Abstract
Aims
Anterior mesh rectopexy is a novel surgical technique for the treatment of complete rectal prolapse, a common disorder in female elderly patients. Aim of the study was to evaluate functional outcomes after ventral mesh rectopexy and conventional suture rectopexy.
Patients and methods
Forty patients have been enrolled in this prospective study. Patients were divided into two groups: 20 patients (group A) had a conventional suture rectopexy with a standard technique and 20 patients (group B) underwent an anterior mesh rectopexy. Each patient had a clinic and defecographic diagnosis of full-thickness rectal prolapse, which was further investigated with manometry and clinical questionnaires (Wexner Constipation and Incontinence Score, Rome III criteria). Postoperative outcomes were evaluated through clinical questionnaires, a rigid rectosigmoidoscopy and a defecography, 1 year after surgery.
Results
Preoperative Wexner constipation score was greater than 15 in all the patients (21 in group A and 22 in group B); median postoperative score was 15 in group A and 11 in group B, and the difference was significant. Median preoperative incontinence score was 11 in group A and 12 in group B; median postoperative score was 9 in group A and 6 in group B. Three patients experienced recurrence in group A and only 1 patient in group B.
Conclusion
Ventral mesh rectopexy is feasible, safe and effective for the treatment of full-thickness rectal prolapse in a well-fit geriatric population. Better functional results have been achieved compared with conventional suture technique with a trend toward a lower recurrence rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Rectal prolapse has a significant social impact, especially in elderly female patients. It is often associated with other pelvic floor disorders, and anorectal symptoms may be associated with urinary and genital dysfunctions. The prolapse is characterized by the intussusception of rectal wall, and it is mainly divided in two main entities: complete (or full-thickness) rectal prolapse and partial (or mucosal) rectal prolapse [1]. Complete rectal prolapse, on the other hand, may be internal or external.
Complete rectal prolapse is mainly associated with the outlet syndrome or ODS (obstructed defecation syndrome) [2]. Surgical therapy is usually indicated in case of persistent bleeding, solitary rectal ulcer, external prolapse, digitation or progressive incontinence [3]. Surgical techniques for the treatment of rectal prolapse include both perineal and abdominal procedures [4].
Aim of the present study is to investigate functional results after conventional suture rectopexy and a more recent “ventral mesh rectopexy,” first described by D’Hoore [5].
Methods
This is a single-institutional study. Forty female patients have been enrolled in the study period (2013–2015); median age was 68. All of them had a complete rectal prolapse. Six patients had a concomitant genital prolapse, and 4 of them had diverticular disease. Patients were divided into two groups: group A had a suture rectopexy (with or without resection) and group B had a more recent suspension technique, the ventral mesh rectopexy first described by D’Hoore. Subsequent patients were alternatively assigned to either group A or B. All the patients were considered fit for surgery with a predicted low surgical risk (ASA I–II).
Conventional technique was based on both anterior and posterior rectal mobilization with sections of lateral ligaments; one or two sutures were then used to fix the rectal wall to the sacral promontory. A sigmoid resection was associated in case of redundant sigmoid colon or concomitant diverticular disease.
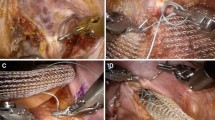
Ventral mesh rectopexy is based on the only anterior rectal mobilization in order to reduce the risk of nerve injuries; a 17-cm-long mesh is then positioned in the rectovaginal space and is fixed both at vagina and, proximally, at sacral promontory.
Preoperative diagnosis was achieved through clinical examination, rigid proctosigmoidoscopy, defecography examination and anal manometry.
Clinical questionnaires were also administered both preoperatively and postoperatively and included Rome III criteria, Wexner Incontinence score and Wexner constipation score. Postoperative evaluation also included a rigid rectosigmoidoscopy and a defecography and was performed 1 year after surgery.
Inclusion criteria were: obstructed defecation syndrome, persistent bleeding, full-thickness rectal prolapse and a squeeze pressure >60 mmHg.
Data were retrospectively analyzed.
Categorical data are presented as frequency counts and associated percentages; comparisons were made by means of Pearson’s Chi-square test. Continuous data are presented as medians and ranges and were compared by using the Wilcoxon rank sum test. A p value equal to or less than 0.05 was considered to be statistically significant. Statistical analyses were performed using STATA 12 statistical software.
Results
All the patients included were found to be constipated at Rome III criteria.
