Abstract
Purpose
Anorexia nervosa (AN) is a poorly understood and often chronic condition. Deviations in the gut microbiota have been reported to influence the gut–brain axis in other disorders. Therefore, if present in AN, it may impact on symptoms and illness progression. A review of the gut microbiota studies in AN is presented.
Method
A literature search on PubMed yielded 27 articles; 14 were selected and based on relevance, 9 articles were included. The findings were interpreted in the larger context of preclinical research and clinical observations.
Results
8 out of 9 included studies analysed microbiota from faeces samples, while the last analysed a protein in plasma produced by the gut. Two studies were longitudinal and included an intervention (i.e., weight restoration), five were cross-sectional, one was a case report, and the last was a case series consisting of three cases. Deviations in abundance, diversity, and microbial composition of the faecal microbiota in AN were found.
Conclusion
There are currently only a few studies on the gut microbiota in AN, all done on faeces samples, and not all describe the microbiota at the species level extensively. The Archaeon Methanobrevibacter smithii was increased in participants with a BMI < 25 in one study and specifically in AN patients in three studies. Methanobrevibacter smithii may, if detected, be a benchmark biomarker for future studies. We propose that microbiota samples could also be collected from the small intestine, where a major exchange of nutrients takes place and where the microbiota may have a biological impact on AN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anorexia nervosa (AN) is a serious and often chronic psychiatric condition. The hallmark feature of AN is a reduction of energy intake relative to energy expenditure leading to low body weight. Potential life-threatening medical complications that affect almost every organ frequently occur contributing to AN having a high standardized mortality ratio of 5.2 (3.7–7.5) [1]. In addition, there are no effective treatments for AN and chronicity is high [2].
Elucidating biomarkers associated with AN could provide guidance for risk stratification, treatment and identify targets for developing novel pharmacological treatments as well as increasing disease understanding. Studies have begun to explore whether the gut microbiota and its associated microbiome might harbour trait biomarkers for AN.
Definitionally, “microbiota” refers to a community of microorganisms, including Bacteria, Viruses, Archaea, and Fungi, and in this review, we have focused on the gut Bacteria and Archaea in AN. The “microbiome” refers to the collective genomes of the present microorganisms [3]. More than 1000 ‘species-level’ phylotypes exist in a human [4]. The majority of these phylotypes are Bacteria, with Faecalibacterium prausnitzii, Roseburia intestinalis, and Bacteroides uniformis dominating in the adult microbiota found in faeces samples [5]. The phylotypes are mostly consistent across individuals, but the relative composition and diversity of organisms can vary markedly.
Gut microbiota not only play a critical role in the development of the gut mucosal immunity [6, 7], but also affect the regulation of the hypothalamic–pituitary–adrenal (HPA) axis [8], serotonergic neurotransmission [9], and signalling mechanisms affecting neuronal circuits involved in motor control and anxiety in mice [10]. This pathway has been described as the gut–brain axis [11]. The mechanism of this interaction is not fully elucidated, and there are as yet no dedicated studies to explore or intervene with this gut–brain axis in AN.
Given the long periods of starvation associated with the core psychopathology of AN, considerable adaptation in intestinal microbiota could occur in people with AN. Alternatively, specific intestinal dysbiosis could predispose to the drive toward negative energy balance in AN. Intestinal dysbiosis has previously been associated with psychological function and mental health including depression and anxiety, both of which are commonly comorbid with AN [12]. AN patients often present with comorbid anxiety (75% lifetime prevalence of anxiety disorder) [13] and depression (more than 34% lifetime prevalence of depression) [14, 15]. As such, the gut–brain axis is of particular interest in understanding the psychopathology of AN.
The intestinal microbiota is involved in both weight gain and weight loss as well as with energy extraction from the diet in both humans and animals [16, 17]. Differences in the composition of the intestinal microbiota between obese and lean individuals have been consistently described, potentially illustrating differences in energy extraction efficiency between obese and lean individuals [18, 19]. Furthermore, in an activity-based mouse model of AN Jésus et al. demonstrated increased permeability in the colon, i.e., “gut leakiness”, in anorexic mice, however the authors also found that the gut leakiness was more related to malnutrition than exercise [20]. In another study examining the role of exercise on gut permeability, Pals et al. found that exercise increases intestinal permeability measured with the lactulose and rhamnose differential urinary excretion test [21]. In contrast to this, a study by Monteleone et al. found reduced urinary recovery of lactulose in AN patients reflecting a reduced permeability in the small intestine, where breakdown and absorption of lactulose take place [22].
Changes in the intestinal permeability may be caused by AN pathophysiology; however, the current results on gut permeability in AN are conflicting. The potential altered gut permeability in AN may underlie the low-grade inflammation and increased risk of autoimmune diseases found in eating disorders [23]. Moreover, starvation has a significant impact on the gut microbiota, and a diet based on animal products used for re-nutrition, may stimulate the growth of Bacteria that trigger inflammation [24].
Studies have shown that the microbiota is highly specific for different gut compartments and even differs within compartments, i.e., within the colon [25,26,27,28].
For example, the microbiota found in ileal effluent, which has a uniquely personal composition [25], differ from that found in faeces samples with lower overall diversity, fast changing profiles, and increased relative abundances of species within the orders Lactobacillales and Clostridiales, and below detection limits of Archaea [29,30,31].
The aim of this article is to conduct a review of the evidence of differences in the faecal microbiota in AN compared to healthy controls. This could provide clues to the pathophysiology of AN, index biomarkers, and generate new ideas for treatment development. In addition, guidance for future research is provided.
Method
Protocol for the review
The protocol is available as a table online at this URL: https://drive.google.com/open?id=0B1bvPK36OlXANVZGNXduSU85RWs.
Eligibility criteria
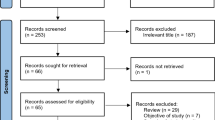
In view of the rather few studies done, all articles were included, except reviews, animal studies, and studies with no relevance to the intestinal microbiota in AN and the gut–brain axis (Fig. 1).
