Abstract
Objectives
To conduct a comprehensive systematic review and meta-analysis to explore the correlation between migraine and the risk of dementia.
Methods
The PubMed, EMBASE, and Cochrane library databases were searched systematically. We selected cohort studies (prospective and retrospective) and case–control studies that reported migraine in patients with dementia, including vascular dementia. The pooled effects were analyzed to evaluate relative risk with 95% confidence intervals.
Results
In total, nine studies (two case–control and seven cohort studies) including 291,549 individuals were identified. These studies indicated that people with migraine (relative risk = 1.33; 95% confidence interval: 1.16–1.53) have an increased risk of all-cause dementia. Additionally, the pooled results of four studies showed that migraine is associated with an increased risk of vascular dementia (relative risk = 1.85; 95% confidence interval: 1.22–2.81; P = 0.004).
Conclusions
Data from observational studies suggest that migraine may be a risk factor for dementia, particularly vascular dementia. More studies are warranted to explore the association between migraine and dementia and the potential common pathophysiological mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migraine is a chronic primary paroxysmal headache, which is mainly characterized by symptoms related to the nervous system, and is accompanied by gastrointestinal and autonomic symptoms [1]. According to a 2016 study, approximately 1 billion people worldwide experience migraine every year [2], and the World Health Organization considers migraine as one of the most disabling illnesses [3]. The prevalence peak is reached between the ages of 30 and 39 years, while the lowest prevalence occurs in individuals over 60 years of age [4]. Migraine affects an individual’s work efficiency and thus negatively affects the economy [5, 6]. Dementia is a common cognitive disorder in the elderly, with a high disability rate [7]. Its etiology includes primary neurological diseases, neuropsychiatric diseases, and other diseases [8]. In the process of disease development, slow progressive memory loss and cognitive impairment may occur, resulting in the inability to carry out personal daily activities and loss of social skills [8, 9]. Importantly, the risk of dementia doubles every year after an individual reaches 65 years of age [10]. Aging, genetic characteristics, and systemic vascular disease are the main risk factors for dementia [11].
Alzheimer’s disease (AD) is the most common neurodegenerative cause of dementia in middle-aged or older individuals. Among individuals 65 years of age and older, the prevalence of AD is 5–6%, and among people over 85 years of age, the incidence is as high as 30% [12]. Cerebrovascular diseases of different etiologies are common causes of cognitive impairment. Vascular dementia (VaD) may be the second leading cause of dementia after AD. It is defined as a vascular cognitive impairment leading to the loss of daily function [8, 13, 14]. VaD has some common risk factors with cerebrovascular diseases, such as hypertension, diabetes, hypercholesterolemia, and coronary heart disease, among others [15,16,17,18].
Although the pathogenesis of migraine and dementia is not well understood, several potential mechanisms have been identified, including vascular disease, increased amyloid plaque formation, and structural cerebral changes concerning overlapping pain and memory networks [19]. In particular, some studies have found that migraine patients with poor vascular conditions have a higher risk of ischemic stroke [20]. In addition, many studies have shown that migraine and cognitive impairment have a number of common risk factors, such as clinically silent brain lesions, white matter abnormalities, subclinical infarct-like lesions, and volumetric cerebral changes [21, 22]. Therefore, in this meta-analysis, we aimed to study the relationship between migraine and the incidence rate of dementia.
Materials and methods
Methods and search strategy
The present meta-analysis was conducted in accordance with the criteria reported by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses group. The study did not require ethics committee approval because of its non-experimental design and search strategy. PubMed, EMBASE, and Cochrane Library databases were systematically searched by two independent reviewers (WJ and GHL) for eligible studies published from the inception of these databases to May 1, 2021 (Supplemental Table 1). The accepted language of publication was restricted to English. The reference list of eligible studies was reviewed to identify additional articles. After excluding clearly irrelevant articles, the authors examined the full text of the selected articles and determined the exact list of literature to be included in the present meta-analysis. The authors resolved disagreements by means of discussion.
Assessment of eligibility
A study was eligible if it fulfilled the following criteria: (1) the exposure of interest, migraine, was reported with a clear definition of the diagnostic criteria; (2) the endpoint of interest was the incidence of dementia, regardless of the type; and (3) the relative risk (RR), hazard ratio (HR), or odds ratio (OR) and the corresponding 95% confidence interval (CI) of total or specific dementia related to the experience of migraine were reported or could be calculated from the data provided. If a further classification of dementia was included in a final screened study, data on the RR and 95% CI were collected. We excluded studies in which the exposure of interest was not migraine but other types of headache or the endpoint of interest was not dementia. We also excluded studies that investigated the relationship between migraine and the risk of dementia but did not report the required data or in which the risk of dementia could not be calculated from the data provided. Duplicate studies were removed. Reviews, case reports, and meta-analyses were also excluded. Two reviewers independently performed the study selection.
