Abstract
Background
The development of atrial fibrillation (AF) during the course of acute coronary syndrome (ACS) is related to poor prognosis. Possible predictors of new-onset AF (NOAF) have not been adequately investigated in elderly patients with ACS undergoing percutaneous coronary intervention (PCI). We aimed to identify the factors associated with NOAF in such patients.
Methods
A total of 308 elderly patients with ACS undergoing PCI were enrolled in the study. Patients were divided into two groups: without NOAF [254 patients, 64.6% men, age: 73.5 (69.0–79.0) years] and with NOAF [54 patients, 70.4% men, age: 75.0 (68.7–81.2) years]. Clinical, angiographic, and laboratory features including neutrophil-to-lymphocyte ratio (NLR) and monocyte-to-high-density lipoprotein ratio (MHR) were compared between the groups.
Results
The percentages of prior myocardial infarction (MI) (20.4 vs. 5.9%) and Killip III/ IV (24.1 vs. 7.1%), NLR [4.5 (2.6–7.2) vs. 3.2 (2.0–6.0)], and MHR [19.4 (15.7–26.5) vs. 12.9 (9.9–18.5)] were higher in patients with NOAF compared to the others (p = 0.020, < 0.001, 0.030, and < 0.001, respectively). In multivariate regression analysis, prior MI (OR 4.509, 95% CI 1.679–12.106, p = 0.003) and MHR (OR 1.102, 95% CI 1.054–1.152, p < 0.001) independently predicted NOAF. In addition, Killip III/IV was found to be an independent predictor of 6-month overall mortality (HR 2.949, 95% CI 1.218–7.136, p = 0.016).
Conclusions
Prior MI and MHR are independent predictors of NOAF in elderly patients with ACS undergoing PCI. Killip III/IV predicts 6-month overall mortality in such patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute coronary syndrome (ACS) is a major cause of death, and older patients with ACS have worse prognosis than younger patients [1, 2]. Despite their high morbidities and complications, percutaneous coronary intervention (PCI) is still beneficial in elderly patients with ACS [3]. Atrial fibrillation (AF) often complicates ACS and the incidence of new-onset AF (NOAF) after ACS ranges from 2.3 to 37% [4,5,6]. Preexisting AF accounts for approximately one-third of all cases of AF observed in patients with acute myocardial infarction (MI), and NOAF for the remaining two-thirds [7]. Patients with acute MI and AF have a higher incidence of reinfarction, cardiogenic shock, heart failure, and asystole [4,5,6]. For these reasons, early identification of individuals at high risk for the development of AF is crucial to prevent complications related to it.
Older age has been found to be an independent predictor of the development of AF in patients with ACS [4,5,6]. In addition, higher Killip class, atrial ischemia, left atrial enlargement, and inflammation are other possible mechanisms of NOAF complicating ACS [4,5,6]. The neutrophil-to-lymphocyte ratio (NLR) and monocyte-to-high-density lipoprotein (HDL) ratio (MHR) have been suggested as indicators of inflammation and oxidative stress, and found to be independent predictors of adverse cardiac events in patients with ACS [8, 9].
Possible predictors of NOAF have not been adequately investigated in elderly patients with ACS undergoing PCI. The aim of the study was to investigate predictors of NOAF in elderly patients with ACS undergoing PCI.
Methods
Study population
Between June 2016 and April 2017, a total of 321 patients older than 65 who underwent primary PCI for ST-segment elevation MI (STEMI) or underwent urgent PCI for non-ST elevation ACS (NSTE-ACS) were enrolled in this prospective study. Patients with end-stage renal disease [estimated glomerular filtration rate (eGFR) < 15 mL/min/1.73 m2] or receiving long-term dialysis (n = 6), patients who died during or early after the procedure (n = 3), patients with acute or chronic infection or inflammatory conditions (n = 1), patients who had an absence of data on serum creatinine during the 72 h after the procedure (n = 3) were excluded. A total of 308 patients were included in this study. Demographic, clinical variables, and medications used after admission were recorded. The diagnosis of STEMI was based on the clinical characteristics, electrocardiographic (ECG) changes consisting of persistent ST-elevation of at least 0.1 mV in at least two contiguous leads or a left bundle branch block with subsequent Q-wave formation, and biochemical markers of cardiac necrosis [creatinine kinase-MB isoenzyme (CK-MB) or troponin-T] [1]. The diagnosis of NSTE-ACS was based on the clinical characteristics, transient ST depression (≥ 0.05 mV) or T-wave inversion (≥ 0.1 mV), and a troponin-T value above the 99th percentile of upper limit of normal range [2]. Previous history of coronary artery disease (CAD) was defined as a definitive history of MI or coronary obstruction ≥ 50% on coronary angiography (CAG) [10]. Contrast-induced nephropathy (CIN) was defined as the elevation of serum creatinine > 0.5 mg/dl or 25% versus baseline serum creatinine within 72 h after PCI [11]. The study was approved by the Ethics Committee of Eskisehir Osmangazi University, Faculty of Medicine, and was conducted in accordance with the principles of the Declaration of Helsinki. All patients were informed about the nature of the study and a written informed consent was obtained.
