Abstract
The current study reports on in vitro propagation and conservation of critically endangered medicinal plant Swertia chirayita. The sterilized explants (leaves) were cultured on Murashige and Skoog’s (MS) medium supplemented with 0.1 mg/L naphthalene acetic acid (NAA) and 3.0 mg/L benzyl adenine (BA) for callus induction, the same medium was used for shoot regeneration and for direct shoot regeneration from in vitro leaves. For in vitro multiplication shoots were cultured on MS medium supplemented with 2.5 mg/L benzyl adenine and 0.1 mg/L Kinetin (Kn). MS half strength medium supplemented with 400 mg/L activated charcoal and 0.1 mg/L naphthalene acetic acid showed 80.30% root induction from in vitro grown shoots. The in vitro raised plantlets were successfully acclimatized. All the regenerated plantlets appeared normal with 70–80% survival rate, while In vitro conservation was carried out by using two different approaches namely slow growth by changing media composition (sucrose and abscisic acid) at low temperature and cryopreservation following vitrification. With increase in concentration of sucrose and ABA decrease in growth of in vitro shoots was observed. At low temperature the in vitro shoots incubated at 4 and 10 °C both showed 100% retrieval. During studies the vitrified shoot gave retrieval of 42.33% when pre-cooled at 4 °C while only 22.77% vitrified shoots were retrieved from those pre-cooled at 10 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Swertia chirayita Buch.-Hams. ex Wall. commonly known as “Chirayita” and belongs to family Gentianaceae. It is an indigenous species of temperate Himalaya found at an altitude of 1200–3000 m from Kashmir to Nepal and also distributed in Bhutan, Khasi hills and Sikkim. It is an annual/biennial medicinal herb (Joshi and Dhawan 2007) but still it is not clear whether the plant behaves differently due to climatic conditions or varying genotype. It can be traced through the medicinal history as a safe ethno medicinal herb. The plant has long been used for its blood-purifying, antifungal and antihelmintic properties (Pant et al. 2011). S. chirayita plants contain several active constituents such as xanthones, flavonoids, iridoids and secoiridoid glycosides that are responsible for its therapeutics properties (Kumar and Chandra 2013).The major phytochemicals of the bitter-tasting plant include swertiamarin, amarogentin and mangiferin, a xanthone C-glucoside (Phoboo et al. 2013). Swertiamarin is reported to be effective against hepatitis (Wang et al. 2011) and shown to exhibit anti-diabetic (Vaidya et al. 2013), anticancer (Kavimani and Manisenthlkumar 2011) and anti-arthritic (Pal et al. 2012) activities. Amarogentin is known to be anti-diabetic (Phoboo et al. 2013), anticancer (Pal et al. 2012). Due to its over exploitation for different medicinal uses and commercial purposes its availability is decreasing day by day so it is becoming extinct. S. chirayita conservation status has been categorized as “critically endangered” (Joshi and Dhawan 2005; Padhan et al. 2015; Shailja 2017.)
Plant tissue culture has significant role in multiplying and conserving plant germplasms. Micropropagation is an alternative to the conventional method. Modifying an in vitro regeneration protocol for Swertia chirayita is urgent to promote large-scale production for ex situ conservation and for satisfying the pharmaceutical needs. The present study was focused to develop an efficient protocol for mass multiplication and conservation of this critically endangered medicinal plant.
Materials and methods
Plant material
Young and healthy leaves of one year old Swertia chirayita plants were procured in the month of August, September and October from nursery of Department of Forest Product, Dr. Y S Parmar University of Horticulture and Forestry Nauni, Solan, Himachal Pradesh, India. The nursery is located at Shilli (30°54′ 18″ N 77°17′ 21″ E), district Solan of Himachal Pradesh at an elevation 1460 m above mean sea level (Fig. S1).