Data extraction
Data extraction was performed independently by two investigators. The following information was extracted from the included studies: the first author, publication year, country, follow-up year, population age range, number of cases, study design, RR or adjusted RR (if possible) with 95% CI, type of dementia, and adjusted factors. Discrepancies were resolved between the two investigators, if necessary, by discussion with a third investigator.
Quality assessment
Quality assessment of all included studies was performed according to the Newcastle–Ottawa scale, which is a validated scale for the assessment of non-randomized studies in meta-analyses. Scores ranged from 0 to 9 for cohort studies, with higher scores indicating studies of a higher quality. Studies with a score of 6 or higher were defined as high-quality studies. Studies that scored between 0 and 5 were regarded as low quality. Two reviewers independently evaluated the quality of the included studies.
Statistical analyses
Stata software version 12.0 (StataCorp LLC., College Station, TX, USA) was used to conduct all statistical analyses. The OR of the case–control study was close to the RR of the cohort study, while the incidence of dementia was relatively low [23]; therefore, we also considered the HR value as the RR value. We weighted logarithmic RRs to obtain a pooled measure of the RRs. The RR with 95% CI was calculated as the effect size of dementia. Cochran’s Q test and the I2 statistic were used to evaluate the heterogeneity across studies. P < 0.1 or I2 > 50% indicates significant heterogeneity. If there was heterogeneity in the data, we used the random effect model and conducted subgroup analysis according to the type of study and sample size to explore the potential source of heterogeneity; otherwise, we used the fixed effect model. Sensitivity analysis was also conducted to test the stability of the results by removing one study at a time. Publication bias was tested by Begg’s rank test and Egger’s regression.
Results
Search results and study characteristics
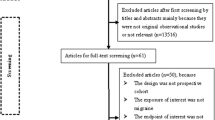
We initially retrieved 2551 articles from PubMed, 13,593 from Embase, and 8871 from Cochrane Library (Fig. 1). After excluding 3001 duplicates, the titles and abstracts of 22,014 citations were screened, of which 21,969 were excluded mainly because they did not comprise original observational studies or were not relevant to migraine and dementia. After a full-text review of the remaining 45 articles, 36 were excluded for various reasons.
A final total of nine eligible studies were included in the present meta-analysis [9, 24,25,26,27,28,29,30,31]. Of these, two [26, 28] were case–control, four [24, 25, 29, 30] prospective, and three [9, 27, 31] retrospective studies. The sample sizes of the studies were as follows: three [25, 26, 28] included > 30,000, three [9, 24, 31] included between 10,000 and 30,000, and three [27, 29, 30] included < 10,000 participants. Only one article [26] classified migraine as with or without aura. Four articles [9, 25, 29, 31] divided the exposure factors of dementia into all-cause dementia and VaD. Of the nine references that were included, only eight were graded because the remaining one was a conference abstract. Most included studies had a quality score > 6 according to the Newcastle–Ottawa Scale, indicating good quality in general. The main basic characteristics and quality assessment of the included studies are summarized in Supplemental Table 2.
Migraine and the risk of all-cause dementia
The nine included studies assessed the association between any type of migraine and the risk of all-cause dementia. The results of a random effects model that incorporated all-cause risk estimates associated with migraine are shown (Fig. 2). Of the nine studies that reported an association between migraine and the risk of dementia, seven reported a significant association of interest, while two suggested no significant association. Overall, a history of migraine was associated with an increased risk of all-cause dementia (RR = 1.33; 95% CI: 1.16–1.53; P < 0.001); however, there was considerable heterogeneity (I2 = 82.1%; P < 0.001).
Subgroup analysis based on study type
Foremost, we performed a subgroup analysis according to the study type, and the results showed that case–control (RR = 1.20; 95% CI: 1.11–1.28; P < 0.001; I2 = 89.7%) and retrospective cohort designs were significant (RR = 1.40; 95% CI: 1.29–1.52; P < 0.001; I2 = 79.5%), while other prospective study types were not significant (RR = 1.11, 95% CI: 0.99–1.25; P = 0.07; I2 = 74.0%). However, after subgroup analysis, the heterogeneity in each group remained considerable (Fig. 3).