Data sources
The PubMed database in the US National Library of Medicine was searched to identify any relevant studies on 27 August 2017.
Search strategy
The following search terms were included: “anorexia nervosa” and “microbiota”. The reference lists of studies were also hand checked for additional articles of interest.
Study selection
We only included original scientific publications, where the human microbiota were analysed in persons with AN. Excluded were review articles, commentaries, preclinical and animal studies and all other types of non-scientific original publications. One case study was also excluded as it examined fungi from a faeces sample instead of Bacteria and Archaea, of which the two latter are the focus point of this review (Fig. 1).
Data collection process
Given the heterogeneity in methods and the limited number of publications, results were evaluated as presented in the source publications (Table 1). No additional analysis were made to the original presentations of data and the corresponding results.
Risk of bias
Risk of bias was assessed by HFS and JMS by reviewing the study designs, the methods used, any selection mechanisms presented, and the consistency of results presentation. Risk of bias is included in the above linked protocol.
Synthesis of results
The full texts were then retrieved and read in full by two authors (HFS and JMS) independently to determine whether the studies met inclusion criteria, and HFS wrote the manuscript with extensive help from the fifth author (JMS). A second author (CK) and a third author (JT) provided important revisions on science and content to the manuscript, and a fourth author (NH) provided expert input on the microbiota and its role in the metabolism and absorption of nutrients, and a critical view on the limitations of the few studies done so far. As raw data were not extracted, there was no handling of data or combining of results.
Results
The search terms “anorexia nervosa” and “microbiota” yielded a total of 27 unique articles. Reviewing the reference lists of all articles did not yield any additional original scientific publications relevant for this review. All 27 articles were screened and assessed for eligibility. 18 of these articles did not meet the eligibility criteria and were excluded (Fig. 1). The main study characteristics of the 9 included studies are summarized in Table 1. During revision of this article for publication another study by Mörkl et al. published in November 2017 was included in this article and in Table 1 [32], and thus a total of 10 articles were included. See the end of the “Results” paragraph for a review of the findings by Mörkl et al.
Of the 10 studies included in this review, two were longitudinal in design [33, 34], six were cross-sectional [32, 35,36,37,38,39], one was a case study involving a severe case of AN [40], and the last study was a case series consisting of three cases that was also longitudinal in its design [41]. The diagnostic criteria used for AN were from Diagnostic and Statistical Manual IV (DSM-IV) in four studies [33, 35,36,37], from DSM-5th Edition in two studies [38, 41], from ICD-10 in one study [32], and not specified in three studies [34, 39, 40]. All studies except one were published in the last 5 years [35].
The number of AN patients included in the six cross-sectional studies ranged between 9 and 25 [32, 35,36,37,38,39], and between 16 and 55 in the two longitudinal studies [33, 34]. Three patients with AN were included in the case series [41]. In the longitudinal studies over the course of renourishment, the second time point (T2) was defined as after approximately 14 weeks in one of the studies [34], and when a mean body mass index (BMI) goal of approximately 17.4 kg/m2 was achieved in the other study [33]. Healthy controls were included in all studies, and in four of the ten studies, the controls were matched to the AN groups both for age and sex [32, 34, 37, 38]. The nine studies that examined the intestinal microbiota all examined it from faeces samples.
The methods used for assessing quantity and type of species in faeces samples were 16S revers transcriptase-PCR based. Morita et al. also included 23S rRNA gene targeted technology. Two studies did not specify whether 16S or 23S rRNA gene targeted PCR was used [35, 36]. Additional measures included organic acids including short-chain fatty acids and pH of faeces (chromatography) [34, 37], and culture growth and mass spectrometry (Matrix-Assisted Laser Desorption-Ionization) [40], and several measures of body fat including anthropometric assessments and ultrasound measurement of subcutaneous adipose tissue thickness [32]. Three studies evaluated the relation between psychiatric measures and changes in abundance and composition of the microbiota [32, 33, 38], and one study examined the relation between caseinolytic peptidase B (ClpB) protein concentrations in plasma and scores on the Eating Disorder Inventory-2 (EDI-2) and the Montgomery-Åsberg Depression Rating Scale (MADRS) [39].
Three studies used employees, relatives and family members of the employees as controls [34, 35, 38]. The other studies recruited controls through public advertisements [32, 33, 39], through previous studies using a snowball approach and from healthy outpatients [36], or recruited controls through non-disclosed methods [37]. Two studies did not include controls, as the study designs were case studies [40, 41].
Bias was assessed in all studies, and the results are shown in the linked online protocol. Furthermore, see Table 1 for a description of the quality of technologies used for collection and handling of faeces samples in the different studies.
Microbiota results
Four studies explored the abundance of gut microbiota in AN, and all investigated AN in the acute stage. Two of these studies described a normal abundance of microbiota in AN [34, 36], while one found a reduced microbiota abundance in AN [37] and another an altered abundance measured on several microbial phyla, genera, and species [38].
Three studies examined the diversity in AN compared to healthy controls. Mack et al. found an overall normal microbial diversity in AN in their weight restoration study at both time point 1 (T1) and time point 2 (T2) [34], and in line with this, Borgo et al. found no significant changes in diversity in acutely ill AN patients compared to controls [38]. The other weight restoration study by Kleiman et al. found a lower alpha (within-sample) diversity at both T1 and T2 compared to controls indicating the number of observed species in the analysed faeces sample [33]. Moreover, Kleiman et al. found a significant association between alpha diversity and depression and eating disorder psychopathology [33]. Changes from T1 to T2 in persons with AN, i.e., beta diversity (between-sample diversity), was also reported by Kleiman et al.; however, the alpha diversity remained significantly lower after weight gain than the observed diversity in controls [33]. In the case series, which followed three female patients with acute AN through hospitalization and weight restoration, significant changes in magnitude of composition and diversity on phylum to genus levels were observed, however, these changes were found to be patient specific and not common changes in the three patients [41]. They also measured the resting energy expenditure (REE) and diet-induced thermogenesis in the three patients, which both increased during weight gain, but was not significantly associated with diversity or composition of the gut microbiota.