Explant preparation
Explants i.e. leaves were excised with help of scalpel blade and collected in jar containing water. The explants were washed under running tap water for about 30 min and then treated with tween-20. Further explants were aseptically transferred to laminar air flow and rinsed with 70% alcohol for about 1–2 min. Thereafter, explants were treated with 0.3% bavistin for different time durations, followed by washing with autoclaved distilled water to remove the traces of bavistin. After that treatment of 0.5% sodium hypochlorite was given for different time durations, followed by washing with autoclaved distilled water to remove the particles of sodium hypochlorite.
In vitro propagation
The sterilized explants were transferred to 100 mL glass flasks containing MS (Murashige and Skoog 1962) basal medium supplemented with different concentrations and combinations of growth regulators i.e. BA (2.0–3.5 to 1 mg/L) and NAA (0.1–0.4 mg/L) for the establishment of callus and shoot bud induction. The pH of the medium was adjusted at 5.8 before gelling and then autoclaved at 121 °C temperature and 15 psi pressure for 15 min. The cultures were incubated at 25 ± 2 °C under 16/8 photoperiod with 50 μmol/m2/s photosynthetic photon flux (PPF), obtained from cool fluorescent tube lights (Philips, Kolkata, India). The effect of different plant growth regulators such as BA (1.0–2.5 mg/L) and Kinetin (0.1–0.4 mg/L) was studied on in vitro shoot multiplication. The leaves were separated from in vitro regenerated shoots and cultured on solid MS basal medium as well as on MS medium supplemented with different combinations of BA (1.0–3.5 mg/L) and NAA (0.1 mg/L). MS medium lacking plant growth regulators served as control.All the experiments were carried out in three replications containing 24 explants in each replication. Data were recorded after an interval of 4 weeks.
In vitro rooting
The plant species follows its natural ‘rosette’ habit of growth even in in vitro cultures, therefore the in vitro shoots were excised on the basis of length of leaf petiole. When the petiole gained a length of 3–4 cm the shoots were separated from the rosette and transferred to culture tubes (15 cm × 150 cm) containing either MS basal medium of half strengths supplemented with 400 mg/L activated charcoal in combination with NAA (0.1–0.4 mg/L) for in vitro root induction. Rooting response was recorded in terms of average of roots, average number of root length and rooting percentage.
Acclimatization and hardening
In vitro regenerated plants were removed from test tubes and agar medium attached was washed off completely. Plants were kept dipped in 0.2% solution of carbendazim for 30 min before transferring to plastic cups containing autoclaved potting mixture consisting of equal amount of different potting mixture soil:sand, soil:FYM, soil:sand:FYM and soil:cocopeat. The pots were covered with perforated polythene bags and watered at 1 day interval and supplied with ¼ strength salts of MS medium twice a week by spraying. Subsequently polythene bags were removed and plants were transferred to earthen pots containing FYM: soil 1:1 and watered as and when required. The survival rate of plantlets was recorded after 1 month of transfer to plastic pots.
In vitro conservation
In vitro maintained shoots subcultured at every 4 week interval were the source of explants for in vitro conservation.
Two approaches have been followed as given below:
-
1.
Slowing down the growth by (a) changing media composition (b) reducing culture temperature.
-
1a.
In vitro raised shoots were cultured on full and half strength MS media with different concentration of sucrose (30–90 g/L) and ABA (1.0–3.0 mg/L) to slow down the growth.
-
1b.
Shoots were incubated for their performance at two lower temperatures i.e. at 4 and 10 °C also. The other culture conditions and medium composition remained same as were for in vitro culture of shoots.
-
1a.
-
2.
Cryopreservation following vitrification of in vitro shoots.