Subgroup analysis based on sample size
A subgroup analysis was conducted according to the sample size (< 10,000; 10,000–30,000; and > 30,000), and the results showed minimal change in heterogeneity (Fig. 4). Subgroup analyses based on sample size were conducted with the purpose of exploring population changes. All the groups were effectual, although the heterogeneity in each group remained significant.
Sensitivity analysis
We performed a sensitivity analysis by removing one study at a time, with the aim of assessing the influence of each included study on the overall results. We observed that none of the individual studies had an evident impact on the pooled effect size (Fig. 5). Therefore, the results of the present meta-analysis are reliable and stable.
Publication bias
A funnel plot indicating the association between migraine and dementia is shown in Fig. 6. In general, no obvious publication bias was observed in our meta-analysis. Considering the small number of studies that were included and subjective differences in funnel plots, we conducted another study using Begg’s rank test and Egger’s regression test. The results showed that there was no obvious publication bias in this meta-analysis based on Begg’s (P = 0.243) and Egger’s (P = 0.379) tests.
Migraine and the risk of VaD
In this meta-analysis, two studies investigated the association between migraine and the risk of AD, and four investigated the association between migraine and the risk of VaD. Since only two AD studies were included, we merged the effect values of VaD (Fig. 7). Pooled results showed that migraine was associated with an increased risk of VaD (RR = 1.85; 95% CI: 1.22–2.81; P = 0.004; I2 = 0%).
Discussion
Our meta-analysis of nine studies (cohort and case–control studies) involving 291,549 participants showed that compared with individuals who have never experienced migraine, those who did experience migraine had a significantly increased risk for dementia (RR = 1.33; 95% CI: 1.16–1.53; P < 0.001). All nine studies were adjusted for sex, and eight were adjusted for age. Unfortunately, the original data of Pavlovic et al.’s study were not available. Nevertheless, we were able to determine that migraine was associated with an increased risk of VaD. These conclusions are consistent with the conclusions of the large prospective cohort studies that were included in this study [25, 29].
There are several common mechanisms between the pathogenesis of migraine and dementia, suggesting that migraine may increase the risk of dementia. First, the most accepted mechanism is that vascular risk factors such as hypertension, diabetes, and stroke are risk factors for dementia, including VaD [32, 33]. These vascular risk factors are strongly interlinked with migraine; they can lead to migraine, which can also lead to cardiovascular and cerebrovascular diseases [34, 35]. Second, white matter hyperintensities are more prevalent in people with migraine, particularly those experiencing migraine with aura, than in the general population. This is relevant because white matter hyperintensities are also associated with an increased risk of dementia [20, 36]. Third, depression and mood disorders may also be linked to migraine and dementia. Several studies have reported that migraine is associated with an increased risk of depression or other mood disorders. Additionally, depression and stress are associated with an increased risk of dementia [37, 38], and these factors influence the occurrence and progression of dementia [39]. Lastly, in terms of brain imaging, there are similar underlying pathophysiological mechanisms involved in migraine and dementia, such as inflammation or reduced blood flow to the brain [40, 41].
Migraine has been reported as a risk factor for stroke [42]. Stroke is an important risk factor for VaD and also a diagnostic criterion for VaD [40, 43]. Therefore, we cannot rule out the potential mediating role of stroke in the association between migraine and VaD. Despite the seemingly reasonable explanation, several studies have demonstrated that there is no correlation between migraine and VaD [27, 31, 44, 45], and whether stroke is just an intermediate variable remains to be determined. Studies have shown that genetic factors may also be related to the cognitive impairment caused by migraine. Previous studies involving chromosomes 1 and 19, as well as presenilin-1 mutations associated with familial AD, suggest a link between migraine and dementia [46, 47].
