Abstract
Renal ectopia and fusion anomalies are Congenital Anomalies of the Kidney and the Urinary Tract (CAKUT) that are usually incidentally detected and asymptomatic. Patients affected present a higher risk of complications like recurrent urinary tract infections or obstruction. Pancake kidney (PK) is one of the rarest types of renal anomaly with complete fusion of the superior, mild and inferior poles of both kidneys in the pelvic cavity. Each kidney has its own excretory system with two ureters that do not cross the midline. In the asymptomatic cases, a conservative approach should be performed. Surgical management may be needed when urological problems occur. PK is often associated with congenital anomalies of other organs. Ultrasound is the first line radiological examination for the diagnosis and the follow-up of kidney malformations. The main sonographic findings suggesting PK diagnosis are a large and lobulated renal mass consisting of two fused lateral lobes without an intervening septum located in the pelvic cavity. Each lobe usually has a separate pelvicalyceal system, the renal pelvis is anteriorly placed and the ureters are usually short and enter the bladder normally without crosses the midline. Ultrasonography gives useful information on the morphology and volume of the organ, and on its vascularization through the use of the Color- and Power-Doppler. Computer Tomography and Magnetic Resonance Urography are second level techniques used to confirm the diagnosis and to evaluate the presence of other abnormalities. The knowledge of the imaging findings and the anatomy of congenital renal malformations is important to avoid diagnostic pitfalls and misinterpretations. We report the case of a 14-years old female with PK who was misdiagnosed with a horseshoe kidney (HSK) during an abdominal ultrasound.
SOMMARIO
L’ectopia e le anomalie di fusione renale rientrano nel gruppo delle Anomalie Congenite del Rene e del Tratto Urinario (CAKUT, Congenital Anomalies of the Kidney and Urinary Tract). Generalmente asintomatiche, queste condizioni vengono descritte come reperti incidentali. I pazienti affetti presentano un rischio più elevato di complicanze dell’apparato urinario, come infezioni e/o ostruzioni. Il rene a focaccia è una rara malformazione congenita dell’apparato urinario caratterizzata dalla fusione dei poli superiori, medi ed inferiori di entrambi i reni all’interno della cavità pelvica. Ogni rene è dotato di un proprio sistema escretore, i cui corrispettivi ureteri non attraversano la linea mediana. Nei casi asintomatici il trattamento è di tipo conservativo; il trattamento chirurgico è indicato solo in caso di complicanze urologiche. Il rene a focaccia può associarsi spesso a malformazioni congenite di altri organi o apparati. L’ecografia è la metodica di imaging di prima istanza utile sia per la diagnosi che per il follow-up delle anomalie renali congenite. I principali segni ecografici che suggeriscono la diagnosi di PK sono una voluminosa e lobulata massa renale in sede pelvica, formata dalla fusione dei due reni in assenza di setti. Ogni reni presenta un sistema calico-pielico autonomo e separato, i cui ureteri non oltrepassano la linea mediana e dopo un breve decorso sboccano normalmente in vescica. L’ecografia fornisce, pertanto, informazioni morfologiche e volumetriche, ma anche sulla vascolarizzazione mediante l’utilizzo del Color- e Power-doppler. Metodiche di imaging di secondo livello sono la Tomografia Computerizzata e la Uro-Risonanza Magnetica, utili per un ulteriore conferma diagnostica e per identificare eventuali altre anomalie. La conoscenza delle singole caratteristiche delle varie malformazioni renali è importante per evitare i frequenti errori diagnostici. In questo articolo descriviamo il caso di una paziente di sesso femminile di 14 anni con rene a focaccia, erroneamente diagnosticato come rene a ferro di cavallo in una precedente ecografia dell’addome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Renal ectopia and fusion anomalies are congenital anomalies of the kidney and the urinary tract (CAKUT) that are usually incidentally detected and asymptomatic [1]. Patients affected present a higher risk of complications such as recurrent urinary tract infections or obstruction [2]. Pancake kidney (PK) or fused pelvic kidney (also known as cake, disc, shield, doughnut kidney, lump or discoid) is a complete fusion renal anomaly with extensive fusion of the superior, mild and inferior poles of both kidneys [2,3,4].
