Abstract
Background and aim
Dietary habits have been associated with the prevalence of the sarcopenia and limited data are available in this field for menopausal women. This study focused on the relationship between dietary patterns and prevalence of the sarcopenia in menopausal women.
Methods
This cross-sectional study was done in 250 menopausal women 45 years old or older. Dietary data were collected using a food-frequency questionnaire and physical activity was assessed by International Physical Activity Questionnaire (IPAQ). Height, weight, skeletal muscle mass, hand grip, and gait speed were measured and sarcopenia was defined based on European Working Group on Sarcopenia in Older People (EWGSOP) guidelines.
Results
Using factor analysis, two major dietary patterns were found: a Western pattern (high in commercial beverage, sugar and dessert, snacks, solid fat, potato, high fat dairy, legume, organ meat, fast food, and sweets) and a Mediterranean pattern (high in olive, low-fat dairy, vegetable, fish, nut, and vegetable oil). After adjusting for confounding variables, for the highest vs the lowest tertiles, the Odds Ratio (OR) for sarcopenia was 1.06 [95% confidence interval (CI), 0.47–2.37] in the Western pattern and 0.40 [95% confidence interval (CI), 0.17–0.89] in the Mediterranean pattern.
Conclusions
Our findings suggest that Mediterranean dietary pattern has a favorable role in the prevention of sarcopenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For the first time, the term “sarcopenia” was created by Rosenberg [1]. Nowadays, the concept of sarcopenia implies the loss of muscle mass while the physical function is impaired with advancing age [2]. Sarcopenia is associated with a number of health consequences including physical disability, falls, fractures, and death [3–5].
Sarcopenia can be caused by several factors, such as nutritional status, physical activity, hormonal changes, insulin resistance, genetic heritability, and pro-inflammatory cytokines [6–8]. Menopause has been linked to a decline in production of ovarian hormones [9], which leads to a reduction in lean mass that is independent of the aging process [10, 11]. Estimated prevalence of sarcopenia in menopausal women varies from 10 to 40% depending on the method of diagnosis used and the reference population [12].
Lifestyle changes such as diet can be appropriate strategies for the prevention of sarcopenia [13]. The relationships between sarcopenia and diet were usually assessed using nutrients and single foods, but because of the complexity of dietary intake and the probable interactions of various nutrients, studies that examine dietary patterns could be more effective than the traditional analysis of single nutrients and foods consumption.
The aims of this observational cross-sectional study were to estimate the prevalence of sarcopenia among menopausal population and to identify major dietary patterns and their association with sarcopenia in this specific population.
Methods
This cross-sectional study was performed on 250 menopausal women 45 years old or older from August to December 2015 in Shariati Hospital in Tehran. Individuals in this study were randomly sampled from the general population. The exclusion criteria were as follows: people with artificial limbs, liver, heart or kidney failure, Chronic Obstructive Pulmonary Disorder (COPD), cancer, and diabetes. The medications taken by each participant was recorded. The study was approved by the research council and ethics committee of the Tehran University of Medical Sciences (code: IR.TUMS.REC.1395.2285) and written informed consent was obtained from all participants before their inclusion in the study.
Interviews and Questionnaire
At first, we recorded their medical history, drug history, family history, alcohol consumption, and smoking habits using an organized questionnaire. Subsequently, to assess dietary intake, a validated food frequency questionnaire (FFQ) of 147 items (dish-based) was completed [14]. Participants were questioned about their dietary intake over the 1 year prior to the interview. It consists of a food list with standard serving size and participants were asked to estimate their frequency of consumption of each food item on a daily, weekly, monthly or yearly basis. Data from the questionnaire were then converted to grams (g/day). Then energy and nutrient content of foods was calculated with the U.S. Department of Agriculture (USDA) food composition table (FCT) included in the Nutritionist 4 software (First Databank Division, The Hearst Corporation, San Bruno, CA, USA). However, for some traditional Iranian food item, such as traditional breads and kashk (Iranian dairy product), that are not included in Nutritionist 4, the Iranian FCT was used [15].