Although in our study we did not analyze the relationship between migraine and AD because of insufficient available data, previous studies have reported conflicting conclusions: one large prospective cohort study indicated that migraine is a risk factor for AD [29], whereas several other studies have reported no such association [25, 48, 49]. For VaD, an association with migraine was consistent with some [25] but not all previous reports [31, 45]. Study design as well as population size and age range may contribute to these contradictory conclusions. Diagnostic criteria for exposures and outcomes may also affect whether a significant relationship between migraine and dementia is found. In addition, the significant association with dementia may reflect bias because individuals with migraine may be more likely to seek medical care and thus more likely to be diagnosed as having dementia. Wang et.al recently published a meta-analysis suggesting that migraine is associated with an increased risk of all-cause dementia [50], the research methods and conclusions of our two research groups are somewhat different. First, this published study included cohort studies only, and we have more case–control studies. Despite the inclusion of case–control studies, our pooled results analysis indicated that the results were reliable and there was no significant publication bias. Second, they included a smaller number of literature, they concluded that there was no association between migraine and VaD when combined effect values. However, we included four articles and reached the opposite conclusion. Due to the low heterogeneity, we believe that the result is relatively reliable. The debate on the possible causal relationship between migraine and AD or VaD will continue in the future. Unlike a previous meta-analysis on headache and dementia [19], we also included case–control studies and divided cohort studies into prospective and retrospective studies. The results of case–control and retrospective cohort analyses were credible, while those of the prospective study type were not significant. Considering that the number of reported studies with related outcomes was small, we suggest that the result was inconclusive; thus, more prospective studies are warranted. A cohort study would provide stronger evidence than a case–control study, while a retrospective cohort design may have more confounding factors and biases than a prospective design. Therefore, more prospective studies are warranted to confirm the link between migraine and dementia.
Our study is meaningful because it provides further evidence of the correlation between migraine and dementia, including VaD. In the future, our findings can be utilized to better understand the common pathophysiological mechanisms of migraine and dementia, which will help determine the type of treatment that may mitigate the risk of dementia.
Limitations
Our meta-analysis has some limitations. First, several included studies were case–control studies, which are prone to recall bias. The patients’ headache recall may be inaccurate and may lead to an incorrect diagnosis of migraine. Second, heterogeneity persisted in our study. Third, migraine and dementia require a long follow-up period; however, due to the characteristics of the included studies, some patients had no follow-up, and some studies did not clearly provide a specific follow-up duration. Therefore, we could not infer a temporal relationship between the onset of migraine and diagnosis of dementia from these studies.
In conclusion, our results suggest that migraine is associated with an increased risk of all-cause dementia and VaD. This brief review suggests that patients with migraine may develop dementia and VaD. Given that many migraine patients have common risk factors with dementia, physicians must be vigilant and effectively intervene in high-risk groups to prevent the development of these risk factors where possible.
References
Silberstein SD (2004) Migraine. Lancet 363:381–391. https://doi.org/10.1016/s0140-6736(04)15440-8
GBD (2018) Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 17:954–976. https://doi.org/10.1016/s1474-4422(18)30322-3
WHO (2001) World Health Report. Available from www.who.int/whr/index.htm
Lipton RB, Bigal ME, Diamond M et al (2007) Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 68:343–349. https://doi.org/10.1212/01.wnl.0000252808.97649.21
Burton WN, Landy SH, Downs KE et al (2009) The impact of migraine and the effect of migraine treatment on workplace productivity in the United States and suggestions for future research. Mayo Clin Proc 84:436–445. https://doi.org/10.1016/s0025-6196(11)60562-4