Blood analysis, electrocardiography, and echocardiography
Venous blood samples were drawn from antecubital veins immediately after the ECG recordings. The NLR, MHR, and mean platelet volume (MPV) were obtained using the same blood samples obtained before PCI. Baseline serum creatinine was measured at admission (before PCI), and on days 1, 2, and 3 after the procedure. The eGFR was calculated using the modification of diet in renal disease formula [12]. The infarct size was estimated by peak CK-MB and peak troponin-T values, defined as the highest serum concentrations within the first 48 h. Transthoracic echocardiography was performed using an echocardiography system (Acuson Sequoia C256, Mountain View, CA, USA) equipped with a broadband transducer (3V2c) for all patients within the first 48 h of hospitalization. Two-dimensional echocardiographic measurements were performed according to the guideline recommendations [13]. The Simpson’s method was used to evaluate left ventricular ejection fraction (LV EF).
Angiographic definitions
All patients underwent CAG preceded by an intracoronary injection of 100 µg of nitroglycerine. All procedures were performed through the femoral or radial approach by experienced interventional cardiologists. Nonionic, low-osmolar contrast medium (iohexol, Omnipaque 350 mg/mL; GE Healthcare, Cork, Ireland) was used. All the patients were treated with a P2Y12 antagonist (clopidogrel 600 mg, ticagrelor 180 mg, or prasugrel 60 mg), and dual antiplatelet therapy (aspirin 100 mg/day and clopidogrel 75 mg/day or ticagrelor 180 mg/day or prasugrel 10 mg/day) continued for at least 12 months. All PCI procedures were performed using unfractionated heparin (100 U/kg). Tirofiban therapy was initiated in the angiography laboratory during PCI in a bailout situation or for thrombotic complications (downstream therapy). Stent thrombosis was defined as the presence of ACS with angiographic evidence of thrombus or occlusion. Target lesion revascularization (TVR) was defined as any repeated PCI of the stented segment, including 5-mm proximal and distal margins [14]. The type of the stents [bare metal or drug-eluting stent] was left to the operator’s discretion. Balloon angioplasty alone was preferred for the patients who developed stent thrombosis (in-stent balloon angioplasty) or who had a vessel diameter of < 2.25 mm. The patients who had a failed coronary intervention or who had no suitable vessel for coronary revascularization were managed conservatively.
Arrhythmic events
The presence of AF was documented by a standard 12-lead ECG. All patients were followed by an inpatient cardiac telemetry (Nihon Kohden Central Monitor System, CNS-9701, Tokyo, Japan and Mortara Surveyor Central System, Milwaukee, WI, USA) for the development of AF during the hospital stay. New-onset AF was defined as the development of at least one episode of AF as assessed by ECG or telemetry during the index hospitalization. Nonsustained ventricular tachycardia (VT) was defined as self-limiting VT events lasting more than 16 consecutive beats with a rate of > 125 beat/min and a duration of 8–30 s. Sustained VT was defined as any VT event lasting more than 16 consecutive beats with a rate of > 125 beat/min and a duration of ≥ 30 s [15].
Clinical endpoints
We evaluated the relationship between NOAF and clinical outcomes during hospitalization, at the 1-month and 6-month follow-up. The MACE (major adverse cardiac event) was defined as cardiovascular death, cardiogenic shock, sudden cardiac death, stent thrombosis, recurrent MI, TVR, or stroke. Major bleeding was defined as intracranial hemorrhage, ≥ 5 g/dL decrease in hemoglobin concentration, or ≥ 15% absolute decrease in hematocrit. Minor bleeding was defined as a blood loss of 3–5 g/dL in the hemoglobin concentration or 10–15% decrease in hematocrit values, or no visible blood loss with ≥ 4 g/dL decrease in the hemoglobin concentration and ≥ 12% decrease in the hematocrit values [16].