Finally, International Physical Activity Questionnaire (IPAQ) was used to assess the Physical activity. Following IPAQ’s guidelines [16], frequency and duration of physical activity were converted to Metabolic Equivalent of Tasks (MET).
Anthropometry
Height and weight were measured using standard equipment, while the participants were wearing light clothes and without shoes. Height was measured to the nearest 1 centimeter (cm) and body weight to the nearest 0.1 kg (kg).
Definition of sarcopenia
To identify sarcopenia, we used the EWGSOP (European Working Group on Sarcopenia in Older People) criteria [2]. The EWGSOP suggests using the presence of both muscle function (performance and strength) and muscle mass for the diagnosis of sarcopenia.
According to the EWGSOP definition, participants who only have low muscle mass were classified as presarcopenia; those who have low muscle mass plus low muscle strength, or low physical performance were diagnosed as sarcopenia and eventually severe sarcopenia was identified by low muscle mass, low muscle strength, and low physical performance.
Muscle mass assessment
Muscle mass was estimated by Bioelectrical impedance analysis (BIA) resistance. BIA resistance (ohms) was measured using a BIA machine (Tanita BC-418 manufactured by Tanita Corporation, Tokyo, Japan) with an operating frequency of 50 kHz at 500 mA. The electric current was supplied from the electrodes on the tips of toes and fingers. Subjects in light clothing stood on the weighing platform with bare feet without bending their knees. Persons touched the electrodes with placing the forepart of the feet in contact with the anterior electrodes and heels on the posterior electrodes while they grasped the grips with both hands.
When the measurements were done, absolute muscle mass was calculated using the formula developed by Janssen and colleagues [17]:
Skeletal muscle mass (kg) = ([height2/BIA resistance × 0.401] + [gender × 3.825] + [age × −0.071] + 5.102).
The height is in centimetres; the BIA resistance is in ohms; for gender, sex = 1 for men and 0 for women; and age is in years.
The muscle mass index was calculated by the following equation: skeletal muscle mass (kg) divided by the square of height (m2). Using the cut-off points proposed in the EWGSOP consensus, women are classified as having normal SMI (≥6.76 kg/m2), moderate low SMI (5.76–6.75 kg/m2), and severe low SMI (≤5.75 kg/m2). These cut-off points were obtained among 2,276 elderly (at least 60 years old) women from the 3rd National Health and Nutrition Examination Survey (NHANES III) [18, 19].
Physical performance assessment
Usual walking speed on a 4-m course was evaluated measuring subjects’ gait speed (meter/second). The participants whose speed was lower than or equal to 0.8 m/s classified as of low physical performance. This cut-off point was obtained among 1,030 persons (age range between 20 and 102 years) from the InCHIANTI study [20]. In this test, we asked participants to gait without assistive devices such as a cane, crutch, and walker.
Muscle strength assessment
Muscle strength was assessed by handgrip strength, which was measured with a squeeze bulb Vigorimeter (Rolyan c7489-02). Three trials for each hand were performed and the average measurements of both hands were used in the analysis. Using the cut-off points suggested for different sex–age groups in the Merkies et al. paper [21], we identified participants with low levels of muscle strength.
Statistical analysis
The food items listed in the dietary FFQ were grouped into 25 predefined food groups based on similarities in food type and nutrient contents (e.g., whole or refined grain and high- or low-fat dairy). Food groups were energy adjusted using the residual method, because over-reporting or under-reporting of food items may result in an increased irrelevant variation [22].
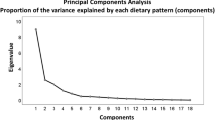
Exploratory factor analysis (principal component analysis) was conducted to reduce the food groups to a small number of underlying factors that could explain the maximum fraction of the variance. In this method, all variables are considered simultaneously, each one related to the others. Factor loadings, i.e. measurements of correlations between each variable and the factors, were analyzed after orthogonal varimax rotation method. The higher the factor loading of a food group, the greater the contribution of that group to the pattern (factor).