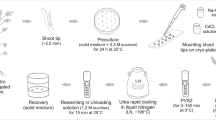
In vitro raised shoots of Viola pilosa were divided into two sets and pre-cooled separately at 4 and 10 °C in incubator for 1 month to slow down the growth by reducing culture temperature. The medium was supplemented with various cryoprotectants such as sucrose, glycerol, ethylene glycol. The pre-cooled in vitro shoot segments were placed on V1 (MS salts + 0.5 M sucrose) medium in petriplates and parafilm sealed petriplates were kept overnight in dark at 4 °C temperature inside a refrigerator. The shoot segments were then transferred to pre-cooled cryovials containing V2 (MS salts + 0.4 M sucrose +3 M glycerol) solution and cryovials were again incubated at 4 °C for 20–30 min. After this V2 solution was pipetted out from cryovials leaving only shoot segments behind and 1.5–2 ml of vitrification solution V3 (MS salts + 30% glycerol + 13% DMSO +15% ethylene glycol) was added to them. The cryovials were ice incubated in laptop cooler for 60 min. Finally, the cryovials were directly immersed in liquid nitrogen. For retrieval the cryovials were taken out thawed rapidly in water bath at 40 °C for 2–3 min. Than V3 solution the was discarded and buds were washed with V4 (MS salts + 1.2 M sucrose) solution to completely eradicate the effects of cryoprotectants and then the shoots were placed on an autoclaved filter paper inside a petridish containing 1 ml of liquid shoot multiplication medium i.e. MS basal + 0.1 mg/L Kinetin +2.5 mg/L BA. The petridish was sealed with parafilm and kept overnight in dark at 25 ± 2 °C. After this, the shoot buds were transferred to fresh, solid shoot multiplication medium in conical flasks and incubated in diffused light for 7–10 days. Finally the shoot tips were transferred to normal culture conditions as is given for in vitro propagation. Retrieval was recorded by counting number of shoot tips regaining growth on multiplication medium.
Statistical analysis
The data recorded was analyzed according to CRD (completely randomized design) analysis using standard statistical software. All experiments were replicated three times. The data of ‘percent’ was converted to arc sine value for the analysis of variance (ANOVA). The critical differences between the treatments were calculated and used to compare the means of treatments and interpretations of results were made accordingly. Each treatment throughout the experimentation consisted of three replicates and the experimental unit was four explant per treatment. The results summarise the data of four independent experiments. Statistical differences between the mean tabulated values were estimated (p ≤ 0.05) using CRD with the OPSTAT online Software (Kumar et al. 2015).
Results and discussion
In vitro establishment and multiplication
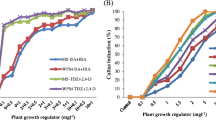
The initial establishment of axillary buds was observed in all the medium compositions used. Both BA and NAA promoted callus establishment, In vitro shoot bud induction and direct shoot regeneration from In vitro leaves. However, BA (3.0 mg/L) in combination with NAA (0.1 mg/L) showed the highest percentage (100%) of callus induction, In vitro shoot bud induction and direct shoot regeneration from in vitro leaves (Tables 1, S1, S2). No callus formation takes place when medium was supplemented with BA (2.0 mg/L) and NAA (0.4 mg/L) (Table 1). The callus was brown and fragile. MS basal media without growth regulators also showed callus induction which was not reported anywhere earlier (Fig. 1a). Similarly, callus induction and shoot regeneration using stem as explant on MS medium containing 3.0 mg/L BA and 0.5 mg/L NAA was reported by (He et al. 2012). Bisht and Bisht (2008) also reported callus induction and shoot regeneration in Swertia angustifolia Buch-Ham. using leaf and petiole as explant. They found that BA and NAA in the range of 0.5–3.0 mg/L exhibited fast proliferation in callus mass and shoot regeneration in both explants whereas lower concentration of BA (0.5 mg/L) produced poor calli in these explants. The effect of varying concentrations of growth regulators (BA and NAA) on in vitro shoot bud formation from callus explant was investigated (Fig. 1b). Out of total, medium MC4 and MC1 (control) showed presence of shoot bud formation while others remained unaffected. The results revealed 100% shoot bud induction on MS medium supplemented with (3.0 