Abstract
The major shortcoming of image-guided navigation systems is the use of presurgically acquired image data, which does not account for intra-operative changes such as brain shift, tissue deformation and tissue removal occurring during the surgical procedure. Intra-operative ultrasound (iUS) is becoming widely used in neurosurgery but they lack orientation and panoramic view. In this article, we describe our procedure for US-based real-time neuro-navigation during surgery. We used fusion imaging between preoperative magnetic resonance imaging (MRI) and iUS for brain lesion removal in 67 patients so far. Surgical planning is based on preoperative MRI only. iUS images obtained during surgery are fused with the preoperative MRI. Surgery is performed under intra-operative US control. Relying on US imaging, it is possible to recalibrate navigated MRI imaging, adjusting distortion due to brain shift and tissue resection, continuously updating the two modalities. Ultrasound imaging provides excellent visualization of targets, their margins and surrounding structures. The use of navigated MRI is helpful in better understanding cerebral ultrasound images, providing orientation and panoramic view. Intraoperative US-guided neuro-navigation adjustments are very accurate and helpful in the event of brain shift. The use of this integrated system allows for a true real-time feedback during surgery.
Sommario
Il principale difetto della neurochirurgia guidata da immagini è il basarsi su immagini acquisite prima dell’intervento, che per ovvie ragioni non possono tenere conto di fenomeni intra-operatori come il brain-shift, la deformazione dei tessuti e l’asportazione di tessuto patologico. L’ecografia intra-operatoria (iUS) sta acquisendo sempre maggior rilevanza in ambito neurochirurgico ma è limitata dalla difficoltosa interpretazione dell’orientamento delle immagini e dalla scarsa panoramicità. In questo articolo descriviamo la nostra tecnica di neuronavigazione real-time basata sull’ecografia intra-operatoria. Fino ad ora abbiamo impiegato la fusione d’immagini tra la risonanza magnetica (MRI) pre-operatoria e l’iUS in 67 pazienti affetti da neoplasie cerebrali. La pianificazione dell’intervento e l’approccio chirurgico è basata sulla (MRI) pre-operatoria mentre l’intervento è guidato dall’iUS. Basandosi sull’iUS è possibile correggere la calibrazione delle immagini (MRI) pre-operatorie correggendo il brain-shift, aggiornando continuamente le due modalità. L’ecografia intra-operatoria permette una eccellente identificazione dei target, dei margini e delle strutture circostanti. L’uso del navigatore basato su (MRI) pre-operatoria è utile nella comprensione delle immagini ecografiche soprattutto per quanto riguarda l’orientazione e la visione panoramica. Le correzione del sistema di neuronavigazione basate sull’iUS sono accurate e utili nel caso di fenomeni intra-operatori come il brain-shift, la deformazione dei tessuti e l’asportazione di tessuto patologico. La neuronavigazione baasata sulla fusione d’immagini tra iUS e (MRI) pre-operatoria permette un vero feeback in real-time durante la chirurgia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Image-guided neuro-navigation systems represent a routine tool in neurosurgery but they are based on preoperative imaging, so they have to be considered a dynamic but not a real-time technique [1]. The accuracy of this system during surgery is maximum before the craniotomy but decreases with the progress of surgical manipulation; this worsening is inevitable, and it is due to two main factors: the first, so-called brain shift, is caused by the effect of the gravity on the brain, brain swelling and escape of cerebrospinal fluid (CSF); the second is due to parenchymal deformation caused by surgical maneuvers [2–4].
Intraoperative MRI (iMRI) and CT (iCT) have been introduced in order to update the imaging data [5] and neutralize this loss of accuracy. On the one hand, these devices have good spatial resolution, wide field of view and the absence of anatomic limitations but on the other hand, they are quite expensive and time-consuming. Furthermore, it is not possible to operate under direct guidance, and for this reason, they cannot be considered true real-time intra-operative imaging systems.
Another technique extensively used during neurosurgical procedures is intra-operative Ultrasounds (iUS); in recent years, multiple studies demonstrated their value in tumor detection during surgery, giving to iUS a foreground position in the field of intraoperative imaging [6–9]. The main point of value of iUS consists in obtaining a real-time scan repeatable as many times as necessary without the cost and the duration of other intraoperative techniques. Surely, iUS have also some limitations: their spatial resolution, width and orientation of the field of view (different from the standard orthogonal planes of CT and MRI) and their scan quality, which are operator dependent [10].