Factors with Eigen values equal to 1.5 or greater were extracted and then scree Plots were used to identify main dietary patterns. A Factor score obtained for each participant was calculated by summing the consumption of each food group that were weighted by factor loading, the higher score indicating consumption of more food groups associated with that respective pattern. Food groups with a factor loading greater than +0.4 were considered key components of each dietary pattern and were used to label patterns. The subjects were then categorized according to the tertiles of their pattern dietary scores.
Residents with sarcopenia were identified using the algorithm developed and suggested by the EWGSOP [2] for sarcopenia case finding and screening in practice. We used one-way analysis of variance for continuous variables and Chi square tests for categorical variables to identify significant differences across tertile categories of dietary pattern scores. To determine the association of dietary patterns with sarcopenia and abnormality of its components, we used multivariable logistic regression. In model 1, we adjusted age, physical activity menopause duration, body mass index (BMI), medical drug use, and history of diseases. The first tertile of dietary pattern scores was considered a reference. SPSS version 16 (SPSS, Inc., Chicago, IL, USA) was used for all analyses, and p < 0.05 was considered statistically significant.
Results
Table 1 presents the baseline characteristics based on the participants’ sarcopenia condition. The mean age of study participants was 57.6 years, and 55 women (22%) were identified as affected by sarcopenia. Women with sarcopenia showed a statistically significant lower skeletal muscle index compared with nonsarcopenic women (6.2 vs 7.0 kg/m2, p < .01, respectively). As well, compared with participants without sarcopenia, they were significantly lower in the hand grip test (61.2 vs 69 Kpa, p < .01, respectively) and slower in the 4-m walking test (0.97 vs 0.86 m/s, p < .01, respectively). Physical activity was lower in sarcopenic women, but this difference was not statistically significant (p = 0.51). Also, no significant difference was observed at the BMI, fat mass, and physical activity. None of the participants were smokers or alcohol drinkers.
Food items and factor loadings for food groups are shown in Table 2. Two food patterns emerged in our sample. The first factor was named Western pattern with high loadings for commercial beverage, sugar and dessert, snacks, solid fat, potato, high-fat dairy, legume, organ meat, fast food, and sweets. The second factor, labeled Mediterranean pattern, consisted of high loadings for olive, low-fat dairy, vegetable, fish, nut, and vegetable oil. Factor 1 explained 14.25% of the variance in intake and factor 2 explained 9.42% of the variance. Together, the two factors explained 23.67% of the variance.
General characteristics of participants across tertiles of the major dietary patterns are presented in Table 3. Mean weight and BMI were lower among women in the highest tertile of Mediterranean pattern as compared with those in the lowest tertile and mean age was highest among subjects in the highest tertile of the Mediterranean pattern. Significant differences were not observed in height, Physical activity, Drug history.
Average of gait speed is higher in the third tertile of the Mediterranean dietary pattern than the first tertile. However, skeletal muscle index and hand grip strength were not significantly different across tertile categories of this patterns. We also did not observe any clear difference in averages of components of sarcopenia between the different categories of the Western dietary pattern.
Table 4 reported Odds ratios (OR) and 95% confidence interval (CI) for abnormality of sarcopenia components across tertiles of major dietary pattern score. The Mediterranean dietary pattern was associated with lower odds of abnormal gait speed and abnormal hand grip strength. Those in the top tertile of the Mediterranean pattern had increased odds of abnormal muscle mass in a crude model. This association was not significant after taking the confounding variables into account. Western dietary patterns were not associated with abnormality of sarcopenia components.
Odds ratios (OR) and 95% confidence interval (CI) for sarcopenia across tertiles of major dietary pattern score are presented in Table 5. In the crude model, we did not find any significant odds ratio of sarcopenia in association with both dietary patterns. Nevertheless, a marginally significant trend in the protective association of the Mediterranean pattern and sarcopenia was found. When we adjusted for model 1 the association between Mediterranean pattern and sarcopenia became significant. Odds ratio for top tertile vs bottom tertile in model 1 was 1.06, 95% CI 0.47–2.37 (Western pattern) and 0.40 95% CI 0.17–0.89 (Mediterranean pattern).