mg/L BA) and (0.1 mg/L NAA )with maximum number of shoots 4.50, having average shoot length 1.72 cm, respectively (Table S1). The control used in the study showed significantly less (66.67%) shoot bud induction with average shoot length 1.32 cm. In vitro leaves were used for direct shoot bud regeneration where, a combination of high cytokinin and low auxin was found to be suitable for the induction of shoot buds directly from the leaf explant. The effect of different concentrations of BA along with 0.1 mg/L NAA on direct shoot regeneration from in vitro leaf was evaluated. The results presented in Table S2 showed 77.78% shoot regeneration on control medium (MS) devoid of any phytohormone. A maximum of 88.89% leaf showed direct shoot regeneration on MS medium supplemented with (3.0 mg/L BA) and (0.1 mg/L NAA) with maximum number of shoots (9.33) having average shoot length of 2.50 cm. During the study it was observed that direct shoot regeneration from leaves initiated after 4 weeks of incubation (Fig. 1d). Similarly, Wang et al. (2009) also observed direct shoot regeneration from mature leaves of Swertia chirayita on MS medium supplemented with 13.32 mM BA and 0.54 mM NAA. Our results were similar with previous study on the shoot formation using leaves taken from in vitro shoot culture of S. chirayita (Chaudhuri et al. 2008). Direct shoot regeneration from shoot tip explant on MS medium containing BA and Kinetin was reported by Balaraju et al. (2009). Subsequently, in vitro shoot multiplication was continued on BA (2.5 mg/L) and Kn (0.1 mg/L) supplemented medium since this proved to be the best composition giving an average number of shoots per explant (5.66) along with shoot length (3.50 cm) after 30 days of incubation (Fig. 1c). No shoot multiplication was observed on treatment with M13 and M17, respectively (Table 2). Similar results were reported by other workers Balaraju et al. (2009) induced multiple shoots in Swertia chirayita on MS medium supplemented with BA 1.0 and 0.1 mg/L kinetin. However, in contrast to this, Dafadar and Jha (2011) reported in vitro shoot multiplication of Swertia bimaculata on half strength MS medium supplemented with 2.22 μM BA, 2.32 μM kinetin and 0.54 μM NAA. Pant et al. (2009) obtained multiple shoots of Swertia chirayita on MS media supplemented with 4.44 μM BAP, 2.85 μM IAA and 271.45 μM Ads with an average of 10.0 numbers of shoots with shoot length 2.0 cm after 4 weeks.
Micropropagation of SwertiachirayitaBuch.- Hams. ex Wall. from leaf explants derived from ex vitro grown plants. a, b Callus and shoot bud induction in MS medium with BA 3.0 mg/L + NAA 0.1 mg/L. c Shoot multiplication on MS medium + BA 2.5 mg/L + Kn 0.1 mg/L. d Direct shoot bud induction on leaf explant on MS medium + BA 3.0 mg/L + NAA 0.1 mg/L. e In vitro rooting on half strength MS medium + activated charcoal 400 mg/L l + NAA0.1 mg/L. f Rooted plantlet after 4 weeks of incubation. g Hardening of in vitro raised plantlet
In vitro conservation by slow growth method using different concentrations of sucrose. a Full strength MS medium + 30 g/L sucrose (control). b Full strength MS medium + 60 g/L sucrose. c Full strength MS medium +90 g/L sucrose. d Half strength MS medium + 30 g/L sucrose. e Half strength MS medium + 60 g/L sucrose. f Half strength MS medium + 90 g/L sucrose
In vitro rooting
Overall, MS basal medium of ½ strength proved better for root induction supplemented with activated charcoal 400 mg/L and 0.1 mg/L NAA (Table 3). The rooting characters like percentage rooting with maximum number of roots and root length have been influenced by the MS strength + Activated charcoal and NAA concentration. 80.30% rooting with maximum number of roots (6.5) and root length (1.5 cm) on this medium (MR2). However, on half strength MS basal medium containing 400 mg/L activated charcoal without NAA, 42.33% of rooting was observed. No rooting was observed on MR4 and MR5 medium (Fig. 1 e, f). Similar results were reported earlier Wawrosch et al. (1999) induced in vitro rooting in Swertia chirayita by dipping the shoots in 15 ppm solution of NAA followed by culturing in half strength MS media. Joshi and Dhawan (2007) reported in vitro rooting in Swertia chirayita on medium containing 1 µM NAA and 500 mg/L activated charcoal. He et al. (2012) and Bisht and Bisht (2008) also reported rooting in Swertia shoots on media containing NAA.