Common findings in the acute stages of AN with regard to specific microbiota were that the phylum Bacteroidetes was low in AN in two studies [33, 34]. Conversely, two other studies showed that Bacteroidetes also was decreased (or trending towards decreased) in obese individuals [35, 36]. The phylum Firmicutes was increased in AN in three studies [33,34,35] and decreased in one study [38].
The genus Methanobrevibacter and specifically, on the species level, M. smithii was increased, when present, in AN patients compared to normal-weight participants in four studies [34,35,36, 38]. Mack et al. detected species belonging to the genus Methanobrevibacter, of which M. smithii is the most common species in the human gut, in 22% of patients with AN at T1, which was higher than the proportion of AN patients with Methanobrevibacter at T2 (14%), and Methanobrevibacter was found in 15% of controls [34]. The relative abundance of Methanobrevibacter was statistically higher in the 22% of AN patients at T1 than the 15% of controls (p = 0.004). In the study by Million et al. M. smithii was detected in 64% of all participants including AN patients, normal-weight controls, and obese participants (BMI > 25), and M. smithii concentrations were higher in participants with BMI < 25 (p = 0.008) with a trend towards a correlation between a higher BMI and lower M. smithii concentration (p = 0.08). In line with this, Armougom et al. detected M. smithii in 100% of the AN patients and 75% of lean participants and found M. smithii statistically increased in AN compared to the lean participants (p = 0.0171) [35], and Borgo et al. found a significantly higher average of genome copy number of M. smithii in their AN group compared to controls [38].
The case study examined the microbial composition of a faeces sample from a 21-year-old Caucasian woman with a severe case of AN with a BMI of 10.4 kg/m2 [40]. 19 new microbial species never previously observed were found, of which 11 were isolated and sequenced. Of these, 7 species belonged to the phylum Firmicutes, 2 belonged to the phylum Bacteroidetes, and 2 belonged to the phylum Actinobacteria. Interestingly, M. smithii was not identified in the faeces of the patient in contrast to the other studies that found an increase in this species in the acute stages of AN [34,35,36, 38].
Apart from that, no clear patterns were detectable with regard to microbiota in the nine selected studies.
Effects of weight restoration
Diversity and richness was initially normal and increased after weight gain in one study [34]. Kleiman et al., reported a lower diversity both at baseline and after inpatient weight restoration [33]. Duration of inpatient stay was defined in one study [34] as 14.0 ± 6.8 weeks (mean ± SD) with BMI at admission of 15.3 ± 1.4 and at the end of treatment 17.7 ± 1.4 (mean ± SD). In the other study [33], duration of stay was 4 weeks (Dr. Ian Carroll, personal communication) and BMI at admission was 16.2 ± 1.5 and 17.4 ± 0.9 (mean ± SD) at endpoint. In the case series duration of stay varied from 34 to 73 days and, as mentioned before, changes in diversity and composition were largely patient specific and no common trend was observed [41].
With regard to specific microbiota, the relative concentration of Bacteroidetes was found low at T1 and further decreased at T2 in AN compared to healthy controls in one study [34]. M. smithii and the mucin-degrading genera Verrumcomicrobia and Bifidobacteria were found to be increased in AN at baseline (T1) compared to controls in one study [34], and the study by Kleiman et al., also found lower abundances of Bacteroidetes in AN after weight restoration compared to controls [33]. Firmicutes was increased compared to controls after weight restoration in both weight restoration studies in AN [33, 34].
Relation to clinical symptoms
One weight restoration study found an improvement in total gastrointestinal (GI) scores (reflecting complaints) after weight restoration, although most upper and a few lower GI symptoms such as abdominal pain and bowel noises did not change [34], and no correlations between GI symptoms and microbiota measures where found. The other weight restoration study found an association between alpha diversity in AN and levels of depression, anxiety, and eating disorder psychopathology at baseline [33].
With regard to correlations between psychiatric symptoms measured on the BDI scale and specific microbiota, Borgo et al. found a negative correlation between Clostridium spp. and depression score, and, in addition, a negative correlation between faecal butyrate concentration and depression and anxiety scores [38].
Breton et al. examined the role of ClpB protein concentrations in plasma and its correlations to clinical symptoms in 24 patients with restrictive AN, 29 patients with bulimia nervosa, 13 patients with binge-eating disorder, and 29 gender-matched controls [39]. ClpB protein is produced by Enterobacteriaceae such as Escherichia coli and has been found as a conformational mimetic of alpha-melanocyte stimulating hormone (alpha-MSH), which is thought to be involved in satiety and anxiety [42]. Indeed, Breton et al. found that ClpB protein concentrations correlated positively with alpha-MSH-reactive IgG for all patients with eating disorders and an increase in ClpB protein concentrations was found in plasma in eating disorder patients compared to plasma in controls, and that ClpB protein concentrations were significantly correlated with several subscales on the Eating Disorder Inventory-2 (EDI-2) for all patients with eating disorders and the Montgomery–Åsberg Depression Rating Scale (MADRS) total score and specifically the anhedonia score for AN patients (p < 0.05). The study adds evidence to the potential role of ClpB protein produced by Enterobacteriaceae in the gut and its impact on the brain and psychopathology in eating disorders.
Mörkl et al. examined the faecal microbiota from five groups; 18 inpatients with AN, 20 athletes, 22 overweight participants, 20 obese participants, and 26 normal-weight controls [32]. They found a lower alpha diversity in AN and obese participants compared to other groups, and that the athletes had the highest alpha diversity. In addition, they found that greater levels of depression measured on the BDI correlated with a lower alpha diversity, when all groups were included in the analysis (p = 0.032). Beta diversity was associated with several parameters, and beta diversity was significantly associated with several different measures of body fat (p < 0.05), smoking status (p = 0.002), and cholesterol–HDL ratio (p = 0.024).