Gilligan AM, Foster SA, Sainski-Nguyen A et al (2018) Direct and indirect costs among United States commercially insured employees with migraine. J Occup Environ Med 60:1120–1127. https://doi.org/10.1097/jom.0000000000001450
Livingston G, Sommerlad A, Orgeta V et al (2017) Dementia prevention, intervention, and care. Lancet 390:2673–2734. https://doi.org/10.1016/s0140-6736(17)31363-6
Gale SA, Acar D, Daffner KR (2018) Dementia. Am J Med 131:1161–1169. https://doi.org/10.1016/j.amjmed.2018.01.022
Chuang CS, Lin CL, Lin MC et al (2013) Migraine and risk of dementia: a nationwide retrospective cohort study. Neuroepidemiology 41:139–145. https://doi.org/10.1159/000353559
Jorm AF, Jolley D (1998) The incidence of dementia: a meta-analysis. Neurology 51:728–733. https://doi.org/10.1212/wnl.51.3.728
Baumgart M, Snyder HM, Carrillo MC et al (2015) Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement 11:718–726. https://doi.org/10.1016/j.jalz.2015.05.016
Hebert LE, Weuve J, Scherr PA et al (2013) Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 80:1778–1783. https://doi.org/10.1212/WNL.0b013e31828726f5
Rockwood K, Wentzel C, Hachinski V et al (2000) Prevalence and outcomes of vascular cognitive impairment Vascular cognitive impairment investigators of the Canadian Study of Health and Aging. Neurology 54:447–451. https://doi.org/10.1212/wnl.54.2.447
O’Brien JT, Erkinjuntti T, Reisberg B et al (2003) Vascular cognitive impairment. Lancet Neurol 2:89–98. https://doi.org/10.1016/s1474-4422(03)00305-3
Shah NS, Vidal JS, Masaki K et al (2012) Midlife blood pressure, plasma β-amyloid, and the risk for Alzheimer disease: the Honolulu Asia Aging Study. Hypertension 59:780–786. https://doi.org/10.1161/hypertensionaha.111.178962
Knopman DS, Penman AD, Catellier DJ et al (2011) Vascular risk factors and longitudinal changes on brain MRI: the ARIC study. Neurology 76:1879–1885. https://doi.org/10.1212/WNL.0b013e31821d753f
Kuller LH, Lopez OL, Jagust WJ et al (2005) Determinants of vascular dementia in the Cardiovascular Health Cognition Study. Neurology 64:1548–1552. https://doi.org/10.1212/01.wnl.0000160115.55756.de
Alonso A, Arenas de Larriva AP (2016) Atrial Fibrillation, cognitive decline and dementia. Eur Cardiol 11:49–53. https://doi.org/10.15420/ecr.2016:13:2
Wang J, Xu W, Sun S et al (2018) Headache disorder and the risk of dementia: a systematic review and meta-analysis of cohort studies. J Headache Pain 19:95. https://doi.org/10.1186/s10194-018-0925-4
Etminan M, Takkouche B, Isorna FC et al (2005) Risk of ischaemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ 330:63. https://doi.org/10.1136/bmj.38302.504063.8F
Bashir A, Lipton RB, Ashina S et al (2013) Migraine and structural changes in the brain: a systematic review and meta-analysis. Neurology 81:1260–1268. https://doi.org/10.1212/WNL.0b013e3182a6cb32
Kruit MC, van Buchem MA, Hofman PA et al (2004) Migraine as a risk factor for subclinical brain lesions. JAMA 291:427–434. https://doi.org/10.1001/jama.291.4.427
Greenland S (1987) Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 9:1–30. https://doi.org/10.1093/oxfordjournals.epirev.a036298
George KM, Folsom AR, Sharrett AR et al (2020) Migraine headache and risk of dementia in the atherosclerosis risk in communities neurocognitive study. Headache 60:946–953. https://doi.org/10.1111/head.13794
Hagen K, Stordal E, Linde M et al (2014) Headache as a risk factor for dementia: a prospective population-based study. Cephalalgia 34:327–335. https://doi.org/10.1177/0333102413513181
Islamoska S, Hansen Å, Wang HX et al (2020) Mid- to late-life migraine diagnoses and risk of dementia: a national register-based follow-up study. J Headache Pain 21:98. https://doi.org/10.1186/s10194-020-01166-7
Kostev K, Bohlken J, Jacob L (2019) Association between migraine headaches and dementia in more than 7,400 patients followed in general practices in the United Kingdom. J Alzheimers Dis 71:353–360. https://doi.org/10.3233/jad-190581
Lee SY, Lim JS, Oh DJ et al (2019) Increased risk of neurodegenerative dementia in women with migraines: a nested case-control study using a national sample cohort. Medicine (Baltimore) 98:e14467. https://doi.org/10.1097/md.0000000000014467
Morton RE, St John PD, Tyas SL (2019) Migraine and the risk of all-cause dementia, Alzheimer’s disease, and vascular dementia: a prospective cohort study in community-dwelling older adults. Int J Geriatr Psychiatry 34:1667–1676. https://doi.org/10.1177/0961203318801672
Pavlovic J, Mowrey W, Hall CB et al (2013) Dementia outcomes in elderly with self-reported history of migraine. Cephalalgia 33:139–140. https://doi.org/10.1177/0333102413490487