Acclimatization and hardening
Amongst different hardening mixtures used, soil: cocopeat (1:1) was best for plant survival. 70–80% survival was achieved on this mixture. When different hardening environments were compared, it was observed that the plants which were kept in glass house covered with green net under controlled light and temperature conditions showed best growth and survival. All the stages of in vitro propagation are shown in Fig. 1g.
In vitro conservation
Slow growth by changing the media composition and reducing temperature.
Slow growth is usually achieved by reducing the culture temperature, by modifying culture media with supplements of osmotic agents and growth inhibitors, striving to maximize the time between subcultures (Gopal et al. 2003; Patil et al. 2004; Goncalves and Romano 2007; Lata et al. 2010; Soni and Kaur 2013).
The effect of variable concentrations (3–9%) of sucrose and ABA (1.0–3.0 mg/L) on growth of in vitro raised shoots of Swertia chirayita was investigated. In vitro raised shoots measuring 0.5 cm were cultured on full and half strength. MS media with different concentration of sucrose and ABA to slow down the growth of in vitro raised shoots.
There was 100% survival of shoots on all the treatments (Tables 4, 5). With increase in concentration of sucrose and ABA, there was decrease in growth rate, number of shoots per explant, shoot length and leaf size was observed (Figs. 2, 3a–f). On MS medium supplemented with 3% sucrose, 5.66 shoots per explant with average shoot length of 2.5 cm and leaf size of 3.67 cm was observed after 4 weeks of incubation. Minimum growth was observed on half strength MS medium supplemented with 9% sucrose as no shoot multiplication occurred and each shoot remained as single shoot with average shoot length of 0.67 cm and leaf size of 0.35 cm. However increase in concentration of ABA from 1.0 to 3.0 mg/L resulted in decreased number of shoots per explants. No shoot proliferation was observed full strength and half strength MS medium supplemented with 3.0 mg/L ABA respectively. Minimum average shoot length (0.83 cm) and leaf size (0.83 cm) was observed with full strength MS medium (3.0 mg/L) ABA. Osmotic regulators, such as sucrose and mannitol, added to the culture medium, these carbohydrates reduce the hydric potential and restrict the water availability to the explants (Fortes et al. 2001; Shibli et al. 2006). Besides osmotic regulators, growth regulators are also routinely used for in vitro germplasm conservation, with ABA being one of the most commonly used. The ABA generally acts as an endogenous growth retardant and has been used for growth reduction of in vitro cultures (Gopal et al. 2004). Carbohydrates strongly affect growth and physiology of plants in all in vitro culture phases, including conservation, as they serve both as carbon sources for cultured tissues and as osmotic regulators in the medium (Pruski et al. 2000). In general a decrease in shoot multiplication rate, shoot length and leaf size was observed with the increase of sucrose concentrations. The survival of shoots at all the concentrations was 100% however, a linear tendency of decrease in shoot length and average number of shoot, leaf size was observed with the increase in sucrose concentrations. Positive effect medium with ABA for in vitro conservation were also reported by Ding et al. (2010) and Manoj et al. (2011), showed that application of exogenous ABA improves in vitro conservation and the adaptive response of plant cell and tissues to various environmental stresses.