Case report
We report a case of a 14-years-old female affected by acute right lower abdominal pain. Her medical and family history was unremarkable; a renal abnormality, classified as horseshoe kidney, was found in another hospital during an abdominal ultrasound (US). The clinical examination revealed localized pain in her right iliac fossa with tenderness and guarding. Laboratory test showed leukocytosis with an increased level of inflammatory markers (CRP); creatinine and urea resulted normal. An abdominal US targeted to the physical exam finding was performed and showed a dilated appendix with thickening of the appendiceal wall layers and periappendiceal reactive nodes enlargement. The patient underwent surgery and the histopathological examination demonstrated a malignant appendiceal carcinoid tumor.
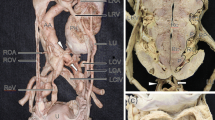
At the first follow-up for the appendiceal carcinoid 1 month after the surgery, Contrast Enhanced Computed Tomography (CECT), performed with special dose reduced protocols in the portal venous phase, resulted negative for residual tumor or metastatic localization but revealed a large kidney mass, consisting of two fused lateral lobes forming a central isthmus of normal tissue, placed in hypogastrium-left iliac region, anteriorly to the plane between L4-S1. The right and the left arteries originate from the aorta just above the level of aortic bifurcation. An accessory upper polar renal artery originated from the right common iliac artery. The kidney was drained by two different veins directly into the inferior vena cava (Fig. 1).
The CECT was executed for the follow-up of the appendiceal carcinoid, so it was done without the excretory phase. We performed additional studies to define better and evaluate the excretory system.
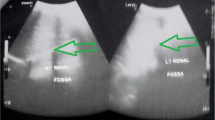
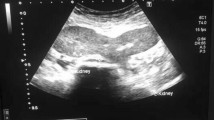
US examination demonstrated a fused pelvic kidney with lobulated contours. Corticomedullary ratio and parenchymal echogenicity were normal. Color-Doppler examination showed normal vascularization (Fig. 2) with a Renal Resistivity Index (IRR) in the normal range, inferior than 0.7 [5].
It was not possible to identify the number of ureters because the pelvis and the calyces were collapsed.
A magnetic resonance urography (MRU) showed similar findings but revealed a renal pelvis anteriorly placed with two short and not dilated ureters that did not cross the midline entering the bladder in abnormal relationship, at the anterolateral wall (Fig. 3).
No evidence of calculi, obstruction or hydronephrosis were found. No anomalies of the abdominal visceral organs were associated.
The patient was discharged in good health condition and it was programmed a CECT scan 6 months later for the follow-up of the appendiceal carcinoid.
Discussion
Congenital urinary system anomalies occur in 3.3–11.1% of the population and they account for about 50% of all congenital abnormalities [6]. Congenital renal malformations include anomalies of number (renal agenesis, supernumerary kidney), shape (HSK and PK), location (simple or ordinary unilateral/bilateral ectopia, crossed ectopia with or without fusion), rotation, renal parenchymal (multicystic dysplastic kidneys and cystic renal diseases) and reduplication of the pelvis and the ureters [1].
The HSK represents the most common abnormality of shape with an incidence of 0.25% in the general population and an M:F ratio of 2,3:1. It is characterized by the presence of two kidneys located symmetrically on either side of vertebral column with a midline fusion of the lower poles, or more rarely of the upper poles, forming an isthmus of normal or fibrous tissue [6, 7]. The isthmus is usually situated anteriorly to L3-L5 [8]. There are two different collecting systems that descend towards the bladder, without crossing the midline [8]. The arteries supplying the HSK are branches of the abdominal aorta, inferior mesenteric or common iliac artery. The veins end, directly or indirectly, in the inferior vena cava. Furthermore, HSK is frequently associated with vascular anomalies of the aortic branches [9].
