Abstract
The foliar application of nutrients is prevailing in most orchards and it has noticeable favorable effects on vegetative traits, yield and fruit quality. The effect of foliar application of zinc (Zn) and boron (B) on qualitative traits of hazelnut fruits was explored in a factorial experiment based on a randomized complete block design with 12 treatments and three replications in Shavak Village of Rudsar County, Iran. The experimental factors included cultivar (including a local variety, ‘Fertile’, and ‘Ronde du Pimount’) and foliar application (control, B at the rate of 3 mg/L, Zn at the rate of 3 mg/L, and a mixture of B and Zn at the same rates of 3 mg/L) in spring. The recorded traits were antioxidant, anthocyanin, catalase, peroxidase, phenolic compounds, fat, ash, protein, Zn, B, and P. The results indicated that the effect of cultivar was significant (p < 0.01) on anthocyanin, catalase, peroxidase, phenolics, fat, ash, Zn, and B. Mean comparison for the effect of the foliar application revealed that the highest anthocyanin was observed in the local variety. In addition, the effect of foliar application was significant (p < 0.01) on anthocyanin, catalase, peroxidase, fat, ash, protein, Zn, B, and P. Means comparison for the effect of cultivar revealed that cv. ‘Ronde du Pimount’ had the highest anthocyanin, catalase, peroxidase, fat, and Zn content in its kernels. The effect of the foliar application of the micronutrients was positive on the measured qualitative traits. The foliar application of B had a more significant impact on the traits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hazelnut (Corylus avellana L.) is a famous nut tree in the world whose leading producers are Turkey, Italy, Spain, USA, Portugal, and France. It can also be produced in several other countries including Azerbaijan, Georgia, Australia, New Zealand, China, Chile, and Iran (Nedim and Damla 2014). In Iran, over 70% of the hazelnut planting area is located in Guilan province, especially in the Eshkavarat region of Rudsar County so that 17,572 ha of the orchards of this province are planted with hazelnut, producing 17,043 tons of hazelnut per year (Anonymous 2016). Hazelnut kernels are mainly used in food industries, and this requires the development of cultivars with the least defects in their morphological and chemical characteristics (Boccacci et al. 2013). Hazelnut has a low average yield in Iran so that it has been reported to be around 912 kg/ha, whereas the global average is 1193 kg/ha with the highest being 2500 kg/ha for Greece and the lowest being 318 kg/ha for Croatia (FAO 2016). To enhance yield, it is imperative to achieve a high rate of fruitset and adequate fruit development. To accomplish this goal, all processes of flower bud formation, flowering, pollination, fruitset and finally fruit growth should be at a certain and suitable level until harvest time (Sotomayor et al. 2002).
Presently, the foliar application of nutrients is prevailing in most orchards and it has noticeable favorable effects on vegetative traits, yield and fruit quality. In rapid growth and development period of fruits during which competition between reproductive parts and roots for the uptake of nutrients reduces the activity of roots, nutrient uptake is also reduced. This competition can be reduced by foliar application of nutrients in a timely manner (Andrade et al. 2009). Foliar spray is usually employed for nutrients, carbohydrates and organic matter (Blandino et al. 2009). Boron (B) is an essential element for the vegetative and reproductive growth of plants and is involved in antioxidant systems of vascular plants. The element also plays a role in changing the concentration and metabolism of phenolic compounds in higher plants in response to its deficiency. It has been reported that B is required more for pollination than for vegetative structures because it is involved in several reproductive processes including flowering, pollen tube elongation and fruit maturity (Christensen et al. 2016). There are various ways to keep B content of plant tissues at an optimal level including its foliar application to crops during the pollination phase. This method has several advantages over soil incorporation including lower consumption rate of fertilizer, its uniform distribution and more rapid response of plants to its application (Saadati et al. 2013). Nonetheless, a major aspect of crop management is to determine its real requirement based on its physiological and biochemical performance to avoid the deficiency or redundancy of a nutrient (Christensen et al. 2016). Mishra et al. (2010) reported the role of B in mitigating oxidative stress induced by an increased level of reactive oxygen species (ROS) in nut cultivars such as European hazelnut. Lakshmipathi et al. (2018) found the potential of zinc and boron in improving various biochemical parameters of cashew including, chlorophyll, carotenoids and leaf area. Zinc (Zn) is another micronutrient related to fruitset and productivity and its role has been documented in flowering and in the synthesis of tryptophan as a precursor of auxin synthesis and flowering enhancement (Sotomayor et al. 2002). It is also involved in the process of metabolite translocation to shoots or the relevant sites and its deficiency will cause the leaves to be thinner, the internodes to be shorter and the shoot tips to wilt (Pandit et al. 2011). Soils in Iran are clearly suffering from Zn deficiency because they are limy, chemical fertilizers are used in an unbalanced manner, P fertilizers have been applied excessively and micronutrients-containing fertilizers have not been used in the past, so Zn deficiency is expectedly a limiting factor of production and its application is necessary to improve crop yields and quality (Asadi Kanqarshahi et al. 2011). Norozi et al. (2019) reported that foliar application of K and Zn fertilizers is necessary for obtaining better fruit yield and quality in pistachio. Foliar application of fertilizers is a necessary practice for the optimal use of chemical fertilizers in rainfed farms to increase yield and quality (biological value). Foliar application of elements such as Boron, Copper, Magnesium and Zinc in the soils of Iran is preferred over their soil incorporation owing to the rapid tackling of deficiency, easiness, lower toxicity of their accumulation in soils and the prevention of their fixation (Malakouti et al. 2008). Though the role of micronutrients including Zn and B, foliar application in improving hazelnut yield is well documented; therefore, this orchard study was designed to evaluate the role of Zn and B application in improving the quality of hazelnut fruits in a hazelnut-producing region of Iran.
