic Society Asthma Management Continuum - 2010 Consensus Summary for children six years of age and over, and adults. Can Respir J. 2010;17:15–24.
Lougheed MD, Garvey N, Chapman KR, Cicutto L, Dales R, Day AG, et al. The Ontario asthma regional variation study: emergency department visit rates and the relation to hospitalization rates. Chest. 2006;129:909–17.
Sanders ME. Probiotics: definition, sources, selection, and uses. Clin Infect Dis. 2008;46:S58–61.
Osborn D, Sinn J. Prebiotics in infants for prevention of allergy ( Review ). Cochrane Database Syst Rev. 2013;CD006474.
Forsythe P, Inman MD, Bienenstock J. Oral treatment with live Lactobacillus reuteri inhibits the allergic airway response in mice. Am J Respir Crit Care Med. 2007;175:561–9.
Lyons A, O’Mahony D, O’Brien F, MacSharry J, Sheil B, Ceddia M, et al. Bacterial strain-specific induction of Foxp3+ T regulatory cells is protective in murine allergy models. Clin Exp Allergy. 2010;40:811–9.
Blumer N, Sel S, Virna S, Patrascan C, Zimmermann S, Herz U, et al. Perinatal maternal application of Lactobacillus rhamnosus GG supresses allergic airway inflammation in mouse offsrping. Clin Exp Allergy. 2007;37:348–57.
Forsberg A, Abrahamsson TR, Björkstén B, Jenmalm MC. Pre- and post-natal Lactobacillus reuteri supplementation decreases allergen responsiveness in infancy. Clin Exp Allergy. 2013;43:434–42.
Cuello-Garcia, CA, Brożek, JL, Fiocchi, A, Pawankar, R, Yepes-Nuñez, JJ, Terracciano, L, et al. Probiotics for the prevention of allergy: A systematic review and meta-analysis of randomized controlled trials. J. Allergy Clin. Immunol. 2015;1–10.Recent most comprehensive systematic review and meta-analysis of randomized controlled trials investigating the efficacy of probiotics in the prevention of allergic diseases published to date. The authors found that while the use of probiotics in pregnancy and/or infancy was associated with protection against infant eczema, no protective effects were found for the use of current probiotics against the development of asthma.
Fiocchi A, Pawankar R, Cuello-Garcia C, Ahn K, Al-Hammadi S, Agarwal A, et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): probiotics. World Allergy Organ J. 2015;8:4.
Prescott SL, Bjorksten B. Probiotics for the prevention or treatment of allergic diseases. J Allergy Clin Immunol. 2007;120:255–62.
Jenmalm MC, Duchén K. Timing of allergy-preventive and immunomodulatory dietary interventions - are prenatal, perinatal or postnatal strategies optimal? Clin Exp Allergy. 2013;43:273–8.
Stiehm M, Peters K, Wiesmüller KH, Bufe A, Peters M. A novel synthetic lipopeptide is allergy-protective by the induction of LPS-tolerance. Clin Exp Allergy. 2013;43:785–97.
Moncayo AL, Vaca M, Oviedo G, Erazo S, Quinzo I, Fiaccone RL, et al. Risk factors for atopic and non-atopic asthma in a rural area of Ecuador. Thorax. 2010;65:409–16.
Hevia A, Milani C, López P, Donado CD, Cuervo A, González S, et al. Allergic patients with long-term asthma display low levels of Bifidobacterium adolescentis. PLoS One. 2016;11.
Acknowledgments
The authors would like to thank Dr. Lisa Reynolds, Kylynda Bauer, and Dr. Marie-Claire Arrieta for their critical review of the manuscript and thoughtful insights.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Rozlyn C.T. Boutin declares that she has no conflict of interest.
Dr. B. Brett Finlay declares that he has no conflict of interest.
Rozlyn C.T. Boutin was supported by a Vancouver Coastal Health-CIHR-UBC MD/PhD Studentship Award during the writing of this review.
Dr. Finlay is the UBC Peter Wall Distinguished Professor and CIFAR Senior Fellow. The Finlay Lab is supported by operating grants from the Canadian Institute for Health Research (CIHR), AllerGen, and CIFAR-HMB.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Allergic Asthma
Rights and permissions
About this article
Cite this article
Boutin, R.C.T., Finlay, B.B. Microbiota-Mediated Immunomodulation and Asthma: Current and Future Perspectives. Curr Treat Options Allergy 3, 292–309 (2016). https://doi.org/10.1007/s40521-016-0087-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40521-016-0087-z