Crossed fused renal ectopia (CFRE) is the second most common fusion renal anomaly with an incidence of 1:7500 [10]. Both kidneys are located on one side [7]; the ectopic kidney is located on the opposite side of the ureteral insertion and its ureter crosses the midline, in contrast to HSK and PK [11]. Left to right ectopia is more common than right to left (3:1) [12]. Six types of CFRE have been described: unilateral fused kidney (distinguished in superior or inferior ectopia), sigmoid or S-shaped kidney, L-shaped kidney, unilateral lump kidney and disc kidney [7].
PK is one of the rarest types of renal ectopia and fusion anomaly and is more common in men than women [13]. Looney and Dodd were the first to define and describe this condition [14]. PK is characterized by a large and lobulated renal mass consisting of two fused lateral lobes without an intervening septum located in the pelvic cavity. Each lobe usually has a separate pelvicalyceal system. The renal pelvis is anteriorly placed and the ureters are usually short and enter the bladder normally [6]. PK usually receives the blood supply from two main arteries originating from the abdominal aorta, below the inferior mesenteric artery, or from the common iliac artery. A single or additional artery may be present. Venous drainage is carried out by vena cava or iliac vein. In some rare cases, a single renal vein may be found [2]. PK should not be confused with the CFRE type disc kidney in which the ureter of the ectopic kidney crosses the midline [7].
Congenital renal malformations are usually incidentally detected and asymptomatic; in these cases, a conservative approach with a long term follow-up must be performed [2].
The alterations of the excretory system predispose patients to recurrent urinary tract infections or obstructions [2].
The alterations of the vascularization are particularly significant in case of abdominal trauma or pelvic surgery, especially for aortic procedures [15, 16]. The knowledge of renal fusion anomalies is also important in emergency medicine because the ectopic kidney is more inclined to be injured by compression in blunt abdominal trauma, both because of the lack of protection by the chest wall and because of its position relative to the lumbar spine. An empty renal fossa, a distorted architecture or an unusual orientation, identified during a FAST scan, should make the sonographer search for an ectopic kidney or an HSK [17].
Extra-renal symptoms may be present because congenital renal malformations are often associated with congenital anomalies of genitourinary, nervous, cardiovascular and skeletal system [13].
An increased risk of developing a renal neoplasm, including Wilms tumor, renal cell carcinoma and rarely rhabdomyosarcoma, is reported in patient with renal fusion anomalies [18, 19].
For these reasons patients should be informed of their condition to receive an appropriate follow-up even when the finding is accidental [17].
Imaging
US is the first noninvasive, low cost and radiation free diagnostic tool readily available to evaluate renal anomalies and their complications, also during the prenatal period [13]. US is especially important in children and in pregnant women and it can be safely used also when Magnetic Resonance Imaging (MRI) is contraindicated. Thanks to the B-mode technique it is possible to evaluate the anatomy, the morphology (anteroposterior and pole to pole length, parenchymal thickness and echogenicity) and the pathology of the kidney (renal stones, hydronephrosis, neoplasms). The color-doppler technique gives information about vascularization, blood flow velocimetry and renal artery resistivity index (RRI) that are useful tools for the evaluation of the complications. Elastography is under development and in course of study for renal tissue characterization [20].
Contrast Enhanced Ultrasound (CEUS) can be used for the study of the complications [21].
Limitations include interference by gaseous bowel loops and subjective errors [22]. US can underestimate the real size of fused kidney and in some cases it cannot distinguish between congenital renal malformation and retroperitoneal tissue [22].
Computed tomography (CT) is the gold standard to delineate the anatomy, the morphology and the vascularity of the kidney, allowing a functional evaluation. Limitations include the exposition to radiation and the risk of reactions to the contrast agent; therefore, it is mostly performed when US findings are equivocal and not for preliminary study. However, CT can provide detailed information about congenital renal malformation complications, such as pyelonephritis, stones, hydronephrosis, and benign or malign lesions [4]. In children, special dose reduced protocols and portal venous phase split-bolus dual-energy CT urography must be considered [23].