Materials and methods
The present study was carried out in Shavak Village in Rudsar County, Iran as a factorial experiment based on a randomized complete block design with 12 treatments and three replications. The experimental factors included cultivar (a local variety, ‘Fertile’ and ‘Ronde du Pimount’’) and foliar application of fertilizers (Control, B 3 mg/L, Zn 3 mg/L and B + Zn at the same concentration of 3 mg/L) in spring. The fertilizers (borax and zinc sulphate) were sprayed on all individual trees. All operations during plant growth were uniformly applied to all treatments during the experiment. The operation of the foliar application was performed in May when leaf activity was peak (Paulasilva et al. 2003). The trees had similar morphological characteristics such as canopy volume, age and trunk diameter. Each plot contained a tree. Thus, 12 trees from each cultivar were evaluated. Ten-year-old trees in the garden were used. Soil type of trial garden was silty clay and it was nutrient- poor.
Trait measurements
Fat content was measured by the Soxhlet method. The kernels were ground after removing their husks. Then, the fat of the powder was extracted by the Soxhlet method (at 45 °C with dry diethyl ether as the solvent). The solvent content of the extracted fat was separated by an oven in vacuum at 41 °C and its fat content was determined (Hamilton and Rossel 1987).
The B content of the kernels was determined by the method of digestion using dry burning applied to the B extract. Then, its absorption was read at 540 nm with a spectrophotometer (Qin 1996). To find out the Zn content of the kernels, they were oven-dried at 75 °C for 24 h. Then, they were ground, 2 g of the dried kernel was put in a container, and it was heated in an electrical furnace at 550 °C for 5 h to be fully dried. After the sample was cooled down, it was added with 10 mL of 2 N hydrochloric acid. After the reaction was completed, it was placed in a bain-marie at 80 °C until the first steam was observed. The solution was, then, filtrated to obtain a transparent solution and its Zn content was measured with an atomic absorption device (Emami 1996).
Phosphorous content of the samples was measured by the Murphy–Riley method. In this method, we first prepared the ammonium molybdate–vanadate solution. Then, 5 mL of the prepared extract was poured into a 25-mL volumetric flask. Next, 5 mL of the ammonium molybdate–vanadate solution was added to it and it was adjusted to a volume of 25 mL by adding distilled water. The absorption of the yellow light was read at 480 nm with a spectrophotometer (an Apel-PD-303UV model) and P content was estimated by using a calibration curve (derived from standard solutions) (Emami 1996).
To determine the enzyme peroxidase (POD) activity, the related extract was prepared. Then, the OD was read by a spectrophotometer at 430 nm every 30 s for two minutes (Addy and Goodman 1972). To measure the activity of the enzyme catalase (CAT), 0.01 M phosphate buffer (pH 7), 0.5 mL of 0.2 M H2O2, and 2 mL of acid reagent (Citric acid/dichromate mixture) were added to 1 g of plant tissue that had been crashed in 4 mL of ethanol. Then, its absorption was read at 610 nm with a spectrophotometer (Chance and Maehl 1995).
To calculate antioxidant capacity, 1 g of plant was wrapped in a piece of foil and was placed in liquid nitrogen for 2–3 min. Then, it was ground with 10 mL of ethanol 85% and was left at room temperature for 1 h. Then, the samples were filtrated with a filter paper and were centrifuged for 5 min. Next, 150 mL of the samples was added with 850 μL of DPPH. The solution was shaken quickly and was kept in darkness at room temperature for 20 min. After placing the blank and setting the device at zero, we first poured only DPPX on a Cuvette and it was read. Then, the sample was read at 517 nm with a spectrophotometer (Ramandeep and Savage 2005). To measure anthocyanin content, 0.5 g of each sample was separated and crashed in a mortar with 50 cc ethanol + hydrochloric acid (85% ethanol 95% + 15% hydrochloric acid). Then, the extract was filtrated and was adjusted to 50 cc. They were poured into small containers and were placed in a refrigerator at 4 °C for 24 h. Then, they were placed in darkness for another 2 h. To find out anthocyanin content, the extracts were read at 535 nm with a spectrophotometer (Mazumdar and Majumder 2003).