The phylotype Coriobacteriaceae was the only enriched phylotype in AN compared to other entities. Collectively, the authors concluded that their results supported the hypothesis of a gut dysbiosis in AN.
Discussion
Individuals with AN are known to have a highly variable weight gain during therapeutic renourishment, and the factors that contribute to this variability are currently unclear [43]. Factors such as increased (and secretive) physical activity [44] and diet-induced thermogenesis have been proposed [45]. Thereby, it is reasonable to hypothesize that AN patients may have a microbial imbalance in their gut that in part contributes to the poor response to weight gain observed during treatment.
Overall, there are few studies investigating the microbiota in AN, all are experimental, and they differ with regard to design and results. In this review, we have attempted to identify both common and divergent features in order to inform future investigations.
Regarding abundance of microbiota, two studies found a normal abundance of microbiota in AN [34, 36], while two studies found a reduced and altered abundance of microbiota [37, 38]. The diverging results make an interpretation difficult.
In four studies, the diversity of the microbiota was compared to controls and explicitly described. Two of these studies found a normal microbial diversity in AN and in the weight restoration study by Mack et al. diversity further increased after weight gain [34, 38]. The third study found a lower alpha diversity compared to healthy controls that was still significantly lower upon weight gain [33], and Mörkl et al. also found a low alpha diversity in AN patients and in obese individuals. There was, however, a major difference between the two weight restoration studies in the duration of follow-up, differing by approximately 10 weeks. This may have had an impact on the ability of the microbiota to adjust to environmental factors. However, with only these four studies, it is too early to draw any firm conclusion on the diversity of the microbiota in AN.
With regard to specific changes in microbiota species, there were some findings that may indicate the trend of change in AN. The phylum Firmicutes was found increased in AN in three studies [33,34,35], and Bacteroidetes was low in AN in two studies [33, 34], although potentially conflicting findings were made in two other studies, where Bacteroidetes was found to be decreased (or trending towards decreased) in obese individuals [35, 36] and Firmicutes was found to be decreased in one study [38]. Another study found an increase in the phylum Coriobacteriaceae [32]. However, these findings may also reflect that it is dysregulation per se, which may be at a similar level and direction in AN and obese individuals, and not specific BMI scores, that is related to the composition of the microbiota. Interestingly, the genus Methanobrevibacter, specifically M. smithii, was also increased, or trending towards an increase when present in patients with AN or participants with BMI < 25 in four studies [34,35,36, 38].
Methanobrevibacter smithii is involved in the breakdown of polysaccharides from vegetable sources and the finding of this specific Archaeon could illustrate an adaptation to a typical diet rich in vegetables and fruits in persons with AN. However, methanogenic Archaea, such as M. smithii, have also been linked to constipation, a common complaint in patients with AN, which statins have been shown to alleviate by suppressing the growth of methanogens [35, 46,47,48]. The evidence of M. smithii in faeces from constipated patients necessitate further investigation of whether this finding in AN patients is only related to constipation or also related to AN psychopathology as a potential biomarker.
There were additional changes found in the different studies, but no clear additional patterns were detectable with regard to specific microbiota in the nine selected studies. 5 out of the 9 studies that examined the microbiota in faeces samples reported microbial changes on the species level extensively, while the remaining four studies reported mainly findings on phylum to genus level, which may be too broad [32,33,34, 41]. Differences in microbial species may better reflect changes that are related to specific biological effects [49].
With regard to the effect of weight gain on the faecal microbiota, Firmicutes was increased after weight restoration in both studies in AN [33, 34]. Furthermore, the Bacteroidetes was found low before weight gain and decreasing in AN in one of the studies [34], while the other found a decreasing level of Bacteroidetes after weight restoration [33]. Only in one of the weight restoration studies were M. smithii and the mucin degraders Verrumcomicrobia and Bifidobacteria increased before weight gain, a finding that was not replicated after weight gain in AN [34]. The only significant finding after weight gain, as interpreted from the two studies, was an increase in the phylum Firmicutes. To conclude on specific microbiota differences in the acute stages of AN compared to controls, only one finding on the species level remains substantial and concrete; an increased concentration of the Archaeon M. smithii. However, on the phylum level Firmicutes was consistently shown to be overrepresented and specific species results from this phylum could be expected in the future.
9 of 10 studies included in this review collected faeces samples, i.e., reflecting mainly the colorectal microbiota. Differences in the microbiota in the distal parts of the gut, when taken from faeces samples, may not relate in a meaningful way to all relevant biological functions, since faeces mainly contains a mixture of Bacteria from the various compartments of the colon and some Bacteria from the distal ileum, and therefore faeces samples are proxies for the gut microbiome rather than displaying true host–microbe interactions [50]. In the large intestine resorption of fluid and the forming of faeces take place and Bacteria are kept at a distance from the epithelial cells by the mucus coat [51, 52]. Theoretically, another interesting location for microbiota sampling in AN, in addition to faeces samples, would be the small intestine, notably the ileum, where there is a microbial flora and where the major breakdown of food and absorption of nutrients take place. It is possible that restrictive dietary intake, a characteristic trait in AN, causes microbial dysbiosis in the small intestine, or microbial dysbiosis in this compartment influences the brain to restrict the food intake via the gut–brain axis.
Bacteria and Archaea from the small intestine are subjected to a harsh environment with fast transit time, digestive enzymes, and bile, and therefore largely contrast the environment in the colon, demanding more resilient inhabitants in the small intestine with different survival strategies, and these microbes are also subjected to breakdown through the digestive tract [53]. Any reported findings in faeces might therefore not represent the microbiota in the small intestine. A study by Vandeputte et al. showed that faecal microbial richness was decreased in female patients with faster intestinal transit time measured with Bristol Stool Scale (BBS) as a proxy of intestinal transit time [54]. Thus, the patients with diarrhoea had the least diverse faecal microbiota, and different enterotypes existed in patients with different BBS scores. In loose stool the Prevotella enterotype dominated, while the Ruminococcaceae–Bacteroides enterotype, which includes the genus Methanobrevibacter with the main species M. smithii, dominates in harder stool. This supports the repeated findings of M. smithii in AN patients and suggests M. smithii to be perhaps correlated mainly with transit time, which is frequently decreased in AN, rather than with AN psychopathology. Interestingly, Archaea including Methanobrevibacter and M. smithii have been found to be below detection limit in ileal effluent from ileostomy patients [29] suggesting this specific Archaeon might dominate mainly in the colorectum and less in the small intestine.