In vitro conservation by slow growth method using different concentration of ABA. a Full strength MS medium + 1.0 mg/L ABA. b Full strength MS medium + 2.0 mg/L ABA. c Full strength MS medium + 3.0 mg/L ABA. d Half strength MS medium + 1.0 mg/L ABA. e Half strength MS medium + 2.0 mg/L ABA. f Half strength MS medium + 3.0 mg/L ABA
In vitro conservation by slow growth method at low temperature and retrieved of shoots after cryopreservation in liquid nitrogen (−196 °C). a Shoots incubation at 4 °C after 4 weeks, b shoots incubation at 10 °C after 4 weeks, c retrieval of shoots preconditioned at 4 °C, d retrieval of shoots preconditioned at 10 °C
In vitro shoots multiplying on MS + 2.5 mg/L BA + 0.1 mg/L Kinetin medium were incubated at two different temperatures i.e. 4 and 10 °C at normal photoperiod of 16/8 h for checking the effect of low temperature on their growth. After 30 days of incubation, the shoots were brought back to normal culture conditions. Shoots kept at 4 and 10 °C no shoot multiplication was observed and shoots remained as single shoot with average shoot length of 0.86 and 0.76 cm. Results also showed that leaf size of 0.34 and 0.23 cm at 4 and 10 °C, respectively. No morphological variation was observed in shoots incubated at both temperatures (Fig. 4a, b). The reduction of incubation temperature has been shown to be very effective in prolonging the subculturing cycle by reducing the growth rate (Engelmann 2011). Under reduced temperature, a high accumulation of unsaturated lipids on cell membranes causes cell membrane thickening and retards cell division and elongation. In vitro slow growth at low temperature has been routinely used for conserving germplasm of plants such as Inula racemosa (Kashyap 2009), Viola pilosa (Soni and Kaur 2013).
Cryopreservation following vitrification technique
The in vitro shoot pre-cooled at 4 °C for 1 month showed multiplication within 3 weeks of culturing. The retrieval rate was 42.33% (Fig. 4c, d). However, they attained their normal growth rate after subculturing. While the per cent retrieval of shoot tips with prior incubation at 10 °C was very less i.e. 22.77% as compared to that of shoots with prior incubation at 4 °C and they showed multiplication after 4 weeks of culturing (Table S4). During this process plant materials are dehydrated by highly concentrated cryoprotectant, then directly dipped in liquid nitrogen (Turner and Singha 1990). Preculturing of explants on MS basal medium supplemented with high concentration of sucrose prior to vitrification had a significant effect on the survival of explants. It is for example known that sugar can maintain the liquid crystalline state of the membrane bilayer and stabilizes proteins under frozen condition Importance of the same has been reported as an important step before vitrification by various researchers (Maubmann et al. 2006; Halmagye and Pinkar 2006) for papaya, (Kashyap 2009; Halmagye and Pinkar 2006) for Inula racemosa and (Soni and Kaur 2013) for Viola pilosa.
Conclusion
Increasing demand and importance to traditional medicine in the recent years has threatened the survival of some plant species. The traditional conventional plant propagation methods are affected by biotic and variable environmental factors. This report describes an efficient in vitro propagation protocol using leaves as explants in Swertia chirayita, where the plant is uprooted before its seed development because it takes long time that is why it is categorized as endangered medicinal plant. The shoots were rooted ex vitro could save time, labor, produces large number of plants and energy in production of plantlets. While in vitro conservation by slow growth, slows down the growth of the plant and it can not be uprooted at earlier stage and by storing at low temperature plant can be conserved for the long time.
References
Balaraju, K., Agastian, P., & Ignacimuthu, S. (2009). Micropropagation of Swertia chirata Buch. Hams. ex Wall: A critically endangered medicinal herb. Acta Physiologiae Plantarum, 31(3), 487–494.
Bisht, S. S., & Bisht, N. S. (2008). Callus induction studies in different explants of Swertia angustifolia (Buch-Ham). Plant Archives, 8(2), 713–716.
Chaudhuri, R. K., Pal, A., & Jha, T. B. (2008). Conservation of Swertia chirata through direct shoot multiplication from leaf explants. Plant Biotechnology Report, 2, 213–221.
Dafadar, A., & Jha, T. B. (2011). In vitro propagation and conservation of Swertia bimaculata Hook.f. and Thomas. Indian Journal of Biotechnology, 11, 295–299.