Raw protein content was found out by the Kjeldahl method. In this method, total protein content was measured by digestion, distillation, collection and Titration methods, and N percentage was converted to protein percentage using the factor of 6.25 (Bradford 1976). Non-structural phenolic compounds have various functions in plants like antioxidants (Elizabeth and Kelly 2007). Total phenolics content was determined by the Folin–Ciocalteu method and spectrophotometry (Meyers et al. 2003). In this method, 50 μL of fruit extract was mixed with 100 μL of Folin reagent (5%). After keeping at 25 °C for 5 min, 100 μL of sodium bicarbonate 7% was added to it. The blank sample used deionized water as the extract. After keeping in darkness for 120 min, the absorption was read at 765 nm with a spectrophotometer and the standard curve was obtained by different concentrations of Gallic acid. Total ash was obtained by oven-drying of the samples at 75 °C for 12 h. Then, the samples were placed in a furnace and it was gradually heated up to 550 °C. After 24 h, the color of the sample changed to white and just ash remained (AOAC 1990).
Statistical analysis
Data analysis was carried out by MSTATC and SPSS and data mean comparison was performed by the LSD method.
Results and discussion
Antioxidants
The analysis of variance (ANOVA; Table 1) showed that the simple effect of cultivar and foliar application of micronutrients and their interaction was not significant for antioxidant activity of hazelnut fruits. Research on nuts, such as walnuts, pecan, chestnuts and pistachios shows that they can be a rich source of antioxidants. Hazelnuts are no exception. Numerous studies have reported antioxidant activities of hazelnut kernel extract, hazelnut fat, and its byproducts (Teresa et al. 2010). We found that antioxidant capacity was not influenced by cultivar or the foliar application of B and Zn so that no statistically significant differences were observed among the experimental factors.
Kornsteiner et al. (2006), Arcan and Yemenicioglu (2009) and Alasalvar et al. (2006) have highlighted the antioxidant activities of nuts. Durak et al. (1999) reported that following the consumption of hazelnut, malondialdehyde (MDA) level was significantly decreased in plasma samples and antioxidant potential (AOP) was increased, implying that antioxidant components of hazelnuts hindered peroxidation reactions greatly.
Anthocyanin
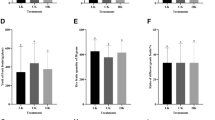
According to the results of ANOVA, the simple and interactive effects of cultivar and micronutrients were significant (p < 0.01) on anthocyanin content of the hazelnuts (Table 1). Cultivars had significantly different anthocyanin contents and the highest was 490.9 mg/100 g observed in ‘Ronde’ and the lowest was 187.1 mg/100 g observed in ‘Fertile’ (Table 2). Means comparison of data (Table 3) indicated that the highest anthocyanin content of the hazelnuts (404.5 mg/100 g) was related to the Zn-treated fruits but differing insignificantly from those treated with B + Zn and control plants. Also, the B-treated plants showed the lowest anthocyanin content (274 mg/100 g) and significantly differed from other treatments. In a study of the effect of foliar application of B on strawberries, Wojcik and Wojcik (2007) stated that B increased soluble solids and anthocyanin content of the fruits irrespective of the application methods. They recommended to foliar application of B to improve the quality and appearance of strawberries. Based on Table 4, highest anthocyanin was obtained under interaction of “Ronde × control”.
Protein
According to the results of ANOVA (Table 1), the effect of cultivar and the interactive effect of treatments were not significant on protein content of hazelnut kernels. But, foliar application of micronutrients influenced kernel protein content significantly (p < 0.01). Means comparison of data (Table 3) revealed that the application of B had the highest fruit protein content of 0.114%, showing significant differences with that of control. But, the application of Zn and Zn + B did not differ significantly. Fruit protein contents in plants treated with them were 0.109 and 0.112%, respectively. The hazelnuts treated with B and Zn exhibited significantly higher fruit protein content than control. This is similar to the report of Nedim and Damla (2014) about the effect of foliar application of Zn on quantitative and qualitative traits of hazelnut (cv. ‘Tombol’), according to which the protein content of the hazelnut kernels was increased as more Zn was applied. Ozdemir and Akinci (2004) state that hazelnut is a rich source of energy and protein. Zinc is a constituent of the structure of many enzymes (Zn-enzymes). Zinc deficiency in plants causes the reduction of protein synthesis and the accumulation of amino acids in plants by reducing transcription and increasing the decomposition of Ribonucleic acids (Marschner 1995). Protein percentage is dictated by plant feeding and is influenced by fertilization treatments. Zinc enhances the translocation of amino acids and the synthesis of RNA, thereby increasing protein synthesis (Whitty and Chambliss 2005). In this respect, researchers have stated that Zn application can increase the protein content of grains. It has been documented that in plants suffering from Zn deficiency, the activity of enzyme RNA polymerase and protein synthase is reduced remarkably and the rate of amino acid conversion diminishes. Therefore, protein content of grains can be increased by foliar application of Zn through its effect on increasing RNA polymerase and protein synthase (Akhtar et al. 2009).