Discussion
Two distinct dietary patterns in women participating in this study were identified, by using the factor analysis technique; these were labeled Mediterranean pattern and Western pattern. The Mediterranean pattern was inversely associated with sarcopenia, whereas no association was found with the Western pattern.
Notably, in our study, we observed the BMI was high in the sarcopenic group, which we knew was related to the high-fat mass in this group. For determining the low muscle mass in our population, we used the highest cut off in EWGSOP criteria, which makes it the high prevalence of sarcopenia in our participants and may explain the higher level of BMI in sarcopenic women.
We have found only three other published studies that have examined dietary patterns and sarcopenia [23–25]. In a study done on the United States urban population [25], ‘‘alcohol’’ pattern was associated with an increased likelihood of sarcopenia and ‘‘poultry’’ pattern had a protective effect on sarcopenia. Any ‘‘healthy’’ cluster was not found in this study. In agreement with our findings, Hashemi et al. found a protective association between Mediterranean dietary pattern and sarcopenia [23] that suggested that higher intakes of a Mediterranean pattern (olives, vegetables, tomatoes, whole grains, nuts, fish, fruits, and pickles) was associated with lower odds of sarcopenia among the Iranian elderly population (in this study, subjects were not categorized based on sex). In contrast, in a recent prospective study, Chan et al. [24] showed that none of the dietary patterns were associated with sarcopenia in the women group of their study.
We observed the protective association of Mediterranean dietary pattern and the risk of sarcopenia in the present study. The Mediterranean dietary pattern haves food groups rich in unsaturated fatty acids such as olive, fish, vegetable oil, and low in saturated fats, which reduce the systemic inflammation [26, 27], and it may decrease the risk of sarcopenia [28, 29].
One of the factors that might play an important role in triggering sarcopenia is the build-up of reactive oxygen species (ROS) [30]. The healthy diet that includes abundant fruit, vegetables, and nuts which provide antioxidants, vitamin C, and vitamin E, may, in turn, scavenge ROS, thereby preventing or attenuating muscle damage [31, 32]. Moreover, there has also been plausible evidence that intake of excess alkaline-producing foods such as fruits and vegetables may prevent acidosis and in consequence lead to preserve muscle mass and strength during aging [33–35]. Also, Mediterranean pattern in our study was associated with consumption of rich dietary sources of vitamin D such as fish and egg. Studies suggested that vitamin D intake may be important for the maintenance of skeletal muscle mass and muscle function in community-dwelling older adults [13, 36].
No significant association was found between the Western pattern and sarcopenia. Although the Western pattern identified in our study is not directly comparable with other studies, they share some similarities with them. We observed that lack of relationship between our Western pattern and sarcopenia is consistent with the Western pattern derived in Hashemi et al. study [23], which had high loadings for fast food, sweets, sugar, and hydrogenated fat.
We also compared the association of components of sarcopenia (muscle mass index, handgrip and gait speed) with dietary patterns by odds ratios for abnormality components of the sarcopenia and means of these components across tertiles of each dietary pattern. We did not find a significant association between Western dietary pattern and components of sarcopenia. But we observed an inverse relation between Mediterranean dietary pattern with gait speed and hand grip strength abnormality, whereas there was no significant relation between the mean of hand grip strength and this pattern. As well, the association between muscle mass and Mediterranean dietary pattern was not seen.
Recent study of Kelaiditi et al. [37], which examined the association between adherence of Mediterranean dietary pattern and skeletal muscle mass in women, concluded that this pattern only prevents from reduction of leg explosive power, but not loss of skeletal muscle and grip strength in women aged over 50 years. This study did not analyze the sarcopenic condition in subjects so that the relationship between Mediterranean pattern with sarcopenia is not determined.
The strengths of our study are that sarcopenia was defined based on EWGSOP criteria, we used from a validated FFQ, energy intake was controlled for in our analysis and to our knowledge, and this is the first study to examine the relation between dietary pattern and sarcopenia among menopausal women.
