Abstract
Purpose
In this work we provide measurements of speed of sound (SoS) and acoustic impedance (Z) of some doped/non-doped rubber-based materials dedicated to the development of ultrasound phantoms. These data are expected to be useful for speeding-up the preparation of multi-organ phantoms which show similar echogenicity to real tissues.
Methods
Different silicones (Ecoflex, Dragon-Skin Medium) and polyurethane rubbers with different liquid (glycerol, commercial detergent, N-propanol) and solid (aluminum oxide, graphene, steel, silicon powder) inclusions were prepared. SoS of materials under investigation was measured in an experimental setup and Z was obtained by multiplying the density and the SoS of each material. Finally, an anatomically realistic liver phantom has been fabricated selecting some of the tested materials.
Results
SoS and Z evaluation for different rubber materials and formulations are reported. The presence of liquid additives appears to increase the SoS, while solid inclusions generally reduce the SoS. The ultrasound images of realized custom fabricated heterogeneous liver phantom and a real liver show remarkable similarities.
Conclusions
The development of new materials’ formulations and the knowledge of acoustic properties, such as speed of sound and acoustic impedance, could improve and speed-up the development of phantoms for simulations of ultrasound medical procedures.
Sommario
Scopo
In questo lavoro sono riportati i valori di velocità del suono (SoS) e impedenza acustica (Z) di alcune gomme nella loro formulazione originale o con l’aggiunta di sostanze droganti (liquide o solide). Le gomme analizzate sono pensate per lo sviluppo di fantocci (phantom) per tecniche ad ultrasuoni. La conoscenza di questi dati può essere utile per accelerare la preparazione di phantom multi-organo che mostrano ecogenicità simili a quelle dei tessuti reali.
Metodi
Differenti siliconi (Ecoflex, Dragon-Skin Medium) e gomme poliuretaniche con diversi dopaggi liquidi (Glicerolo, detergente commerciale, N-Propanolo) e inclusioni solide (Ossido di Alluminio, Grafene, Acciaio, Polvere di Silicio) sono stati preparati. La velocità del suono è stata misurata in un banco di prova sperimentale e l’impedenza acustica (Z) è stata ottenuta moltiplicando la densità e la SoS di ogni materiale. Infine, è stato fabbricato un phantom anatomicamente realistico, inteso a riprodurre un fegato ed alcune sue caratteristiche, selezionando alcuni dei materiali testati.
Risultati
Le misure della SoS e di Z di diverse gomme a differenti formulazioni sono riportate. In generale, la presenza di additivi liquidi aumenta la SoS, mentre le inclusioni metalliche la riducono. Le immagini ecografiche del phantom e di un fegato reale mostrano somiglianze significative.
Conclusioni
La realizzazione di nuovi materiali e la conoscenza delle proprietà acustiche quali la velocità del suono e l’impedenza acustica può dare un importante contributo per quanto riguarda la realizzazione di phantom per simulazioni di procedure mediche che utilizzano ultrasuoni.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of phantoms to progress both diagnostics and therapeutic strategies based on Ultrasound (US) is constantly increasing. Phantoms can be used for different purposes: (1) calibration of new devices, strategies and technologies [1]; (2) validation of computational models [2]; (3) training of physicians and radiologists [3]; (4) achievement of in vitro, standardized, reproducible experiments to demonstrate scientific hypotheses [4].
At the moment ultrasound phantoms available in the market suffer from some limitations such as high cost, simplified anatomy, coarse morphology and—very often—short lifetime [5].
For these reasons, there is a huge interest in the development of custom phantoms, and in particular multi-element patient-specific phantoms that mimic the complex anatomical structures with different acoustic properties [6].
Speed of Sound (SoS) and the correlated acoustic impedance (Z) play an important role among the different acoustic properties of materials. In fact, the energy reflected at the interface between two different media, that produce the typical echoes of US imaging, is caused by (and is proportional to) the difference in impedance of the materials at that interface. The coefficient of reflection (γ r ), is calculated as follow:
where Z 1 and Z 2 are the acoustic impedances of the first and second medium for longitudinal waves, respectively.
In the human body, small differences in impedance are observed (see Table 1). Therefore, a precise knowledge of the acoustic properties of the phantom materials and the possibility to slightly tune them can be extremely helpful for the development of multi-organ phantoms.
Most of the current phantoms are realized with biodegradable materials, so they have high instability over time and low mechanical strength [7, 10].