Ding, W., Song, L., Wang, X., & Bi, Y. (2010). Effect of ABA and heat stress tolerance in the calli form two ecotypes of phragmits communis. Biologia Plantaerum, 54, 67–613.
Engelmann, F. (2011). Use of biotechnologies for the conservation of plant biodiversity. In vitro Cellular and Development Biology—Plant, 47, 5–16.
Fortes, G., Rde, L., & Pereira, J. E. S. (2001). Effect of low temperature and growth retardants on in vitro conservation of asparagus. Revista Cientifica Rural, 6(2), 181–186.
Goncalves, S., & Romano, A. (2007). In vitro minimum growth for conservation of Drosophyllum lusitanicum. Biologia Plantarum, 51, 795–798.
Gopal, J., Anjali, C., & Debabrata, S. (2003). Use of microtubers for slow growth in vitro conservation of potato. Journal of the Indian Potato Association, 30(1/2), 35–36.
Gopal, J., Chamail, A., & Sarkar, D. (2004). In vitro production of microtubers for conservation of potato germplasm: Effect of genotype, abscisic acid, and sucrose. In vitro Cellular and Developmental Biology—Plant, 40, 485–490.
Halmagye, A., & Pinkar, I. (2006). Cryopreservation of Rosa shoot tips: Importance of precultured conditions. Acta Horticulturae, 725, 351–356.
He, T., Xu, J., Yang, L., & Wang, H. (2012). An efficient method for plant regeneration from calli of Swertia mussotii, an endangered medicinal herb. American Journal of Plant Sciences, 3(7), 904–908.
Joshi, P., & Dhawan, V. (2005). Swertia chirayita—An overview. Current Science, 89, 635–640.
Joshi, P., & Dhawan, V. (2007). Axillary multiplication of Swertia chirayita: A critically endangered medicinal herb of temperate Himalayas. In Vitro Cellular and Developmental Biology Plant, 43(6), 631–638.
Kashyap, A. (2009). Studies on in vitro propagation and conservation of Inula racemosa. In Hook. F (Ed.). Ph. D. Thesis. Dr. Y S Parmar University of Horticulture and Forestry, 158 p.
Kavimani, S., & Manisenthlkumar, K. T. (2011). Effect of methanolic extract of Enicostemma littorale on Dalton’s ascitic lymphoma. Journal of Ethnopharmacology, 71, 349–352.
Kumar, V., & Chandra, S. (2013). Efficient regeneration and antioxidant activity of the endangered species Swertia chirayita. International Journal of Pharma and Bio Sciences, 4(4), 823–833.
Kumar, A., Guleria, S., Mehta, P., Walia, A., Chauhan, A., & Shirkot, C. K. (2015). Plant growth-promoting traits of phosphate solubilizing bacteria isolated from Hippophae rhamnoides L. (Sea-buckthorn) growing in cold desert Trans-Himalayan Lahul and Spiti regions of India. Acta Physiologiae Plantarum, 37(3), 1–12.
Lata, H., Moraes, R. M., Bertoni, B., & Pereira, A. M. S. (2010). In vitro germplasm conservation of Podophyllum peltatum L. under slow growth conditions. In vitro Cellular and Developmental Biology—Plant, 46, 22–27.
Manoj, K., Rai, N. S., Shekhowat, H. K., & Gupta, A. (2011). The role of ABA in plant tissue culture: A review of recent progress. Plant Cell, Tissue and Organ Culture, 106, 179–190.
Maubmann, V., Serek, M., & Winkelmann, T. (2006). Cryopreservation of embryogenic suspension of cultures of Cyclamen persicum Mill. Acta Horticulturae, 725, 391–396.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantareum, 15, 473–497.
Padhan, J. K., Kumar, V., Sood, H., Singh, T. R., & Chauhan, R. S. (2015). Contents of therapeutic metabolites in Swertia chirayita correlate with the expression profiles of multiple genes in corresponding biosynthesis pathways. Phytochemistry, 116, 38–47.