This study has several limitations: first, the study design was cross-sectional, which makes the inference of causality difficult. Second, due to budget constraints, we used from BIA machine for muscle mass estimation, while computed tomography (CT-scan), magnetic resonance imaging (MRI) and dual energy X-ray absorptiometry (DEXA) are more accurate than BIA [2]. Nevertheless, it considered a valid and reliable method, and commonly implemented in previous studies [38]. Moreover, the squeeze bulb vigorimeter was used instead of the more commonly used Jamar handheld dynamometer. Third, although FFQ is a standard instrument to assess long-term dietary intake, estimates of food consumption from an FFQ are not precise and there is always the possibility of measurement error [39]. Fourth, the limited number of participants may have caused reduced statistical power in multivariable analyses thus making it impossible to conduct a stratified analysis by age.
In conclusion, findings from this study show that Mediterranean pattern has a protective association for sarcopenia in menopausal women, but there is no association between Western pattern and sarcopenia.
References
Rosenberg IH (1997) Sarcopenia: origins and clinical relevance. J Nutr 127:990S–991S
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel J-P, Rolland Y, Schneider SM (2010) Sarcopenia: European consensus on definition and diagnosis Report of the European Working Group on Sarcopenia in Older People. Age Ageing 39:412–423
Cawthon PM, Blackwell TL, Cauley J, Kado DM, Barrett-Connor E, Lee CG, Hoffman AR, Nevitt M, Stefanick ML, Lane NE (2015) Evaluation of the usefulness of consensus definitions of sarcopenia in older men: results from the Observational Osteoporotic Fractures in Men Cohort Study. J Am Geriatr Soc 63:2247–2259
Woo J, Leung J, Morley J (2015) Defining sarcopenia in terms of incident adverse outcomes. J Am Med Dir Assoc 16:247–252
Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, Cederholm T, Coats AJS, Cummings SR, Evans WJ (2011) Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc 12:403–409
Bauer J, Sieber C (2008) Sarcopenia and frailty: a clinician’s controversial point of view. Exp Gerontol 43:674–678
Balagopal P, Proctor D, Sreekumaran Nair K (1997) Sarcopenia and hormonal changes. Endocr 7:57–60
Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS (2001) Sarcopenia. J Lab Clin Med 137:231–243
Douchi T, Yamamoto S, Yoshimitsu N, Andoh T, Matsuo T, Nagata Y (2002) Relative contribution of aging and menopause to changes in lean and fat mass in segmental regions. Maturitas 42:301–306
Aloia JF, McGowan DM, Vaswani AN, Ross P, Cohn SH (1991) Relationship of menopause to skeletal and muscle mass. Am J Clin Nutr 53:1378–1383
Douchi T, Yamamoto S, Nakamura S, Ijuin T, Oki T, Maruta K, Nagata Y (1998) The effect of menopause on regional and total body lean mass. Maturitas 29:247–252
Van Kan GA (2009) Epidemiology and consequences of sarcopenia. J Nutr Health Aging 13:708–712
Morley JE, Argiles JM, Evans WJ, Bhasin S, Cella D, Deutz NE, Doehner W, Fearon KC, Ferrucci L, Hellerstein MK (2010) Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc 11:391–396
Hosseini Esfahani F, Asghari G, Mirmiran P, Azizi F (2010) Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran Lipid and Glucose Study. J Epidemiol 20:150–158
Azar M, Sarkisian E (1980) Food composition table of Iran: National Nutrition and Food Research Institute. Shaheed Beheshti University, Tehran
Committee IR (2005) Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ)–short and long forms. Retrieved September 17:2008
Janssen I, Heymsfield SB, Baumgartner RN, Ross R (2000) Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol 89:465–471
Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R (2004) Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol 159:413–421
Janssen I, Heymsfield SB, Ross R (2002) Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 50:889–896
Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L (2003) Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol 95:1851–1860