Catalase
As the results of ANOVA indicated, the simple and interactive effects of the experimental factors (cultivar and micronutrients) were significant (p < 0.05) on catalase activity (Table 1). Means comparison for the effect of cultivar revealed that the highest catalase activity was related to ‘Ronde’ differing from that of the local variety and ‘Fertile’ significantly (Table 2). Also, means comparison for the effect of micronutrients (Table 3) reflected that the plants treated with Zn + B had the highest catalase activity in their fruits, showing significant differences with control and Zn treatment but insignificant difference with the treatment of B. The lowest catalase activity was obtained from the control plants.
Catalase is an antioxidant enzyme that is necessary for activating defensive responses against stress and the scavenging of free radicals (Lee et al. 2007). The higher activity of this enzyme in foliar application treatments can be attributed to the role of Zn in strengthening plants’ antioxidant system which makes plants more tolerant to stress (Zand et al. 2010). In this respect, Jung et al. (2006) pointed to the role of catalase in improving stress tolerance of maize mutants when exposed to post-pollination stress. In another study, a higher level of catalase was observed in drought-resistant cultivars of wheat than in susceptible cultivars (Feng et al. 2004).
Means comparison for the interaction of cultivar and foliar application of micronutrients (Table 4) indicated that the highest catalase activity was obtained from cv. ‘Ronde’ fertilized with Zn + B and it was significantly different from the other treatments. The lowest catalase activity was observed in the local variety × control.
Peroxidase
According to the results of means comparison (Table 2), the simple effect of cultivar was not significant on peroxidase activity in hazelnut fruits among cultivars, but the simple effect of micronutrients and the interactive effect of cultivar and micronutrients were significant for this trait. Means comparison for foliar application of micronutrients (Table 3) revealed that the highest content of peroxidase was related to the treatment of B, showing significant differences with other experimental treatments. The lowest content was observed in Zn-sprayed plants, but it did not differ significantly from control and Zn + B.
Peroxidases generally act as ROS detoxifying enzymes because hydrogen peroxide is a compound involved in a wide range of reactions that are dependent on peroxidase as an electron receiver (Zand et al. 2010). In the meantime, peroxidases are involved in breaking down H2O2 via several mechanisms (Kawano 2003). Thus, it is inferred that the foliar spray of treatments that are superior in terms of peroxidase activity level will lead to the breakdown of hydrogen peroxide in cells. With respect to the interactive effect of cultivar and foliar application levels on peroxidase activity of the fruits, it was found the highest activity was obtained from ‘Ronde’ × B treatment, showing statistically significant differences with other treatments. The activity of catalase and peroxidase is the second most important defensive mechanism against oxidative stresses (Sanitaditoppi and Gabbrieli 1999). Zinc can act as a stabilizer and protector of vital membranes against oxidative stress and peroxidase damages. Also, Zn applies its impacts by changing plasma cohesion and membrane permeability (Hassan et al. 2005).
Phenolic compounds
According to the results of ANOVA for the effect of cultivar on phenolics content (Table 1), cultivar significantly (p < 0.01) influenced this trait. But, the trait was not significantly changed by foliar application of micronutrients. As means comparison (Table 3) revealed, the highest phenolics content of 2.49 mg/g fruit was obtained from cv. ‘Fertile’ with a significant difference with the other two studied cultivars. But, no significant difference was observed between the local variety (1.7 mg/g fruit) and cv. ‘Ronde’ (1.6 mg/g fruit). In a report on the need for soil incorporation and foliar application of zinc sulfate in potato gardens, Souri and Malakouti (2003) note that Zn is a major factor of potato quality and highlight its role in reducing phenolics compounds and possibly in increasing antioxidants of apple fruit tissue and juice. The phenolics of seeds and husks are involved in yield. They are natural antioxidants and, along with vitamin E, help seed protection against oxidations As well, phenolics may influence nut flavor and taste by making them bitter (Bignami et al. 2005).
Fat
As ANOVA (Table 1) showed, the effect of cultivar and micronutrients was significant (p < 0.01) on fat content of hazelnut fruits. Means comparison for the effect of cultivar on fat content (Table 2), the highest fat content of 57.43% was related to cv. ‘Ronde’, showing significant differences with the other two cultivars. The fat content of the local variety and cv. ‘Fertile’ did not differ to one another significantly. According to the results of means comparison (Table 3), the effect of foliar application of micronutrients was significant on the fat content of fruits versus control. The highest fat content was related to the fruits treated with B with a significant difference with control but insignificant difference with the treatments of Zn and Zn + B. In other words, all foliar application levels improved fat content of the hazelnuts as compared to control.