Vandeputte et al. concluded that transit time may be a selective force on microbial life strategies. In line with this, different transit times within the individual GI tract may promote growth of different species and offer less diversity in compartments with faster transit, i.e., the small intestine. In addition, several studies have found significant differences in microbiota composition between different compartments in the GI tract in patients with ileostomy, autopsy patients, and patients undergoing both gastro-duodenoscopy and colonoscopy [29,30,31, 55], and studies have found separate clusters of bacteria at both family and species taxonomic levels, when comparing colonic and rectal mucosal samples with faeces in healthy control persons and patients with IBS [25,26,27,28, 56]. Thus, different microbial compositions exist within different intestinal compartments and even between faeces and rectal mucosal samples indicating the importance of intestinal mucosal biopsies for microbiota analysis in specific compartments.
From an immunological point of view, the small intestine is also an interesting location as the microbiota is important for immunological homeostasis and susceptibility to immune-mediated diseases and disorders through the Peyer’s patches and other parts of the gut-associated lymphoid tissue (GALT), which are prominent in the small intestine and constitute a major line of defence against pathogens in the GI tract [57, 58].
To retrieve samples from the small intestine, one will need to use pinch or submucosal biopsy from endoscopy [50], alternatively using more novel technologies, such as capsule endoscopy, which need further research before implementation [59].
However, despite the several studies that point at the small intestine as an immunologically and metabolically important anatomical location for AN, faeces samples remain the easiest to collect and should continue to be the standard approach to analysing the gut microbiota until the use of more minimally invasive approaches, such as capsule endoscopy, are further developed.
The mechanistic link between gastrointestinal illnesses and psychiatric disorders has been well-established [60]. Raevouri et al. found an increased prevalence of autoimmune disorders in patients with eating disorders, which could possibly be caused by alterations in the gut microbiota, while another study found that early life stress altered the microbiota, the systemic immune responses, and resulted in an elevated HPA-axis function in a rat model [23, 61]. It has also been established in a systematic review that more than 50% of patients with IBS also meet the criteria for mood disorders [62].
The relation between weight gain and clinical symptoms were assessed in two studies [33, 34] and one weight restoration study found that total GI scores (reflecting complaints) were improved by weight gain, although individual symptoms did not change [34], while the other weight restoration study described an association between within-sample alpha diversity and levels of depression, anxiety, and eating disorder psychopathology in AN at baseline [33]. Borgo et al. also found a negative correlation between depression and Clostridium spp. (p = 0.089) and an inverse correlation between faecal butyrate concentration and depression (p = 0.0379) and anxiety (p = 0.0206) scores in AN patients compared to controls, while Breton et al. found correlations between ClpB in plasma and several subscales on the EDI-2 in all eating disorder patients and with MADRS total score and anhedonia score in AN patients when compared to controls (both p < 0.05) [38]. These findings may support that it is the gut–brain axis that is underlying, or at least involved, in these symptoms in AN, and ClpB in plasma potentially provides an interesting link between the gut and the brain. The gut–brain axis has been described as a bidirectional communication network that monitors and integrates gut functions and connects them to cognitive and emotional centres of the brain. This network includes the central, autonomic, and enteric nervous systems, in addition to the neuroendocrine, enteroendocrine, and neuroimmune systems [63]. Furthermore, the network might mediate both the effects of genetic and environmental factors on brain development and function, and has been proposed to be involved in the aetiology of several psychiatric disorders [64]. Albeit early findings, which require validation in repeated and larger studies before any clear conclusions can be made, they point in a direction for the design of future microbiota studies, which should include the assessment of psychiatric symptoms in AN over time.
There were a number of potential weaknesses in all studies included. All studies included were conducted in adults except the study by Mack et al. and Patient C in the case series [41], and AN usually has an onset in the adolescence, which may imply that the results were the effects of long standing undernutrition, and/or a selected diet, i.e., as caused by mainly external factors and less from any internal inherent morphological or structural deviation. However, it may also be inherent morphological or structural deviations that explain differences between adolescents and adults with AN, or why the disease has persisted into adulthood. It is also possible that observed abnormalities in the microbiota in adults with AN are absent in their adolescent counterparts as a result of different hormonal levels, or it may relate to other maturational effects. Another limitation of the studies is that all studies have included females only, neglecting the fact that 10% of AN patients are males, which make the results hard to extrapolate to a male population with AN, though limiting the population to females only reduces the risk of sex as a confounder in the studies.
All of the studies were experimental and albeit several were well designed, the studies were in general not designed to adjust for potential bias. For the sake of compliance with the rules of systematic reviews, we included a bias analysis (see link in methods section to our online protocol). We found that recruitment was selective, e.g., based upon available patients at the ward, and that selection of healthy controls was biased in some studies, e.g., including available staff or family members of staff. This may imply that the controls used may not reflect changes in a general/normal population, and using control persons that are genetically related might underestimate potential findings as the intestinal microbiota might also be genetically based and certain Bacteria and Archaea might be shared within families. Furthermore, the choice of timepoint for investigation in the cross-sectional studies and other factors such as sample collection procedures, calorie intake and contents of the diet [65], activity level, and medication were not always specified and may account for variation between studies. In addition, transit time, i.e., stool consistency, varies from person to person and has been shown to have a significant impact on microbial composition [54], and only one study accounted for stool frequency measured with the Gastro-Questionnaire [66].