In group A, 9 patients received a sigmoid resection, cause of concomitant diverticular disease or redundant sigmoid colon. Group B patients underwent a ventral mesh rectopexy without resection.
Preoperative Wexner constipation score was >15 in all the patients (group A: median 21/group B: median 22). Median postoperative Wexner constipation score was 15 in group A and 11 in group B, and the difference between group was found to be significant (p < 0.05). In group A, 5 patients out of 20 still showed a constipation score above 15 postoperatively; that being said, 25% of patients still showed some degree of constipation after the procedure, while all the patients receiving the ventral rectopexy had their constipation solved (p < 0.05) (Table 1).
Regarding incontinence, in group A, median preoperative incontinence score was 11 and median postoperative score was 9; in group B, on the other hand, median preoperative Wexner incontinence score was 12, while postoperative assessment showed a median postoperative score of 6 (p < 0.05), and the difference was found to be significant (Table 1). More, while in group A, only 12 patients out of 20 showed an improvement in their continence score, all the patients receiving a ventral rectopexy (group B) showed a better continence function after surgery (p < 0.05).
Regarding recurrence, we had one patient in group A (5%), which showed a clinical and endoscopic recurrence one year after surgery; two more patients in the same group (10%) only showed an endoscopic mucosal recurrence not requiring any further treatment. In group B, we had only one mucosal recurrence assessed endoscopically one year after surgery.
Discussion
Functional outcomes and quality of life are nowadays considered key factors after colorectal surgery even in the setting of malignant diseases [6, 7]; this is particularly true, if we considered the lessons learned from the implementation and success of minimally invasive and laparoscopic surgery for the treatment of colorectal diseases [8–12]. Nevertheless, an improvement in quality of life and patient satisfaction are even more important and crucial aspects after surgery for benign and functional conditions.
Management of rectal prolapse may be really challenging, and a clear consensus among experts does not exist; more, no clear guidelines basically may be easily applied [13].
Clinical situations and degree of prolapse may be very heterogeneous. First-line therapy is usually based on conservative management, made of biofeedback and diet correction; most patients with internal or mucosal prolapse usually manage to deal with their symptoms with this non-operative management.
Aim of surgery is to correct anatomical alterations, mitigate symptoms (constipation, incontinence or obstructed defecation symptoms) and prevent urinary or sexual dysfunction. Surgery is mainly based on abdominal and perineal procedures. Abdominal procedures usually require a rectal suspensions (sutured or with a mesh) and may be associated with sigmoid resection. Perineal procedures aim to re-establish the function of the pelvic floor and may include mucosal or rectal resection from below [14].
Abdominal procedures (both sutured and mesh rectopexy) may be associated with sigmoidal resection that is sometimes performed in patients with constipation and redundant sigmoid colon, even if a clear benefit from sigmoidal resection has not been proved. Rectal mobilization may be completed, including the section of lateral ligaments, which is usually associated with postoperative constipation despite a lower recurrence rate. Suture rectopexy requires the fixation of the rectum to the sacral promontory with non-absorbable suture (usually one or two stitches); fixation will also depend on the fibrosis that will develop in the retro-rectal space between rectum and sacrum. Data on postoperative continence and constipation are quite variable [15, 16]. Recurrence rate has been described between 0–27%, and sometimes recurrence might be due to the disconnection of the stitches to the sacrum [17].
Ventral mesh rectopexy was first described by D’Hoore [18]; it is based on an inverted J-shaped pelvic peritoneal incision with only anterior rectal mobilization down to the recto-vaginal space, thus preventing lateral and posterior nerve damage. A 3 × 17 cm mesh is anchored down to the rectovaginal space (posterior vaginal fornix) and proximally to the sacral promontory; the peritoneal flap is then closed; and the mesh is hidden in the infra-peritoneal space.
The choice of the best operation to correct rectal prolapse depends on many factors: patients’ fitness for surgery, age, previous abdominal or perineal operations, history of pelvic radiation and concomitant cardiopulmonary diseases. Abdominal rectopexy is usually associated with a lower recurrence rate compared with perineal procedures; particularly, D’Hoore operation is associated with a 5% recurrence rate and this might also depends on the learning curve.