Instead, silicone phantoms have been commonly considered because of their longevity combined with the possibility to mimic the mechanical properties of real tissues [11, 12]; on the other hand, they were often discharged because of the high attenuation and the low SoS, compared with values exhibited by soft tissues [13]. However, the addition of some substances to the silicone allows to modify acoustic parameters, such as SoS and Z [11, 14]. If these variations in properties are assessed accurately, it could be possible to tune the parameters in order to obtain tailored materials that mimic tissues under US imaging or elastography [15, 16].
This paper reports the measurements of SoS and Z of some doped/non-doped rubber-based materials (silicone and polyurethane materials) to be used in the production of multi-organ phantoms.
The experimental system for acoustic characterization has been validated and an example of a heterogeneous phantom exhibiting small differences in impedance is shown.
Materials and methods
Sample preparation
All the materials used in the experiments were purchased from Smooth-On Inc. (Easton, USA). The Room Temperature Vulcanization silicones are the Ecoflex00-10® (EF) and the Dragon-Skin Medium® (DSM).
EF and DSM are double-component silicones; each silicone comes with a Part A and a Part B component, the latter containing the catalyst. EF and DSM samples were prepared with an A:B mix ratio by volume. The additives were added only to one component (Part A), and accurately stirred before mixing with Part B.
Curing time for both EF and DSM samples was of 4 h without additives and 6 h with additives. The polyurethane rubber (PU) is a double component: a liquid part (Part A) and a more viscous part (part B, the catalyst). PU samples were prepared with an A:B mix ratio by weight. Additives were added to Part A. The PU samples have a curing time of 16 h.
Glycerol (C3H8O3), a commercial detergent, and N-Propanol have been used as liquid additives in different percentages. Aluminum Oxide (Al2O3), Graphene, Steel, and Silicon powder have been used as solid inclusions.
All the samples were molded in cylinders measuring 40 mm in height (h) and 40 mm in diameter (d).
Different combinations have been tested, whose formulation is reported in Table 2.
Acoustic measurements
Hardware
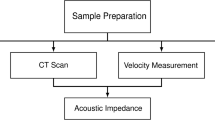
The experimental system used for SoS measurements, shown in Fig. 1, is composed of a home-made tank, a 3-axis step-by-step motorized positioning frame (XXYZ BiSlide, Velmex, Bloomfield, NY, US), a direct digital synthesis wave generator (33220A, Agilent Technologies Inc., Santa Clara, CA, USA), a 50-dB power amplifier (240L, Electronics & Innovation Ltd, Rochester, NY, USA), a single element ceramic focused transducer (PA260, Precision Acoustics Ltd, Dorchester, UK with 60 mm diameter, 75 mm radius of curvature and 1 MHz of nominal frequency), a 0.2-mm needle hydrophone (Precision Acoustics Ltd, Dorchester, UK) connected in series with a submersible pre-amplifier, a DC coupler and an oscilloscope (InfiniiVision 7034B, Agilent Technologies, Santa Clara, CA, US).
A custom-made holder with a cylindrical hole for embedding the samples has been 3D printed. During the experiments, it is rigidly fixed with the hydrophone so that the distance between the back surface of the sample and the sensitive element remains constant.
Experimental procedure
Materials’ SoS is determined using a through-transmission substitution technique, in which the unknown properties of the sample under test are obtained from the comparison with the properties of water, used as reference [17, 18].
The hydrophone is aligned parallel to the acoustic beam axis of the transmitting transducer and measurements are performed (both in the presence and absence of the sample) at 11 different axial distances, all located in the far field. Each single burst is repeated 10 times and averaged to reduce the noise.
A custom-made LabView Graphic User Interface (GUI) allows the control and the synchronization between the gantry, the wave generator, and the oscilloscope.
Data are post-processed and the Time of Flight (TOF) is identified by using a threshold method; this method is supposed to be accurate for high signal-to-noise ratio [19].
SoS is obtained from TOF thanks to the following relation:
where h is the thickness of the sample and subscripts w and s refer to water and sample, respectively. SoS in water (SoS w ) is calculated as a function of the temperature only [20]. Z is obtained by simply multiplying the density and the SoS for each material. Density is calculated by weighting each sample before experiments and measuring it with a precision caliper.
At least two samples (S1 and S2 in Table 2) for each formulation have been measured to determine variability in the fabrication process.
The system has been validated in advance by measuring the SoS of castor oil (Eur. Pharm. Grade, produced by Acros Organics, Geel, Belgium), commonly used as reference material [21, 22]. The value of SoS has been calculated to be 1491.8 ± 2.6 at 28.8 °C which is in line with literature and references [23].