In the weight restoration studies, calory intake was only controlled for in the study by Mack et al., apart from the case series, where calory intake was measured extensively throughout hospitalization [41]. Additionally, activity level were in general also not controlled for, which may have influenced bowel movements and energy expenditure. Furthermore, not all studies examined the abundance of microbiota, and the duration of weight restoration in the two large longitudinal studies was not equal [33, 34], and in the cross-sectional studies severity and duration of AN were not consistently specified throughout the studies, why these may have differed substantially and contributed to differences in the results [32, 35,36,37,38,39]. Only one individual was studied in the case study [40] and three individuals in the case series [41] and the relevance on a great scale to the characteristics of the microbiota in AN and replicability therefore remain limited.
Potential differences in the methods used to determine the microbiota, i.e., differences in sampling, nucleic acid extraction, and analysis techniques, etc., can also have contributed to the variability in results [50].
Other limitations were that not all studies examined the abundance and diversity of microbiota, why a potential link between overall abundance and diversity of microbiota and AN remain undefined. In addition, different clinical aspects were investigated in the two large weight restoration studies making their results hard to compare.
Conclusion
Few studies have examined the microbiota in AN, and all studies thus far have been experimental, and hypothesis generating. Larger, controlled studies will strengthen the validity of the results and should be a clear recommendation for future studies. Future studies should focus more on reporting specific microbial species either through marker gene analysis based on an amplicon of a single gene or through metagenomic sequencing, which attempts to sequence all or most genes in a sample.
An issue raised in this systemic review is that faeces samples may not optimally reflect differences in the microbiome that are biologically relevant for AN as they are proxies for the microbiome in the intestinal microbiota rather than reflecting true host–microbe interactions in the various gut compartments. It is proposed that future studies on the microbiota in AN in addition to faeces samples consider collecting, when possible, faeces biopsies from the small intestine, where breakdown and absorption of nutrients occur, and where a large impact of the microbiota on biological functions, and thereby symptoms and signs, is likely to occur. However, analysing the microbiota from faeces samples remains to date the most convenient, minimally invasive, and easiest obtainable way to analysing the microbiota in AN and potentially finding a biomarker. The intestinal microbiota in AN is an interesting field and has yet to be fully unraveled.
References
Keshaviah A, Edkins K, Hastings ER, Krishna M, Franko DL, Herzog DB, Thomas JJ, Murray HB, Eddy KT (2014) Re-examining premature mortality in anorexia nervosa: a meta-analysis redux. Compr Psychiatry 55(8):1773–1784. https://doi.org/10.1016/j.comppsych.2014.07.017
Steinhausen HC (2009) Outcome of eating disorders. Child Adolesc Psychiatr Clin N Am 18(1):225–242. https://doi.org/10.1016/j.chc.2008.07.013
Quigley EMM (2013) Gut bacteria in health and disease. Gastroenterol Hepatol (N Y) 9(9):560–569
Claesson MJ, O’Sullivan O, Wang Q, Nikkila J, Marchesi JR, Smidt H, de Vos WM, Ross RP, O’Toole PW (2009) Comparative analysis of pyrosequencing and a phylogenetic microarray for exploring microbial community structures in the human distal intestine. PLoS One 4(8):e6669. https://doi.org/10.1371/journal.pone.0006669
Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Dore J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, Meta HITC., Bork P, Ehrlich SD, Wang J (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464(7285):59–65. https://doi.org/10.1038/nature08821
Sudo N, Sawamura S, Tanaka K, Aiba Y, Kubo C, Koga Y (1997) The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol 159(4):1739–1745
Guarner F, Malagelada JR (2003) Gut flora in health and disease. Lancet 361(9356):512–519. https://doi.org/10.1016/S0140-6736(03)12489-0
Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y (2004) Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J Physiol 558(Pt 1):263–275. https://doi.org/10.1113/jphysiol.2004.063388
Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF (2013) The microbiome–gut–brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry 18(6):666–673. https://doi.org/10.1038/mp.2012.77
Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S (2011) Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA 108(7):3047–3052. https://doi.org/10.1073/pnas.1010529108
Cryan JF, O’Mahony SM (2011) The microbiome–gut–brain axis: from bowel to behavior. Neurogastroenterol Motil 23(3):187–192. https://doi.org/10.1111/j.1365-2982.2010.01664.x
Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S (2016) From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry 21(6):738–748. https://doi.org/10.1038/mp.2016.50
Godart NT, Flament MF, Lecrubier Y, Jeammet P (2000) Anxiety disorders in anorexia nervosa and bulimia nervosa: co-morbidity and chronology of appearance. Eur Psychiatry 15(1):38–45
Fernandez-Aranda F, Pinheiro AP, Tozzi F, Thornton LM, Fichter MM, Halmi KA, Kaplan AS, Klump KL, Strober M, Woodside DB, Crow S, Mitchell J, Rotondo A, Keel P, Plotnicov KH, Berrettini WH, Kaye WH, Crawford SF, Johnson C, Brandt H, La Via M, Bulik CM (2007) Symptom profile of major depressive disorder in women with eating disorders. Aust N Z J Psychiatry 41(1):24–31. https://doi.org/10.1080/00048670601057718