Hazelnut has a high fat content. The most important compounds of hazelnut are composed of fatty acids with monounsaturated fat (MUFA) or polyunsaturated fat (PUFA). These compounds may be good for lipid profile of blood serum so that they protect low-density lipoprotein (LDL) against oxidations and reduce the oxidized LDL level in plasma (Teresa et al. 2010). Bignami et al. (2005) determined fruit quality and compounds of six hazelnut cultivars over a 2-year period and reported that fat content varied from 61 to 68%, but no difference was observed among years in this trait. In a study of the effect of different levels of iron and zinc sulfate on qualitative and quantitative traits of soybean, Adeli and Rafiee (2018) stated that Zn application increased fat and protein content of soybean cv. ‘L17’ significantly. In another study on almond, Bybordi and Malakouti (2006) revealed that the simple effect of Zn application was significant (p < 0.01) on fruit fat percentage and the highest fat content of 53% was observed in Zn-sprayed plants.
Ash
The results of ANOVA (Table 1) showed that the simple and interactive effects of the experimental factors were significant (p < 0.01) on hazelnut ash content. According to means comparison (Table 2), the lowest ash content (1.77% of DM) was related to cv. ‘Ronde’. The local variety and ‘Fertile’ did not differ significantly in ash content. Means comparison (Table 4) indicated that the highest ash content was obtained from the plants treated with B and they differed significantly from the control and other treatments. The lowest ash content was obtained from the control (1.83% DW), but it was not different from Zn and B + Zn treatments significantly. Many studies have shown that the composition of minerals and protein, fat content and ash content of hazelnut are determined by climate, diversity, soil composition, fertilization, irrigation, planting method, harvest year, and local geography (Ozkutlu et al. 2011). In our experiment, the ash content of the hazelnut fruits treated with micronutrients was increased versus control, which is consistent with Nedim and Damla (2014). They reported that the total ash content of hazelnut ‘Tombol’ in Turkey was significantly increased with the increase in Zn application from 20.5 to 25.1 g/kg. According to means comparison (Table 4), hazelnuts ‘Fertile’ treated with B had the highest ash content (2.43% of DM) and this combination was different from ‘Fertile’ × control (1.95% of DM) and other experimental treatments significantly.
Zinc
According to the results of ANOVA (Table 1), the simple effect of cultivar and micronutrients was significant on Zn content of fruits significantly (p < 0.01). As means comparison (Table 2) shows, the highest Zn content was obtained from cv. ‘Ronde that differed from that of local variety insignificantly and that of ‘Fertile’ significantly.
According to means comparison (Table 3), the highest Zn content of hazelnut fruits (0.26 mg/kg) was obtained from Zn treatment and this differed from control (with Zn content of 0.19 mg/kg) significantly but from other treatments insignificantly. The lowest Zn content was related to control whose Zn content was increased by about 36% when it was sprayed with Zn. Saadati et al. (2013) found that the application of zinc sulfate and boric acid to olive fruits increased fruit-setting and B content of leaves and fruits significantly so that the application of these two micronutrients improved fruit quality. However, Zn was more influential on qualitative traits, especially fruit size. Cakmak (2008) stated that the application of Zn fertilizer increased grain Zn content and seed vigor. Although plants require a little Zn, the unavailability of this trace element will cause the plants to suffer from physiological stresses emanating from the inefficiency of numerous enzymatic systems (Bybordi and Jasarat 2010).
Boron
The results of ANOVA (Table 1) indicated that the simple and interactive effects of cultivar and micronutrients were significant on B content of hazelnut fruits significantly. Means comparison (Table 2) showed that the cultivars differed significant in terms of fruit B content so that the highest B content was related to ‘Fertile’ and the lowest to the local variety.
Among the fertilization treatments, the highest B content of 5.82 mg/kg was obtained from the treatment of B, which was significantly different from the control and the treatments of Zn and Zn + B. The control displayed the lowest B content. The measurement of Zn and B content of the fruits showed that foliar spray of the hazelnuts in spring increased the concentration of these elements in the fruits. This is consistent with the results of Ghaderi et al. (2003) about the increase in Zn and B content of almond fruits following their foliar treatment with boric acid and zinc sulfate and inconsistent with their results about the increase in B content of hazelnut leaves. This may show that fruit acts as a strong sink. The interaction of hazelnut cultivar and foliar application level for fruit B content (Table 4) showed that the highest B content was related to cv. ‘Fertile’ × B application, differing from that of control × ‘Fertile’ significantly. The lowest B content (2.81) was obtained from the interaction of cv. ‘Ronde’ × control.