Phantom fabrication
An anatomically realistic liver phantom has been built selecting some of the materials reported in Table 2. To obtain a reflection coefficient close to the one of the vessel/healthy liver interface (γ r = 1.6e-3, [17]), taking the data from Table 2 and using Eq. (1), the parenchyma has been fabricated with the DSM° + °30 % N-Propanol° + °5 % Graphene formulation and the vessels were fabricated with pure DSM, revealing a reflection coefficient of 1.5e-3.
Results
SoS and Z measurements
Materials described in “Sample preparation” section have been tested and results are reported in Table 2.
Phantom visualization
The US scanner images of the liver phantom realized with materials described in “Phantom fabrication” section and a real liver are shown in Fig. 2.
Discussion and conclusions
In this work, we reported the results of the acoustic characterization (speed of sound and acoustic impedance measurements) of different materials to be used for US phantoms.
The materials under investigations were silicone rubbers (Ecoflex and the Dragon-Skin Medium) and a polyurethane rubber doped with liquid additives (Glycerol, commercial detergent and N-Propanol) and/or solid inclusions (Aluminum Oxide, Graphene, Steel and Silicon powder).
The results of SoS and Z were reported in Table 2. Standard deviation is negligible within each single test (almost 0.1 %) and it is extremely low also for different tests on the same sample (0.5 %). The fabrication process was demonstrated to be extremely reproducible, with an average percentage error lower than 1 %.
Values for the SoS and Z are low compared to soft tissues [13], but they are in line with previous measurements made on silicones [8].
The investigated materials are promising for the development of US imaging phantoms for their longevity, simple fabrication and good mechanical properties, but they present low SoS respect to tissues.
However, new generation of US scanners are provided with more advanced software for data acquisition and processing to take into account, e.g., speed of sound variations [24]. These advances are expected to partially compensate problems of rubber-based materials and possibly push them forward, in particular for multi-organ, anatomically realistic phantoms, which mimic the tissue for elastography and for training for US imaging.
The presence of doping materials modifies the value of speed of sound and acoustic impedance. Results reported in this work show that the addition of liquid additives in silicone matrices increases the SoS, in agreement with previous literature on, e.g., agar-based phantoms [5]. On the other hand, metal inclusions generally reduce the SoS.
It has been previously shown that rubber-based phantoms exhibit mechanical properties similar to those of tissues [11, 12] and the difference in impedance inside heterogeneous phantoms can be tuned, thanks to an accurate knowledge of the speed of sound. The small differences in impedance among, e.g., healthy and diseased tissues, produce values of reflection similar to the ones that can be obtained by the newly built material for US phantoms reported in Table 2.
As for example, a liver phantom has been built starting from these results. The selected materials have a reflection coefficient close to that of vessel/liver interface, which enforces the similitude observed in Fig. 2.
In conclusion, in this work, silicone rubber and polyurethane rubber materials with different inclusions have been tested in order to find out the combination which produces echogenicity similar to that of real tissues, in the attempt to develop patient-specific phantoms.
The use of these materials shows several advantages including stability, long duration and the possibility to mimic elastic properties of different tissues, making silicone a valid solution for US phantoms in general and elastography in particular. These phantoms can be useful for surgical training in procedures such as biopsies and needle insertion and also for the development of new strategies of diagnosis. To this aim, it is essential to characterize acoustic properties during silicone doping in order to tailor specific properties for each need.
Precise measurements of SoS and acoustic impedance can effectively speed-up the preparation of materials mimicking the different targets, and allow the determination of the effects of the different inclusions both in the acoustical properties and in echogenic appearance.