Kask J, Ekselius L, Brandt L, Kollia N, Ekbom A, Papadopoulos FC (2016) Mortality in women with anorexia nervosa: the role of comorbid psychiatric disorders. Psychosom Med. https://doi.org/10.1097/PSY.0000000000000342
Flint HJ (2011) Obesity and the gut microbiota. J Clin Gastroenterol 45(Suppl):S128–S132. https://doi.org/10.1097/MCG.0b013e31821f44c4
Cox LM, Blaser MJ (2013) Pathways in microbe-induced obesity. Cell Metab 17(6):883–894. https://doi.org/10.1016/j.cmet.2013.05.004
Aguirre M, Jonkers DM, Troost FJ, Roeselers G, Venema K (2014) In vitro characterization of the impact of different substrates on metabolite production, energy extraction and composition of gut microbiota from lean and obese subjects. PLoS One 9(11):e113864. https://doi.org/10.1371/journal.pone.0113864
Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI (2009) A core gut microbiome in obese and lean twins. Nature 457(7228):480–484. https://doi.org/10.1038/nature07540
Jesus P, Ouelaa W, Francois M, Riachy L, Guerin C, Aziz M, Do Rego JC, Dechelotte P, Fetissov SO, Coeffier M (2014) Alteration of intestinal barrier function during activity-based anorexia in mice. Clin Nutr 33(6):1046–1053. https://doi.org/10.1016/j.clnu.2013.11.006
Pals KL, Chang RT, Ryan AJ, Gisolfi CV (1997) Effect of running intensity on intestinal permeability. J Appl Physiol (1985) 82(2):571–576
Monteleone P, Carratu R, Carteni M, Generoso M, Lamberti M, Magistris LD, Brambilla F, Colurcio B, Secondulfo M, Maj M (2004) Intestinal permeability is decreased in anorexia nervosa. Mol Psychiatry 9(1):76–80. https://doi.org/10.1038/sj.mp.4001374
Raevuori A, Haukka J, Vaarala O, Suvisaari JM, Gissler M, Grainger M, Linna MS, Suokas JT (2014) The increased risk for autoimmune diseases in patients with eating disorders. PLoS One 9(8):e104845. https://doi.org/10.1371/journal.pone.0104845
Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB (2012) Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 487(7405):104–108. https://doi.org/10.1038/nature11225
Zoetendal EG, von Wright A, Vilpponen-Salmela T, Ben-Amor K, Akkermans AD, de Vos WM (2002) Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from feces. Appl Environ Microbiol 68(7):3401–3407
Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA (2005) Diversity of the human intestinal microbial flora. Science 308(5728):1635–1638. https://doi.org/10.1126/science.1110591
Carroll IM, Ringel-Kulka T, Keku TO, Chang YH, Packey CD, Sartor RB, Ringel Y (2011) Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 301(5):G799–G807. https://doi.org/10.1152/ajpgi.00154.2011
Durban A, Abellan JJ, Jimenez-Hernandez N, Ponce M, Ponce J, Sala T, D’Auria G, Latorre A, Moya A (2011) Assessing gut microbial diversity from feces and rectal mucosa. Microbial Ecol 61(1):123–133. https://doi.org/10.1007/s00248-010-9738-y
Booijink CC, El-Aidy S, Rajilic-Stojanovic M, Heilig HG, Troost FJ, Smidt H, Kleerebezem M, De Vos WM, Zoetendal EG (2010) High temporal and inter-individual variation detected in the human ileal microbiota. Environ Microbiol 12(12):3213–3227. https://doi.org/10.1111/j.1462-2920.2010.02294.x
Hayashi H, Takahashi R, Nishi T, Sakamoto M, Benno Y (2005) Molecular analysis of jejunal, ileal, caecal and recto-sigmoidal human colonic microbiota using 16S rRNA gene libraries and terminal restriction fragment length polymorphism. J Med Microbiol 54(Pt 11):1093–1101. https://doi.org/10.1099/jmm.0.45935-0
Wang M, Ahrne S, Jeppsson B, Molin G (2005) Comparison of bacterial diversity along the human intestinal tract by direct cloning and sequencing of 16S rRNA genes. FEMS Microbiol Ecol 54(2):219–231. https://doi.org/10.1016/j.femsec.2005.03.012
Morkl S, Lackner S, Muller W, Gorkiewicz G, Kashofer K, Oberascher A, Painold A, Holl A, Holzer P, Meinitzer A, Mangge H, Holasek S (2017) Gut microbiota and body composition in anorexia nervosa inpatients in comparison to athletes, overweight, obese, and normal weight controls. Int J Eat Disord 50(12):1421–1431. https://doi.org/10.1002/eat.22801
Kleiman SC, Watson HJ, Bulik-Sullivan EC, Huh EY, Tarantino LM, Bulik CM, Carroll IM (2015) The intestinal microbiota in acute anorexia nervosa and during renourishment: relationship to depression, anxiety, and eating disorder psychopathology. Psychosom Med 77(9):969–981. https://doi.org/10.1097/psy.0000000000000247
Mack I, Cuntz U, Gramer C, Niedermaier S, Pohl C, Schwiertz A, Zimmermann K, Zipfel S, Enck P, Penders J (2016) Weight gain in anorexia nervosa does not ameliorate the faecal microbiota, branched chain fatty acid profiles, and gastrointestinal complaints. Sci Rep 6:26752. https://doi.org/10.1038/srep26752
Armougom F, Henry M, Vialettes B, Raccah D, Raoult D (2009) Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS One 4(9):e7125. https://doi.org/10.1371/journal.pone.0007125
Million M, Angelakis E, Maraninchi M, Henry M, Giorgi R, Valero R, Vialettes B, Raoult D (2013) Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. Int J Obes (Lond) 37(11):1460–1466. https://doi.org/10.1038/ijo.2013.20
Morita C, Tsuji H, Hata T, Gondo M, Takakura S, Kawai K, Yoshihara K, Ogata K, Nomoto K, Miyazaki K, Sudo N (2015) Gut dysbiosis in patients with anorexia nervosa. PLoS One 10(12):e0145274. https://doi.org/10.1371/journal.pone.0145274
Borgo F, Riva A, Benetti A, Casiraghi MC, Bertelli S, Garbossa S, Anselmetti S, Scarone S, Pontiroli AE, Morace G, Borghi E (2017) Microbiota in anorexia nervosa: the triangle between bacterial species, metabolites and psychological tests. PLoS One 12(6):e0179739. https://doi.org/10.1371/journal.pone.0179739