Tzeng NS, Chung CH, Lin FH et al (2017) Headaches and risk of dementia. Am J Med Sci 353:197–206. https://doi.org/10.1016/j.amjms.2016.12.014
Hébert R, Lindsay J, Verreault R et al (2000) Vascular dementia : incidence and risk factors in the Canadian study of health and aging. Stroke 31:1487–1493. https://doi.org/10.1161/01.str.31.7.1487
Patterson C, Feightner J, Garcia A et al (2007) General risk factors for dementia: a systematic evidence review. Alzheimers Dement 3:341–347. https://doi.org/10.1016/j.jalz.2007.07.001
Bigal ME, Kurth T, Santanello N et al (2010) Migraine and cardiovascular disease: a population-based study. Neurology 74:628–635. https://doi.org/10.1212/WNL.0b013e3181d0cc8b
Adelborg K, Szépligeti SK, Holland-Bill L et al (2018) Migraine and risk of cardiovascular diseases: Danish population based matched cohort study. BMJ 360:k96. https://doi.org/10.1136/bmj.k96
Debette S, Schilling S, Duperron MG et al (2019) Clinical significance of magnetic resonance imaging markers of vascular brain injury: a systematic review and meta-analysis. JAMA Neurol 76:81–94. https://doi.org/10.1001/jamaneurol.2018.3122
Chen YC, Tang CH, Ng K et al (2012) Comorbidity profiles of chronic migraine sufferers in a national database in Taiwan. J Headache Pain 13:311–319. https://doi.org/10.1007/s10194-012-0447-4
Jensen R (2003) Diagnosis, epidemiology, and impact of tension-type headache. Curr Pain Headache Rep 7:455–459. https://doi.org/10.1007/s11916-003-0061-x
Hatch DJ, Schwartz S, Norton MC (2015) Depression and antidepressant use moderate association between widowhood and Alzheimer’s disease. Int J Geriatr Psychiatry 30:292–299. https://doi.org/10.1002/gps.4140
Hamed SA, Hamed EA, Ezz Eldin AM et al (2010) Vascular risk factors, endothelial function, and carotid thickness in patients with migraine: relationship to atherosclerosis. J Stroke Cerebrovasc Dis 19:92–103. https://doi.org/10.1016/j.jstrokecerebrovasdis.2009.04.007
Scher AI, Gudmundsson LS, Sigurdsson S et al (2009) Migraine headache in middle age and late-life brain infarcts. JAMA 301:2563–2570. https://doi.org/10.1001/jama.2009.932
Hachinski VC, Iliff LD, Zilhka E et al (1975) Cerebral blood flow in dementia. Arch Neurol 32:632–637. https://doi.org/10.1001/archneur.1975.00490510088009
Kurth T, Schürks M, Logroscino G et al (2009) Migraine frequency and risk of cardiovascular disease in women. Neurology 73:581–588. https://doi.org/10.1212/WNL.0b013e3181ab2c20
Stræte Røttereng AK, Bosnes O, Stordal E et al (2015) Headache as a predictor for dementia: the HUNT Study. J Headache Pain 16:89. https://doi.org/10.1186/s10194-015-0573-x
Morton RE, St John PD, Tyas SL (2019) Migraine and the risk of all-cause dementia. Alzheimer’s disease, and vascular dementia: a prospective cohort study in community-dwelling older adults. Int J Geriatr Psychiatry 34:1667–1676. https://doi.org/10.1002/gps.5180
Breslau N, Rasmussen BK (2001) The impact of migraine: Epidemiology, risk factors, and co-morbidities. Neurology 56:S4–S12. https://doi.org/10.1212/wnl.56.suppl_1.s4
Ringman JM, Romano JD, Medina LD et al (2008) Increased prevalence of significant recurrent headache in preclinical familial Alzheimer’s disease mutation carriers. Dement Geriatr Cogn Disord 25:380–384. https://doi.org/10.1159/000121986
French LR, Schuman LM, Mortimer JA et al (1985) A case-control study of dementia of the Alzheimer type. Am J Epidemiol 121:414–421. https://doi.org/10.1093/oxfordjournals.aje.a114013
No authors listed (1994) The Canadian Study of Health and Aging: risk factors for Alzheimer’s disease in Canada. Neurology 44:2073–2080. https://doi.org/10.1212/wnl.44.11.2073
Wang L, Wu JC, Wang FY et al (2022) Meta-analysis of association between migraine and risk of dementia. Acta Neurol Scand 145:87–93. https://doi.org/10.1111/ane.13528
Funding
This work was supported by a grant from the National Natural Science Foundation of China to Ming Dong (Grant No. 31872772).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics approval
The study did not require ethics committee approval, because of its non-experimental design and search strategy.
Statement of human and animal rights
This review does not contain any experiments involving human participants or animals performed by any authors.
Informed consent
For this review, informed consent forms were not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiang, W., Liang, GH., Li, JA. et al. Migraine and the risk of dementia: a meta-analysis and systematic review. Aging Clin Exp Res 34, 1237–1246 (2022). https://doi.org/10.1007/s40520-021-02065-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-021-02065-w