Statistical analysis
Statistical analysis was performed using the SPSS version 16.0 software (SPSS Inc., Chicago, IL, USA). The categorical variables are expressed in frequencies and percentages. The Pearson’s Chi-square test and Fisher’s exact test were used to compare categorical variables. Continuous variables were tested for normal distribution by the Shapiro–Wilk test. Normally distributed variables were presented as mean ± SD and compared using independent samples t test. Abnormally distributed variables were expressed in median and interquartile ranges [25th–75th percentile levels], and Mann–Whitney U test was used to determine the significant differences among the groups. Multiple binary logistic regression analysis, Enter method was used to define the independent predictors of NOAF. The goodness-of-fit assumption was assessed using the Hosmer–Lemeshow method (Chi-square = 4.128, df = 8, p = 0.845). Receiver operating characteristic (ROC) curve was generated to define the cut-off value of MHR for NOAF and area under the curve (AUC) was calculated. Time-dependent Cox-proportional hazards regression model was used to examine the independent predictors of 6-month overall mortality. A p value of < 0.05 was considered statistically significant.
Results
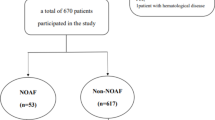
A total of 308 elderly patients were enrolled in the study [204 were males (66.2%) and mean age was 74.4 ± 6.5 (range 65–90)]. Of these patients, 204 (66.2%) had STEMI and NOAF was present in 54 (17.5%). The MACEs were observed in 69 patients (22.4%) and 48 (15.5%) died during follow-up.
Clinical, laboratory, and angiographic features of the groups are presented in Table 1. Patients with NOAF were more likely to have prior MI, Killip III/ IV, inotropic support, triple vessel disease, and CTO compared with patients without NOAF. The LV EF, eGFR, HDL-cholesterol, and lymphocyte count were lower in the NOAF group. Contrast volume, white blood cell count, peak CK-MB, peak troponin-T, NLR, MHR, and MPV were higher in patients with NOAF.
Table 2 presents arrhythmic events and clinical endpoints. Contrast-induced nephropathy, MACE at 1-month follow-up, MACE at 6-month follow-up, overall mortality at 1-month follow-up and overall mortality at 6-month follow-up were more prevalent in patients with NOAF. Rates of cardiogenic shock and recurrent MI at 6-month follow-up were higher in such patients. Rates of arrhythmic events and bleeding were similar between the groups.
In the multiple logistic regression analysis, prior MI and MHR were independent predictors for NOAF (Table 3). The ROC curve analysis showed that the best cut-off point of MHR was 15.87 to identify patients with NOAF (sensitivity: 75.9%, specificity: 65.0%, AUC 0.750, 95% CI 0.698–0.798, p < 0.001) (Fig. 1).
Receiver operating characteristic curve analysis suggested that the optimal MHR level cut-off point for patients with NOAF was 15.87 with a sensitivity and specificity of 75.9 and 65.0%, respectively (AUC 0.750; 95% confidence interval 0.698–0.798). MHR monocyte-to-high-density lipoprotein ratio, NOAF new-onset atrial fibrillation
In the Cox-proportional hazards modeling, Killip III/IV (HR 2.949, 95% CI 1.218–7.136, p = 0.016) predicted 6-month overall mortality (Table 4).
Discussion
The present study showed that prior MI and MHR were independent predictors of the development of AF in elderly patients with ACS undergoing PCI. Using a cut point of > 15.87, MHR predicted NOAF with a sensitivity of 75.9% and specificity of 65.0%. When a cut-off point of 4.41 was used, NLR predicted NOAF with a sensitivity of 53.7% and specificity of 65.0%. We found that Killip III/IV was an independent predictor of 6-month overall mortality in such patients.
Despite advanced treatment options, adverse cardiovascular events, such as NOAF, can occur during the course of ACS and it is related to poor prognosis [4,5,6]. Older age was found to be a risk factor for NOAF in patients with ACS [4,5,6]. In our elderly population, the rate of patients who developed AF at the course of ACS was 17.5%. For this reason, NOAF during the course of ACS is very important in elderly population.