Breton J, Legrand R, Akkermann K, Jarv A, Harro J, Dechelotte P, Fetissov SO (2016) Elevated plasma concentrations of bacterial ClpB protein in patients with eating disorders. Int J Eat Disord 49(8):805–808. https://doi.org/10.1002/eat.22531
Pfleiderer A, Lagier JC, Armougom F, Robert C, Vialettes B, Raoult D (2013) Culturomics identified 11 new bacterial species from a single anorexia nervosa stool sample. Eur J Clin Microbiol Infect Dis 32(11):1471–1481. https://doi.org/10.1007/s10096-013-1900-2
Kleiman SC, Glenny EM, Bulik-Sullivan EC, Huh EY, Tsilimigras MCB, Fodor AA, Bulik CM, Carroll IM (2017) Daily changes in composition and diversity of the intestinal microbiota in patients with anorexia nervosa: a series of three cases. Eur Eat Disord Rev J Eat Disord Assoc 25(5):423–427. https://doi.org/10.1002/erv.2524
Kishi T, Elmquist JK (2005) Body weight is regulated by the brain: a link between feeding and emotion. Mol Psychiatry 10(2):132–146. https://doi.org/10.1038/sj.mp.4001638
Van Wymelbeke V, Brondel L, Marcel Brun J, Rigaud D (2004) Factors associated with the increase in resting energy expenditure during refeeding in malnourished anorexia nervosa patients. Am J Clin Nutr 80(6):1469–1477
Kaye WH, Gwirtsman HE, Obarzanek E, George DT (1988) Relative importance of calorie intake needed to gain weight and level of physical activity in anorexia nervosa. Am J Clin Nutr 47(6):989–994
Moukaddem M, Boulier A, Apfelbaum M, Rigaud D (1997) Increase in diet-induced thermogenesis at the start of refeeding in severely malnourished anorexia nervosa patients. Am J Clin Nutr 66(1):133–140
Samuel BS, Gordon JI (2006) A humanized gnotobiotic mouse model of host–archaeal–bacterial mutualism. Proc Natl Acad Sci USA 103(26):10011–10016. https://doi.org/10.1073/pnas.0602187103
Gottlieb K, Wacher V, Sliman J, Pimentel M (2016) Review article: inhibition of methanogenic archaea by statins as a targeted management strategy for constipation and related disorders. Aliment Pharmacol Ther 43(2):197–212. https://doi.org/10.1111/apt.13469
Triantafyllou K, Chang C, Pimentel M (2014) Methanogens, methane and gastrointestinal motility. J Neurogastroenterol Motil 20(1):31–40. https://doi.org/10.5056/jnm.2014.20.1.31
Lapage SP, Sneath PHA, Lessel EF et al (1992) International code of nomenclature of bacteria: bacteriological code. ASM Press, Washington (DC)
Claesson MJ, Clooney AG, O’Toole PW (2017) A clinician’s guide to microbiome analysis. Nat Rev Gastroenterol Hepatol. https://doi.org/10.1038/nrgastro.2017.97
Johansson ME, Hansson GC (2011) Microbiology. Keeping bacteria at a distance. Science 334(6053):182–183. https://doi.org/10.1126/science.1213909
Belkaid Y, Grainger J (2013) Immunology. Mucus coat, a dress code for tolerance. Science 342(6157):432–433. https://doi.org/10.1126/science.1246252
Zoetendal EG, Raes J, van den Bogert B, Arumugam M, Booijink CC, Troost FJ, Bork P, Wels M, de Vos WM, Kleerebezem M (2012) The human small intestinal microbiota is driven by rapid uptake and conversion of simple carbohydrates. ISME J 6(7):1415–1426. https://doi.org/10.1038/ismej.2011.212
Vandeputte D, Falony G, Vieira-Silva S, Tito RY, Joossens M, Raes J (2016) Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 65(1):57–62. https://doi.org/10.1136/gutjnl-2015-309618
Finegold SM, Sutter VL, Boyle JD, Shimada K (1970) The normal flora of ileostomy and transverse colostomy effluents. J Infect Dis 122(5):376–381
Rangel I, Sundin J, Fuentes S, Repsilber D, de Vos WM, Brummer RJ (2015) The relationship between faecal-associated and mucosal-associated microbiota in irritable bowel syndrome patients and healthy subjects. Aliment Pharmacol Ther 42(10):1211–1221. https://doi.org/10.1111/apt.13399
Rooks MG, Garrett WS (2016) Gut microbiota, metabolites and host immunity. Nat Rev Immunol 16(6):341–352. https://doi.org/10.1038/nri.2016.42
Cebra JJ (1999) Influences of microbiota on intestinal immune system development. Am J Clin Nutr 69(5):1046 s-1051 s
Singeap AM, Stanciu C, Trifan A (2016) Capsule endoscopy: the road ahead. World J Gastroenterol 22(1):369–378. https://doi.org/10.3748/wjg.v22.i1.369
Neufeld KA, Foster JA (2009) Effects of gut microbiota on the brain: implications for psychiatry. J Psychiatry Neurosci 34(3):230–231
O’Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, Cryan JF, Dinan TG (2009) Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry 65(3):263–267. https://doi.org/10.1016/j.biopsych.2008.06.026
Whitehead WE, Palsson O, Jones KR (2002) Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology 122(4):1140–1156
Grenham S, Clarke G, Cryan JF, Dinan TG (2011) Brain–gut–microbe communication in health and disease. Front Physiol 2:94. https://doi.org/10.3389/fphys.2011.00094
Cryan JF, Dinan TG (2012) Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13(10):701–712. https://doi.org/10.1038/nrn3346
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature 505(7484):559–563. https://doi.org/10.1038/nature12820
Leibbrand R, Cuntz U, Hiller W (2002) Assessment of functional gastrointestinal disorders using the Gastro-Questionnaire. Int J Behav Med 9(2):155–172
Acknowledgements
We are thankful to Psychiatric Center Ballerup and the Capitol Region of Denmark, for providing support for this study.
Funding
No funding was received in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, no formal consent is required.
Rights and permissions
About this article
Cite this article
Schwensen, H.F., Kan, C., Treasure, J. et al. A systematic review of studies on the faecal microbiota in anorexia nervosa: future research may need to include microbiota from the small intestine. Eat Weight Disord 23, 399–418 (2018). https://doi.org/10.1007/s40519-018-0499-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-018-0499-9