Pal, D., Sur, S., Mandal, S., Das, A., Roy, A., Das, S., et al. (2012). Prevention of liver carcinogenesis by amarogentin through modulation of G1/S cell cycle check point and induction of apoptosis. Carcinogenesis, 33, 2424–2431.
Pant, M., Bisht, P., & Gusain, M. P. (2009). De novo shoot organogenesis from cultured root explants of Swertia chirata Buch.-Ham.ex Wall: An endangered medicinal plant. African Journal of Biotechnology, 11(29), 7408–7416.
Pant, M., Bisht, P., & Gusain, M. P. (2011). In vitro propagation through root derived callus culture of Swertia chirata Buch. Ham. ex Wall. African Journal of Biotechnology, 11(29), 7408–7416.
Patil, M. S., Adiga, J. D., Reddy, B. S., Kulkarni, B. S., Hegde, L., & Mulge, R. (2004). Effect of slow growth strategies for in vitro conservation of gladiolus. Karnataka Journal of Horticulture, 1(1), 39–43.
Phoboo, S., Pinto, M. D. S., Barbosa, A. C. L., Sarkar, D., Bhowmik, P. C., Jha, P. K., et al. (2013). Phenolic-linked biochemical rationale for the anti-diabetic properties of Swertia chirayita (Roxb. ex Flem.) Karst. Phytotherapy Research, 27, 227–235.
Pruski, K., Kozai, T., Lewis, T., Astakie, T., & Novak, J. (2000). Sucrose and light effects on in vitro cultures of potato, chokecherry and Saskatoon berry during low temperature storage. Plant Cell, Tissue and Organ Culture, 63, 215–221.
Shailja, (2017). A mini review on in vitro propagation of Swertia chirayita an endangered medicinal plant. Bioscience Biotechnology Research Communication, 10(1), 8–12.
Shibli, R. D., Shatnawi, M. A., Subaih, W. S., & Ajlouni, M. M. (2006). In vitro conservation and cryopreservation of plant genetic resources: A review. World Journal of Agricultural Sciences, 2, 372–382.
Soni, M., & Kaur, R. (2013). Rapid in in vitro propagation, conservation and analysis of genetic stability of Viola pilosa. Physiology and Molecular Biology Plants. doi:10.1007/s1298-013-0200-8.
Turner, S. R., & Singha, S. (1990). Vitrification of crabapple, pear, and geum on gellan gum solidified culture medium. Hortscience, 25(12), 1648–1649.
Vaidya, H., Goyal, R. K., & Cheema, S. K. (2013). Anti-diabetic activity of swertiamarin is due to an active metabolite, gentianine, that upregulates PPAR-c gene expression in 3T3-L1 cells. Phytotherapy Research, 27, 624–627.
Wang, L., An, L., Hu, Y., Ping, W., Lixin, W., & Li, Y. (2009). Influence of phytohormones and medium on the shoot regeneration from leaf of Swertia chirata Buch. Ham. ex Wall in vitro. African Journal of Biotechnology, 8(11), 2513–2517.
Wang, J., Zhao, C., Liu, C., Xia, G., & Xiang, F. (2011). Introgression of Swertia mussotii gene into Bupleurum scorzonerifolium via somatic hybridization. BMC Plant Biology, 11, 71.
Wawrosch, C., Maskay, N., & Koop, B. (1999). Micropropagation of the threatened Nepalese medicinal plant Swertia chirata Buch-Ham. Ex Wall. Plant Cell Report, 18(12), 997–1001.
Acknowledgement
We thanks Dr Y S Parmar Univ. of Horticulture and Forestry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest among them.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shailja, Kanwar, K., Soni, M. et al. In vitro propagation and conservation of an endangered high value medicinal herb Swertia chirayita of temperate Himalayas. Ind J Plant Physiol. 22, 247–257 (2017). https://doi.org/10.1007/s40502-017-0294-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-017-0294-z