In their study of the effect of Zn and B application on fruit setting and quality of olives, Saadati et al. (2013) stated that the application of boric acid and zinc sulfate enhanced B content of the studied tree leaves considerably. Also, the treatment with boric acid had the same increasing effect on B content of fruits. They reported that B can be partitioned to younger parts of the plants in spite of the demand by the growing sinks. It can be concluded that B is supplied to growing tissues via phloem and that the B content of phloem may be supplied from growing leaves indirectly or from xylem directly. The rapid emergence of B deficiency symptoms in sink tissues after the withdrawal of B from the growing medium shows that the rate of B mobilization in phloem is partially dictated by the current rate of B uptake (Hosseini and Malakouti 2005).
According to Abdel-Karim et al. (2015), the foliar application of B and Zn promoted the quantitative and qualitative traits of avocado fruits significant and the foliar application of B was more effective in most treatments. As well, the mixture of B and Zn in the same proportion (1 g/L Zn + 1 g/L B) had positive synergistic effects so that it caused more changes versus control.
Phosphorous
The results of ANOVA (Table 1) showed that the effect of cultivar was not significant on fruit P content, but the application of micronutrients and its interaction with cultivar was significant (p < 0.01) for this trait. Means comparison for the effect of fertilization (Table 2) showed that the highest hazelnut P content (0.28%) was obtained from the treatment of Zn, showing significant difference with control and other treatments. The lowest P content was related to control.
Means comparison for the interaction of cultivar and foliar application of micronutrients (Table 4) revealed that the highest P content (0.28%) was obtained from the local variety treated with Zn showing insignificant differences with that of the local variety treated with B. However, it differed from the other treatments significantly. The lowest one was 0.17% observed in the local variety × control. The effect of the foliar application of Zn and B was significant for hazelnut kernel P content so that all treatments improved it versus control.
In a study of the effect of Zn foliar application on nutrient contents of two safflower (Carthamus tinctorius L.) cultivars, Moradi et al. (2015) observed an increase in the Zn content of the fertilized seeds whereas the P content of the seeds showed a decrease. Phosphorous may interact with B. Boron is essential for the development and elongation of root apexes, which improves P uptake (Paulasilva et al. 2003). However, P affects B uptake too. It has been reported that P redundancy lessens B uptake in citrus. B and P may be subject to surface absorption by solid particles or be involved in alluvial reactions with sesquioxides and clay minerals of soil. (Bignami et al. 2005).
Conclusion
A key factor for fruit formation and the improvement of its quantitative and qualitative traits is the supply of trace elements. We found that the foliar application of zinc sulfate and Borax improved hazelnut fruit quality in the studied cultivars significantly. In total, the foliar application of B was more influential on the studied traits than other treatments. Given the positive effect of B and Zn application and their interaction on the hazelnut fruits, it is suggested to apply to meet plant requirements in a timely manner and thereby improve its qualitative traits.
References
Abdel-Karim, H. A., Nehad, M. A., El-Rouby, K. M., & Roshdy, K. A. (2015). Effect of foliar application of Boron and Zinc on fruit set, yield and some fruit characteristics of Fuerte avocado. Research Journal of Pharmaceutical, Biological and Chemical Sciences,6(5), 443–449.
Addy, S. K., & Goodman, R. N. (1972). Polyphenol oxidase and peroxidase in apple leaves inoculated with a virulent or an avirulent strain for erwinia amylovora. Indian phytopathology,25, 575–579.
Adeli, S., & Rafiee, M. (2018). Influence of different levels of zinc and iron sulfate on quantitative and qualitative characteristics of soybean L17 cultivar. Journal of Applied Research of Plant Ecophysiology,4(2), 49–66.
Akhtar, N., Sarker, M. A., Akhter, H., & Nada, K. (2009). Effect of planting time and micronutrient as zinc chloride on the growth: yield and oil content of Mentha piperita. Bangladesh Journal of Scientific and Industrial Research,44(1), 125–130.
Alasalvar, C., Karamac, M., Amarowicz, R., & Shahidi, F. (2006). Antioxidant and antiradicalactivities in extracts of hazelnut kernel (Corylus avellana L.) and hazelnutgreen leafy cover. Journal of Agricultural and Food Chemistry,54, 4826–4832.
Andrade, S., Priscila, L., Gratao, L., Schiavinato, M., & Silveira, A. (2009). Zn uptake, physiological response and stress attenuation in mycorrhizal jack bean growing in soil with increasing Zn concentration. Chemospher,75, 1363–1370.
Anonymous. (2016). Statistic book of the Ministry of Jihad-e Agriculture: Office of Statistics, IT, Networking, and GIS of Guilan Province.
AOAC. (1990). Official methods of analysis (17th ed.). Washington, DC: Association of Official Analytical Chemists.
Arcan, I., & Yemenicioglu, A. (2009). Antioxidant activity and phenolic content of fresh and dry nuts with or without the seed coat. Journal of Food Composition and Analysis,22, 184–188.