MRU is a non-invasive technique with high spatial resolution that allows anatomical and functional imaging of urinary system [24]. Static-fluid and excretory MRU can be combined with conventional MRI to evaluate urinary tract complications in case of renal failure, severe allergic reaction to iodinated contrast, especially in children and in pregnant women. Disadvantages include high cost, claustrophobia, metal implants, foreign bodies and the need for a child sedation procedure [25].
Conclusion
Knowing the imaging findings of congenital fused renal malformations is important to avoid diagnostic pitfalls and misinterpretations.
PK is a rare kidney malformation and, as such, needs to undergo differential diagnosis with other congenital malformations such as HSK and CFRE.
Usually, they are accidental findings. In the asymptomatic cases a conservative approach, with an appropriate follow-up, should be performed.
US is the first line radiological examination for the diagnosis and the follow-up of kidney malformations. Ultrasonography gives useful information on the morphology and volume of the organ and on its vascularization through the use of the Color- and Power-Doppler. The presence of a fused pelvic kidney with two ureters that do not cross the midline is suggestive for a PK. In contrast to PK, HSK present two kidneys located symmetrically on either side of vertebral column with a midline fusion of the lower poles, while CFRE present both kidneys on one side and ureter that crosses the midline.
CT and MRU are second level techniques used to confirm the diagnosis and to evaluate the presence of anatomical variants to reduce post-operative complications.
Surgical management may be needed when urological problems occur.
References
Ramanathan S, Kumar D, Khanna M, Al Heidous M, Sheikh A, Virmani V, Palaniappan Y (2016) Multi-modality imaging review of congenital abnormalities of kidney and upper urinary tract. World J Radiol. https://doi.org/10.4329/wjr.v8.i2.132
Ghawanmeh HM, Al-Ghazo M, Halalsheh OM, Al-Ghazo OM, Alshammari AK, Al-Karasneh AI, Al-Okour R (2017) Pancake kidney found inside abdominal cavity: rare case with literature review. Urol Case Rep 13:123–125. https://doi.org/10.1016/j.eucr.2016.11.020
Wong HYF, Lee KH (2018) The pancake kidney. Abdom Radiol. https://doi.org/10.1007/s00261-018-1706-x
Prasad DVSR, Srinivas S (2016) Problem of fused kidneys—our observations. IAIM 3(9):182–188
Tublin ME, Bude RO, Platt JF (2003) The resistive index in renal Doppler sonography: where do we stand. Am J Roentgenol. https://doi.org/10.2214/ajr.180.4.1800885
Srinivas MR, Adarsh KM, Jeeson R, Ashwini C, Nagaraj BR (2016) Congenital anatomic variants of the kidney and ureter: a pictorial essay. Jpn J Radiol. https://doi.org/10.1007/s11604-015-0514-2
Babu CSR, Sharma V, Gupta OP (2015) Renal fusion anomalies: a review of surgical anatomy. Anat Physiol. https://doi.org/10.4172/2161-0940.s5-001
Oktem H, Gozil R, Calguner E, Bahcelioglu M, Mutlu S, Kurkcuoglu A, Kadioglu D (2008) Morphometric study of a horseshoe kidney. Med Princ Pract 17(1):80–83. https://doi.org/10.1159/000109596
Schiappacasse G, Aguirre J, Soffia P, Silva CS, Zilleruelo N (2015) CT findings of the main pathological conditions associated with horseshoe kidneys. Br J Radiol 88(1045):20140456. https://doi.org/10.1259/bjr.20140456