Merkies I, Schmitz P, Samijn J, Van Der Meché F, Toyka K, Van Doorn P (2000) Assessing grip strength in healthy individuals and patients with immune-mediated polyneuropathies. Muscle Nerve 23:1393–1401
Willett W, Stampfer M (1998) Implications of total energy intake for epidemiologic analysis. Monogr Epidemiol Biostat 1:273–301
Hashemi R, Motlagh AD, Heshmat R, Esmaillzadeh A, Payab M, Yousefinia M, Siassi F, Pasalar P, Baygi F (2015) Diet and its relationship to sarcopenia in community dwelling Iranian elderly: a cross sectional study. Nutrition 31:97–104
Chan R, Leung J, Woo J (2016) A prospective cohort study to examine the association between dietary patterns and Sarcopenia in Chinese Community-Dwelling Older People in Hong Kong. J Am Med Dir Assoc 17:336–342
Fanelli Kuczmarski M, Mason MA, Beydoun MA, Allegro D, Zonderman AB, Evans MK (2013) Dietary patterns and sarcopenia in an urban African American and white population in the United States. J Nutr Gerontol Geriatr 32:291–316
Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC (2007) Dietary patterns and markers of systemic inflammation among Iranian women. J Nutr 137:992–998
Ticinesi A, Meschi T, Lauretani F, Felis G, Franchi F, Pedrolli C, Barichella M, Benati G, Di Nuzzo S, Ceda GP (2016) Nutrition and inflammation in older individuals: focus on vitamin D, n-3 polyunsaturated fatty acids and whey proteins. Nutrients 8:186
Schaap LA, Pluijm SM, Deeg DJ, Visser M (2006) Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med 119:526 e529–517
Jensen GL (2008) Inflammation: roles in aging and sarcopenia. J Parenter Enteral Nutr 32:656–659
Fulle S, Protasi F, Di Tano G, Pietrangelo T, Beltramin A, Boncompagni S, Vecchiet L, Fanò G (2004) The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp Gerontol 39:17–24
Kim J-S, Wilson JM, Lee S-R (2010) Dietary implications on mechanisms of sarcopenia: roles of protein, amino acids and antioxidants. J Nutr Biochem 21:1–13
Kim J, Lee Y, Kye S, Chung Y-S, Kim K-M (2015) Association of vegetables and fruits consumption with sarcopenia in older adults: the Fourth Korea National Health and Nutrition Examination Survey. Age Ageing 44:96–102
Chan R, Leung J, Woo J (2014) Association between estimated net endogenous acid production and subsequent decline in muscle mass over four years in ambulatory older Chinese people in Hong Kong: a prospective cohort study. J Gerontol Series A Biol Sci Med Sci 70:905–911
Mithal A, Bonjour J-P, Boonen S, Burckhardt P, Degens H, Fuleihan GEH, Josse R, Lips P, Torres JM, Rizzoli R (2013) Impact of nutrition on muscle mass, strength, and performance in older adults. Osteoporos Int 24:1555–1566
Dawson-Hughes B, Harris SS, Ceglia L (2008) Alkaline diets favor lean tissue mass in older adults. Am J Clin Nutr 87:662–665
Wintermeyer E, Ihle C, Ehnert S, Stöckle U, Ochs G, de Zwart P, Flesch I, Bahrs C, Nussler AK (2016) Crucial role of vitamin D in the musculoskeletal system. Nutrients 8:319
Kelaiditi E, Jennings A, Steves C, Skinner J, Cassidy A, MacGregor A, Welch A (2016) Measurements of skeletal muscle mass and power are positively related to a Mediterranean dietary pattern in women. Osteoporos Int 27:3251–3260
Cruz-Jentoft AJ, Landi F, Schneider SM, Zúñiga C, Arai H, Boirie Y, Chen L-K, Fielding RA, Martin FC, Michel J-P (2014) Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 43:748–759
Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE (1985) Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 122:51–65
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Funding
This study was supported by the Tehran University of Medical Sciences (Grants Id: 94-03-161-30350).
Ethical approval
The study was approved by the research council and ethics committee of the Tehran University of Medical Sciences (code: IR.TUMS.REC.1395.2285).
Informed consent
Written informed consent was obtained from all participants before their inclusion in the study.
Rights and permissions
About this article
Cite this article
Mohseni, R., Aliakbar, S., Abdollahi, A. et al. Relationship between major dietary patterns and sarcopenia among menopausal women. Aging Clin Exp Res 29, 1241–1248 (2017). https://doi.org/10.1007/s40520-016-0721-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-016-0721-4