Various mechanisms may contribute to postoperative constipation; nerve injuries are one of the most advocated. In the ventral rectopexy, dissections are performed behind the Denonvillier fascia and risk of nerve damages is limited; more, there is no posterior or lateral dissection; thus, the risk of hypogastric or sacral nerves is virtually absent. The mesh is positioned to reinforce the rectovaginal septum, and this might help to correct the obstruction defecation symptoms and the rectocele, when associated. Another technical aspect is that the mesh is positioned only anteriorly and does not surround the rectum; this might prevent the postoperative risk of rectosigmoidal kinking, which is usually believed to be associated with postoperative constipation secondary to other mesh techniques.
Further randomized trial with a longer follow-up is necessary in order to establish if the ventral rectopexy might be considered as a gold standard treatment for the treatment of complete rectal prolapse.
Conclusion
In this study, ventral mesh rectopexy has demonstrated to be safe and feasible to treat complete rectal prolapse. Patients satisfaction was very good. Postoperative functional outcomes in terms of constipation and incontinence were significantly better compared with suture rectopexy with a trend toward a lower recurrence rate at one year follow-up.
References
Felt-Bersma RJ, Cuesta MA (2001) Rectal prolapse, rectal intussusception, rectocele, and solitary rectal ulcer syndrome. Gastroenterol Clin N Am 30:199–222
D’Hoore A, Vanbeckevoort D, Penninckx F (2008) Clinical, physiological and radiological assessment of rectovaginal septum reinforcement with mesh for complex rectocele. Br J Surg 95:1264–1272
Parks AG, Swash M, Urich H (1977) Sphincter denervation in anorectal incontinence and rectal prolapse. Gut 18:656–665
Azimuddin K, Khubchandani IT, Rosen L et al (2001) Rectal prolapse: a search for the best operation. Am Surg 67:622–627
D’Hoore A, Cadoni R, Penninckx F (2004) Long-term outcome of laparoscopic ventral rectopexy for total rectal prolapse. Br J Surg 91:1500–1505
Luglio G, Masone S, Quarto G et al (2013) Functional results after TME: J-pouch vs straight coloanal anastomosis and role of neoadjuvant radiochemotherapy. Ann Ital Chir 84:571–574
Giglio MC, Persico M, Quarto G et al (2013) Intersphinteric resection for rectal cancer: role in fecal continence and quality of life. Ann Ital Chir 84:287–290
De Palma GD, Luglio G (2015) Quality of life in rectal cancer surgery: what do the patient ask? World J Gastrointest Surg 7:349–355
West NP, Kennedy RH, Magro T et al (2014) Morphometric analysis and lymph node yield in laparoscopic complete mesocolic excision performed by supervised trainees. Br J Surg 101:1460–1467
Milone M, Elmore U, Di Salvo E et al (2015) Intracorporeal versus extracorporeal anastomosis. Results from a multicentre comparative study on 512 right-sided colorectal cancers. Surg Endosc 29:2314–2320
Luglio G, De Palma GD, Tarquini R et al (2015) Laparoscopic colorectal surgery in learning curve: role of implementation of a standardized technique and recovery protocol. A cohort study. Ann Med Surg (Lond) 4:89–94
Luglio G, Nelson H (2010) Laparoscopy for colon cancer: state of the art. Surg Oncol Clin N Am 19:777–791
Kim DS, Tsang CB, Wong WD et al (1999) Complete rectal prolapsed. Evolution of management and results. Dis Colon Rectum 42:460–468
Gourgiotis S, Baratsis S (2007) Rectal prolapse. Int J Dis 22:231–243
Slawik S, Soulsby R, Carter H et al (2008) Laparoscopic ventral rectopexy, posterior colporrhaphy and vaginal sacrocolpopexy for the treatment of recto-genital prolapse and mechanical outlet obstruction. Colorectal Dis 10:138–143
Portier G, Iovino F, Lazorthes F (2006) Surgery for rectal prolapse. Dis Colon Rectum 49:1136–1140
Luukkonen P, Mikkonen U, Järvinen H (1992) Abdominal rectopexy with sigmoidectomy vs. rectopexy alone for rectal prolapse: a prospective, randomized study. Int J Colorectal Dis 7:219–222
D’Hoore A, Penninckx F (2006) Laparoscopic ventral recto (colpo) pexy for rectal prolapse: surgical technique and outcome for 109 patients. Surg Endosc 20:1919–1923
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human and animal rights
This article does not contain any studies with animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Luglio, G., Tarquini, R., Giglio, M.C. et al. Ventral mesh rectopexy versus conventional suture technique: a single-institutional experience. Aging Clin Exp Res 29 (Suppl 1), 79–82 (2017). https://doi.org/10.1007/s40520-016-0672-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-016-0672-9