Asadi Kanqarshahi, A., Akhlaghi Amiri, N., & Malakouti, M. (2011). Effect of rates and methods of application of zinc on yield, and fruit quality in Satsuma Mandarin. Iranian Journal of Soil and Water Research,42(1), 77–86.
Bignami, C., Cristofori, V., & Troso, D. (2005). Kernel quality and composition of hazelnut (Corylus avellana L.). Acta Horticulturae,686, 477–484.
Blandino, M., Pilati, A., & Reyneri, A. (2009). Effect of foliar treatment to durum wheat on flag leaf senescence, grain yield, quality and deoxynivalenol contamination in North Italy. Field Crop Research,114(2), 214–222.
Boccacci, P., Armamini, M., Valentini, N., Bacchetta, L., Rovira, M., Drogoudi, P., et al. (2013). Molecular and morphological diversity of on-farm hazelnut (Corylus avellana L.) landraces from southern Europe and their role in the origin and diffusion of cultivated germplasm. Tree Genetics & Genomes,9, 1465–1480.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of dye binding. Analytical Biochemistry,72, 248–254.
Bybordi, A., & Jasarat, A. (2010). Effects of the foliar application of magnesium and zinc on the yield and quality of tree grape cultivars grown in the clcareous soils of Iran. Notulae Scientia Biologicae,2(2), 81–86.
Bybordi, A., & Malakouti, M. J. (2006). Effects of foliar applications of nitrogen, boron and zinc on fruit setting and quality of aalmonds. Acta Horticulturae,726, 351–358.
Cakmak, I. (2008). Enrichment of cereal grains with zinc. Agronomic or genetic biofortification. Plant and Soil,302, 1–17.
Chance, B., & Maehl, A. C. (1995). Assay of catalase and peroxidase. In S. P. Colowick & N. D. Kaplan (Eds.), Methods in enzymology (Vol. 2, pp. 764–791). New York: Academic Press.
Christensen, P., Beede, R. H., & Peacock, W. (2016). Fall foliar sprays prevent boron deficiency symptomps in grapes. California Agriculture,60(2), 100–103.
Durak, I., Köksal, I., Kacmaz, M., Büyükkocak, S., Cimen, B. M. Y., & Öztürk, H. S. (1999). Hazelnut supplementation enhances plasma antioxidant potential and lowersplasma cholesterol levels. Clinica Chimica Acta,284, 113–115.
Elizabeth, A., & Kelly, M. (2007). Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nature Protocol,2(4), 875–877.
Emami, A. (1996). Plant analysis method. Tehran: Soil and Water Research Institute.
FAOSTAT. (2016). Food and agriculture data. http://Faostat.fao.org/site/565/desktop.
Feng, Z., Jin-Kui, G., Ying-Li, Y., Wen-Liang, H., & Li-in, Z. (2004). Changes in the pattern of antioxidant exzymes in wheat exposed to water deficit and rewatering. Acta Physiologiae Plantarum,26(3), 345–352.
Ghaderi, N., Vazvaei, A., Talaei, A., & Babalar, M. (2003). The effect of foliar application of boron and zinc on the concentration of these elements in the leaves and fruits and some traits of almond fruits. Iran Journal of Agricultural Sciences,34(1), 127–135.
Hamilton, R. J., & Rossel, J. B. (1987). Analysis of oils and fats. Elsevier Applied Science, 18–19, 49–55, 103, 245, 313–336.
Hassan, M. J., Zhang, G., Wei, K., & Chen, Z. (2005). Zinc alleviates growth inhibition and oxidative stress caused by cadmium in rice. JPNSS,168, 255–261.
Hosseini, Y., & Malakouti, M. (2005). Boron dynamism in plants. Higher council of policymaking for development of application of biological compounds and optimal use of fertilizers and herbicides in agriculture. Tehran: Sena Press.
Jung, S., Chon, S. U., & Kuk, Y. I. (2006). Differential antioxidant responses in catalase deficient maize mutants exposed to norflurazon. Biologia Plantarum,50(3), 383–388.
Kawano, T. (2003). Roles of the reactive oxygen species-generating peroxidase reactions in plant defense and growth in duction. Plant Cell Reports,21, 829–837.
Kornsteiner, M., Wagner, K. H., & Elmadfa, I. (2006). Tocopherols and total phenolics in10 different nut types. Food Chemistry,98, 381–387.
Lakshmipathi, J., Adiga, D., Kalaivanan, D., Muralidhara, B. M., & Preethi, P. (2018). Effect of zinc and boron application on leaf area, photosynthetic pigments, stomatal number and yield of cashew. International Journal of Current Microbiology and Applied Sciences,7, 1786–1795.
Lee, S. H., Choi, J. H. W. S., Park, Y. S., & Gemma, H. (2007). Effect of calcium chloride spray on peroxidase activity and stone cell development in pear fruit (Pyrus pyrifolia, Nnitaka). Journal of the Japanese Society for Horticultural Science,76, 191–196.