We found that NOAF was associated with increased incidence of adverse events during hospitalization, 1-month and 6-month follow-up in elderly patients with ACS. This finding emphasizes the prognostic significance of NOAF in such patients. However, NOAF was not independent predictor for overall mortality in our study, perhaps associated with confounding factors. Killip III/IV was the only independent predictor for overall mortality in the present study.
Prior MI was an independent predictor of NOAF in elderly patients with ACS in the study. The number of patients with higher Killip class, complex coronary anatomy, and CIN were higher in elderly patients with NOAF in the present study. The LV EF and eGFR were lower, and biomarkers of myocardial damage were higher in such patients. These findings are consistent with the literature [4,5,6, 17,18,19]. However, they were not independent predictors for NOAF. In our study, white blood cell count at admission was higher, and HDL was lower in the NOAF group. These results may be related to extensive inflammation in patients with ACS who developed AF [20]. Interestingly, contrast media volume was higher in patients with NOAF in the study. These findings can be explained by the more complex coronary anatomy in such patients. Previous studies have also demonstrated VT, VF, and complete atrioventricular block are more prevalent in patients with NOAF [17, 18]. We have not found a similar result and this could be due to the low-number of patients with NOAF in our study.
Inflammation plays a substantial role in atherosclerotic plaque rupture [21] and it has also an important role in the onset of AF complicating ACS [4, 22]. Some biomarkers have been studied for prediction of NOAF in patients with ACS. The NLR has been found to be an independent predictor of NOAF in patients with STEMI [23, 24]. Circulating monocytes account for the major source of various proinflammatory and prooxidant factors [25, 26]. On the other hand, HDL-cholesterol has well known anti-inflammatory, antioxidant, and antithrombotic effects [27]. The MHR has been suggested as a novel vascular inflammatory marker and it has been shown to predict NOAF in patients with STEMI [22]. The MPV is an important biomarker of platelet activity and it has a prognostic value in patients with ACS treated with PCI [28]. Karatas et al. have shown an association between MPV and NOAF in patients with STEMI [23].
However, such biomarkers have not been adequately investigated in elderly patients with ACS undergoing PCI. We found that pre-procedural MHR was an independent predictor for NOAF in such patients. This result indicates the importance of inflammation on NOAF in the course ACS. As an inflammatory marker, the MHR has the advantages of being able to be obtained before the procedure and to work in most centers. Pre-procedural MHR measurement may help to early identify elderly patients with ACS having the highest risk of NOAF. In our study, NLR and MPV were significantly higher in the NOAF group, but they were not independent predictors for NOAF.
Limitations
Our study is a single-center study. The number of elderly patients with NOAF was small, which could limit the number of independent predictors identified. Unfortunately, our study lacks information about CRP. Pre-procedural CRP has been demonstrated to be a risk factor for NOAF in patients undergoing PCI, but we were not able to study pre-procedural CRP levels of the patients.
Conclusion
Prior MI and MHR are predictors of NOAF in elderly patients with ACS undergoing PCI. Given the poor prognosis of NOAF, early identification of such patients by means of the above-mentioned variables may help to prevent poor outcomes. However, our results should be further confirmed in multicenter, larger studies.