References
Browne J, Ramnarine K, Watson A, Hoskins P (2003) Assessment of the acoustic properties of common tissue-mimicking test phantoms. Ultrasound Med Biol 29(7):1053–1060
Hamaluik K, Moussa W, Ferguson-Pell M (2014) Numerical Characterization of Quasi-Static Ultrasound Elastography for the Detection of Deep Tissue Injuries. IEEE Trans Med Imaging 33(7):1410–1421
Farjad Sultan S, Shorten G, Iohom G (2013) Simulators for training in ultrasound guided procedures. Medical ultrasonography 15(2):125–131
Dasgupta S, Banerjee RK, Hariharan P, Myers MR (2011) Beam localization in HIFU temperature measurements using thermocouples, with application to cooling by large blood vessels. Ultrasonics 51(2):171–180
Cannon LM, Fagan AJ, Browne JE (2011) Novel tissue mimicking materials for high frequency breast ultrasound phantoms. Ultrasound Med Biol 37(1):122–135
Cuccaro R, Musacchio C, Albo PAG, Troia A, Lago S (2015) Acoustical characterization of polysaccharide polymers tissue-mimicking materials. Ultrasonics 56:210–219
Culjat MO, Goldenberg D, Tewari P, Singh RS (2010) A review of tissue substitutes for ultrasound imaging. Ultrasound Med Biol 36(6):861–873
Zell K, Sperl J, Vogel M, Niessner R, Haisch C (2007) Acoustical properties of selected tissue phantom materials for ultrasound imaging. Phys Med Biol 52(20):N475
Zhang D, Gong X-F (1999) Experimental investigation of the acoustic nonlinearity parameter tomography for excised pathological biological tissues. Ultrasound Med Biol 25(4):593–599
Dabbagh A, Abdullah BJJ, Ramasindarum C, Kasim NHA (2014) Tissue-mimicking gel phantoms for thermal therapy studies. Ultrason Imaging 36(4):291–316
Maggi L, von Kruger M, Pereira W, Monteiro E (2009) Development of silicon-based materials for ultrasound biological phantoms. In: IEEE International Ultrasonics Symposium (IUS) IEEE, pp 1962–1965
Piazza R, Condino S, Carbone M, Mattei L, Ferrari V, Di Puccio F, Caramella D, Forte P (2012) Materials characterization for elastosonographic phanthoms. Int J CARS 7 (Suppl 1) (1):S51-S57
Duck FA (1990) Physical properties of tissues: a comprehensive reference book. Academic Press, San Diego, London
Carbone M, Condino S, Mattei L, Forte P, Ferrari V, Mosca F (2012) Anthropomorphic ultrasound elastography phantoms—characterization of silicone materials to build breast elastography phantoms. In: 34th annual international conference of the IEEE Engineering in medicine and biology society (EMBC). IEEE, pp 492–494
Botticelli A, Mazzotti E, Di Stefano D, Petrocelli V, Mazzuca F, La Torre M, Ciabatta FR, Giovagnoli RM, Marchetti P, Bonifacino A (2015) Positive impact of elastography in breast cancer diagnosis: an institutional experience. J Ultrasound 18(4):321–327
Giannetti A, Biscontri M, Matergi M (2014) Feasibility of real-time strain elastography in colonic diseases. J Ultrasound 17(4):321–330
Hill CR, Bamber JC, Haar G (2004) Physical principles of medical ultrasonics, vol 2. Wiley Online Library,
Zeqiri B, Scholl W, Robinson SP (2010) Measurement and testing of the acoustic properties of materials: a review. Metrologia 47(2):S156
Arnold W, Hirsekorn S (2013) Acoustical Imaging, vol 27. Springer Science & Business Media
Marczak W (1997) Water as a standard in the measurements of speed of sound in liquids. J Acoust Soc Am 102(5):2776–2779
He P (1998) Determination of ultrasonic parameters based on attenuation and dispersion measurements. Ultrason Imaging 20(4):275–287
Shaw A (2008) A buoyancy method for the measurement of total ultrasound power generated by HIFU transducers. Ultrasound Med Biol 34(8):1327–1342
Timme R (1972) Speed of sound in castor oil. J Acoust Soc Am 52(3B):989–992
Fontanarosa D, van der Meer S, Bloemen-van Gurp E, Stroian G, Verhaegen F (2012) Magnitude of speed of sound aberration corrections for ultrasound image guided radiotherapy for prostate and other anatomical sites. Med Phys 39(8):5286–5292
Acknowledgments
The work leading to these results has received funding from the European Union under grant agreement n° 611963—FUTURA project (Focused Ultrasound Therapy Using Robotic Approaches) and from the Fondazione Pisa in the framework of the MicroVAST project (Microsystems for VAscular diagnosticS and inTervention).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Andrea Cafarelli, Piero Miloro, Antonella Verbeni, Marina Carbone, Arianna Menciassi declare that they have no conflict of interest.
Informed consent
Real patients' images used in this work were selected with the help of expert clinicians from the anonymized archive of the University of Pisa. All the diagnostic exams stored in the archive previously received approval for storing and use for research purposes by the patients.
Rights and permissions
About this article
Cite this article
Cafarelli, A., Miloro, P., Verbeni, A. et al. Speed of sound in rubber-based materials for ultrasonic phantoms. J Ultrasound 19, 251–256 (2016). https://doi.org/10.1007/s40477-016-0204-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40477-016-0204-7