Pupca G, Miclăuş GD, Bucuraş V, Iacob N, Sas I, Matusz P, Loukas M (2014) Left crossed fused renal ectopia L-shaped kidney type, with double nutcracker syndrome (anterior and posterior). Romanian J Morphol Embryol 55:1237–1241
Bhatnagar V, Gupta A, Kumar R, Solanki S (2013) Crossed fused renal ectopia: challenges in diagnosis and management. J Indian Assoc Pediatr Surg 18(1):7. https://doi.org/10.4103/0971-9261.107006
Mudoni A, Caccetta F, Caroppo M, Musio F, Accogli A, Zacheo MD, Nuzzo V (2017) Crossed fused renal ectopia: case report and review of the literature. J Ultrasound 20(4):333–337. https://doi.org/10.1007/s40477-017-0245-6
Tiwari AK, Choudhary AK, Khowal H, Chaudhary P, Arora MP (2014) Pancake kidney: a rare developmental anomaly. J Can Urol Assoc 8:7–8. https://doi.org/10.5489/cuaj.1933
Looney WW, Dodd DL (1926) An ectopic (pelvic) completely fused (cake) kidney associated with various anomalies of the abdominal viscera. Ann Surg 84:522–524
Hollis HW, Rutherford RB, Crawford GJ, Cleland BP, Marx WH, Clark JR (1989) Abdominal aortic aneurysm repair in patients with pelvic kidney: technical considerations and literature review. J Vasc Surg 9(3):404–409. https://doi.org/10.1016/S0741-5214(89)70001-X
Pasquali M, Sciascia N, Liviano GDA, La Manna G, Zompatori M (2018) Case report pancake kidney: when it is not a problem. BJR Case Rep 4:3–6
Rosenthal AA, Ditchek JJ, Lee SK, Sanchez R, Kiffin C, Davare DL, Carrillo EH (2016) Congenital renal fusion and ectopia in the trauma patient. Case Rep Emerg Med 2016:1–4. https://doi.org/10.1155/2016/5203872
Sado HN, Duarte PS, Sapienza MT (2016) Pancake kidney with cysts and a single ureter Dear. Bcad. Radiol Brasil 49(2):127–128
Walther A, Cost NG, Garrison AP, Geller JI, Alam S, Tiao GM (2013) Renal rhabdomyosarcoma in a pancake kidney. Urology 82(2):458–460. https://doi.org/10.1016/j.urology.2013.03.003
Grenier N, Gennisson JL, Cornelis F, Le Bras Y, Couzi L (2013) Renal ultrasound elastography. Diagnostic and Interventional Imaging 94(5):545–550. https://doi.org/10.1016/j.diii.2013.02.003
Putz FJ, Erlmeier A, Wiesinger I, Verloh N, Stroszczynski C, Banas B, Jung EM (2017) Contrast-enhanced ultrasound (CEUS) in renal imaging at an interdisciplinary ultrasound centre: possibilities of dynamic microvascularisation and perfusion. Clin Hemorheol Microcirc 66(4):293–302. https://doi.org/10.3233/CH-179103
Nahm A, Ritz E (1999) Horseshoe kidney. Nephrol Dial Transplant 14:2740–2741. https://doi.org/10.1093/ndt/14.11.2740
Chen CY, Hsu JS, Jaw TS, Shih MCP, Lee LJ, Tsai TH, Liu GC (2015) Split-bolus portal venous phase dual-energy CT urography: protocol design, image quality, and dose reduction. Am J Roentgenol 15:25–26. https://doi.org/10.2214/ajr.14.13687
Napolitano M, Damasio MB, Grumieri G (2012) Ruolo dell’ uro-risonanza magnetica in urologia pediatrica : stato dell’ arte 42:163–169
Leyendecker JR, Barnes CE, Zagoria RJ (2008) MR urography: techniques and clinical applications. RadioGraphics 28(1):23–46. https://doi.org/10.1148/rg.281075077
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Human and animal rights
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Lomoro, P., Simonetti, I., Vinci, G. et al. Pancake kidney, a rare and often misdiagnosed malformation: a case report and radiological differential diagnosis. J Ultrasound 22, 207–213 (2019). https://doi.org/10.1007/s40477-018-0331-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40477-018-0331-4