References
O’Gara PT, Kushner FG, Ascheim DD et al (2013) 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 61:e78–e140
Amsterdam EA, Wenger NK, Brindis RG et al (2014) 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 64:e139–e228
Vandecasteele EH, De Buyzere M, Gevaert S et al (2013) Reperfusion therapy and mortality in octogenarian STEMI patients: results from the Belgian STEMI registry. Clin Res Cardiol 102:837–845
Wang J, Yang YM, Zhu J (2015) Mechanisms of new-onset atrial fibrillation complicating acute coronary syndrome. Herz 40:18–26
Schmitt J, Duray G, Gersh BJ et al (2009) Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. Eur Heart J 30:1038–1045
Gorenek B, Blomström Lundqvist C, Brugada Terradellas J et al (2014) Cardiac arrhythmias in acute coronary syndromes: position paper from the joint EHRA, ACCA, and EAPCI task force. Europace 16:1655–1673
Jabre P, Jouven X, Adnet F et al (2011) Atrial fibrillation and death after myocardial infarction: a community study. Circulation 123:2094–2100
Kaya MG, Akpek M, Lam YY et al (2013) Prognostic value of neutrophil/lymphocyte ratio in patients with ST-elevated myocardial infarction undergoing primary coronary intervention: a prospective, multicenter study. Int J Cardiol 168:1154–1159
Cetin MS, Ozcan Cetin EH, Kalender E et al (2016) Monocyte to HDL cholesterol ratio predicts coronary artery disease severity and future major cardiovascular adverse events in acute coronary syndrome. Heart Lung Circ 25:1077–1086
Kurtul A, Yarlioglues M, Duran M (2017) Predictive value of CHA2DS2–VASC score for contrast-induced nephropathy after percutaneous coronary intervention for acute coronary syndrome. Am J Cardiol 119:819–825
Mehran R, Nikolsky E (2006) Contrast-induced nephropathy: definition, epidemiology, and patients at risk. Kidney Int Suppl 100:S11-S15
Levey AS, Bosch JP, Lewis JB et al (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130:461–470
Lang RM, Badano LP, Mor-Avi V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28:1–39
Mauri L, Hsieh WH, Massaro JM et al (2007) Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med 356:1020–1029
Ruwald AC, Bloch Thomsen PE, Gang U et al (2013) New-onset atrial fibrillation predicts malignant arrhythmias in post-myocardial infarction patients—a Cardiac Arrhythmias and Risk Stratification after acute Myocardial infarction (CARISMA) substudy. Am Heart J 166:855–863
Chesebro JH, Knatterud G, Roberts R et al (1987) Thrombolysis in Myocardial Infarction (TIMI) Trial, phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation 76:142–154
Mrdovic I, Savic L, Krljanac G et al (2012) Incidence, predictors, and 30-day outcomes of new-onset atrial fibrillation after primary percutaneous coronary intervention: insight into the RISK-PCI trial. Coron Artery Dis 23:1–8
Crenshaw BS, Ward SR, Granger CB et al (1997) Atrial fibrillation in the setting of acute myocardial infarction: the GUSTO-I experience. Global utilization of streptokinase and TPA for occluded coronary arteries. J Am Coll Cardiol 30:406–413
Raposeiras Roubín S, Abellas-Sequeiros RA, Abu Assi E et al (2015) Relation of contrast induced nephropathy to new onset atrial fibrillation in acute coronary syndrome. Am J Cardiol 115:587–591
Annoura M, Ogawa M, Kumagai K et al (1999) Cholesterol paradox in patients with paroxysmal atrial fibrillation. Cardiology 92:21–27
van der Wal AC, Becker AE, van der Loos CM et al (1994) Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation 89:36–44
Guo Y, Lip GY, Apostolakis S (2012) Inflammation in atrial fibrillation. J Am Coll Cardiol 60:2263–2270
Karataş MB, Çanga Y, İpek G et al (2016) Association of admission serum laboratory parameters with new-onset atrial fibrillation after a primary percutaneous coronary intervention. Coron Artery Dis 27:128–134
Chavarria N, Wong C, Hussain H et al (2015) Persistent elevation of neutrophil/lymphocyte ratio associated with new onset atrial fibrillation following percutaneous coronary intervention for acute ST segment elevation myocardial infarction. J Ayub Med Coll Abbottabad 27:441–447
Mestas J, Ley K (2008) Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med 18:228–232
Woollard KJ, Geissmann F (2010) Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol 7:77–86
Murphy AJ, Woollard KJ (2010) High-density lipoprotein: a potent inhibitor of inflammation. Clin Exp Pharmacol Physiol 37:710–718
Wasilewski J, Desperak P, Hawranek M et al (2016) Prognostic implications of mean platelet volume on short- and long-term outcomes among patients with non-ST-segment elevation myocardial infarction treated with percutaneous coronary intervention: a single-center large observational study. Platelets 27:452–458
Acknowledgements
We are grateful for the assistances from Mehmet Eren Altınbaş, Özlem Karabulut, Nurcan Göküz and Sinem Tekin.
Funding
No financial support from any organization was used in the preparation of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Ethical approval
The study was approved by the Ethics Committee of Eskisehir Osmangazi University, Faculty of Medicine. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
All patients were informed about the nature of the study and a written informed consent was obtained.
Rights and permissions
About this article
Cite this article
Ulus, T., Isgandarov, K., Yilmaz, A.S. et al. Predictors of new-onset atrial fibrillation in elderly patients with acute coronary syndrome undergoing percutaneous coronary intervention. Aging Clin Exp Res 30, 1475–1482 (2018). https://doi.org/10.1007/s40520-018-0926-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-018-0926-9