Malakouti, M., Keshavarz, P., & Karimian, N. (2008). A comprehensive method to diagnose and propose optimal fertilization for sustainable farming. Tehran: Tarbiat Maddares University Press.
Marschner, H. (1995). Nutrition of higher plants (2nd ed.). London: Academic Press.
Mazumdar, B. C., & Majumder, K. (2003). Methods on physcochemical analysis of fruits (pp. 136–150). Calcutta: University College of Agriculture.
Meyers, K. J., Watkins, C. B., Pritts, M. P., & Liu, R. H. (2003). Antioxidant and antiproliferative activities of strawberries. Journal of Agricultural and Food Chemistry,51, 6887–6892.
Mishra, N., Dubey, A., Mishra, R., & Barik, N. (2010). Study on antioxidant activity of common dry fruits. Food and Chemical Toxicology,48, 3316–3320.
Moradi, M., Roushan, F., & Siadat, S. (2015). Effect of foliar application of zinc sulfate on minerals content, seed and oil yields of two safflower cultivars (Carthamus tinctorius L.). Iranian Journal of Crop Science,17(2), 153–164.
Nedim, Ö., & Damla, B. Ö. (2014). Effect of iron fertilization on nut traits and nutrient composition of Tombul hazelnut (Corylus avellana L.) and its potentialvalue for human nutrition. Acta Agriculturae Scandinavica Section B-Soil and Plant Science,64(7), 633–643.
Norozi, M., ValizadehKaji, B., Karimi, R., & Nikoogoftar Sedghi, M. (2019). Effects of foliar application of potassium and zinc on pistachio (Pistacia vera L.) fruit yield. International Journal of Horticultural Science and Technology,6(1), 113–123.
Ozdemir, F., & Akıncı, I. (2004). Physical and nutritional properties of fourmajor commercial Turkish hazelnut varieties. Journal of Food Engineering,63, 341–347.
Ozkutlu, F., Dogru, Y. Z., Ozenc, N., Yazıcı, G., Turan, M., & Akcay, F. (2011). The importance of Turkish hazelnut trace and heavy metal contents for human nutrition. Journal of Soil Science and Environmental Management,2, 25–33.
Pandit, A. H., Wani, M. S., Mir, M. A., Bhat, K. M., Wani, S. M., & Malik, A. R. (2011). Effect of foliar application of boron and zink on fruit set and productivity of almond. Acta Horticulturae,903, 1007–1009.
Paulasilva, A., Eduardo, R., & Silivia, H. (2003). Infuence of foliar Boron on fruit set and yield of hazelnut. Journal of Plant Nutrition,26(3), 561–569.
Qin, X. (1996). Foliar sprays of B, Zn, and Mg and their effects on fruit production and quality of Jincheng organ. Journal of Southwest Agricultural University,18(1), 40–45.
Ramandeep, K. T., & Savage, P. G. (2005). Antioxidant activity in different factions of tomatoes. Food Research International,38, 487–494.
Saadati, S., Moallemi, N., Mortazavi, S. M. H., & Seyyednejad, S. M. (2013). Foliar applications of zinc and boron on fruit set and some fruit quality of olive. Scientia Horticiculturae,164, 30–34.
Sanitaditoppi, L., & Gabbrieli, R. (1999). Response to cadmium in higher plants. Environmental and Experimental Botany,41, 105–130.
Sotomayor, C., Silva, H., & Castro, J. (2002). Effectiveness of boron and zinc foliar sprays on fruit setting of two almond cultivars. Acta Horticiculturae,591, 437–440.
Souri, M., & Malakouti, M. (2003). The role of zinc sulfate and calcium chloror foliar application on alleviating apple juice browning. Technical Report No. 192.
Teresa, D., Ricardo, M., José, A. P., & Elsa, R. (2010). Hazelnut (Corylus avellana L.) kernels as a source of antioxidants and their potential in relation to other nuts. Industrial Crops and Products,32, 621–626.
Whitty, E. N., & Chambliss, C. G. (2005). Fertilization of field and forage crops (pp. 21–24). Reno: Nevada State University Publication.
Wojcik, P., & Wojcik, W. (2007). Effect of boron fertilization on sweet cherry tree yield and fruit quality. Journal of Plant Nutrition,29(10), 1755–1766.
Zand, B., Sorooshzadeh, A., Ghanati, F., & Moradi, F. (2010). Effect of zinc and auxin foliar application on some antioxidant enzymes activity in corn leaf. Iranian Journal of Plant Biology,2(1), 35–48.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alidust, M., Sedaghathoor, S. & Abedi Gheshlaghi, E. The effect of foliar application of boron and zinc on qualitative traits of hazelnut cultivars. Plant Physiol. Rep. 25, 131–139 (2020). https://doi.org/10.1007/s40502-019-00470-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-019-00470-y