Furthermore, most neurosurgeons have difficulty interpreting iUS imaging mainly for the lack of specific training, which leads to a long learning curve when adopted.
Based on these premises, a real-time intraoperative fusion imaging (FI) between preoperative imaging (MRI) and intraoperative ultrasound for virtual navigation has been introduced and promoted by some authors [11, 12].
Our aim with this article is to present our method for intra-operative US guidance and how we perform surgery for brain lesion removal using iUS system with virtual navigation technology, which fuses iUS exam with a reference modality (preoperative MRI), emphasizing on the positive aspects of this approach.
Materials and methods
System architecture
We use a last generation US equipment (Esaote MyLab, Esaote, Italy), which includes an US scanner equipped with an electromagnetic tracking system and a dedicated software for Virtual Navigation (MedCom, Germany). The probe is a variable band linear array with operating bandwidth: 3–11 MHz (Esaote-LA332), covered with a sterile probe cover (Civco, USA) for iUS evaluation in sterile conditions. The tracking system consists of a transmitter, positioned on a dedicated support, that generates a magnetic field, and a receiver attached to a pointer during the registration phase and to the US probe during navigation. The system provides the position and orientation of the US probe in relation to the transmitter in a 3-D frame, based on which an oblique plane is cut through the 3-D MRI dataset in order to generate the corresponding 2-D MR images. As a preoperative reference imaging, we ordinarily use a volumetric T1-weighted contrast-enhanced MRI (Siemens, Holland).
Preoperative procedure
The first step is to acquire a scan of the patient with the reference modality (usually volumetric T1-weighted contrast-enhanced MRI) and to transfer the obtained images in the navigation system in DICOM format, using a LAN connection. The virtual navigation system processes every slice of the exam, evaluating the slice thickness and dimension, generating a three-dimensional (3-D) volume; the exam is showed in three orthogonal planes and in a 3-D reconstruction, on which the surgeon plans the surgical approach, as with a standard neuro-navigation system (Fig. 1a2).
a Craniotomy planning using the navigated US probe; a1 US probe is used like a neuro-navigator pointer; a2 screenshot of the VN unit: in the superior right box, it is visualized the MRI corresponding to the probe orientation; the superior left box usually displays the US imaging that it is not visualized because of bone shielding, the inferior boxes depict different orthogonal planes. b intraoperative procedure, bone flap has been removed, and trans-dural US scan is performed; b1 US probe is approached to dura mater, to perform a two-axis B-mode evaluation of the lesion and a first comparison between iUS and preoperative MRI; b2 screenshot of the VN unit: in the superior right box, it is visualized the MRI corresponding to the probe orientation; the superior left box displays the US imaging; the inferior boxes depict different orthogonal planes
The patient is then positioned on the surgical table with the head held in a three-pin headholder; the transmitter is bonded to the clamp and is kept steady and correctly oriented toward the patient by a proper support. The transmitter is considered the origin of the reference system, while position and orientation of US probe in the generated 3-D space is provided by the receiver. This tracking system is based on an electromagnetic field that attenuates with distance, having the highest accuracy in 15–20 cm far from the transmitter (Fig. 1a1).
The registration procedure between the MRI volume and the real-time US scan consists of an initial rigid registration of corresponding external anatomical landmarks that is subsequently refined with a fine-tuning registration. The first phase (rigid registration) is performed as with standard neuro-navigator, using eight anatomical landmarks selected on the volume’s surface (tip of the nose, glabella, lateral canthus, tragus, ear attachment). The receiver of the tracking system is linked to a registration pen and is used to mark the anatomical landmarks on the patient’s skin, matching them with the equivalent points indicated on the MRI volume rendering on the navigation system.
The second level of the registration phase (fine tuning) is carried out during surgery.
The aim of the registration is to align the patient’s position with the 3-D dataset position in a known and fixed coordinate system in a 3-D frame of which the transmitter is the origin. When all anatomical landmarks defined on the MRI reconstructed surface are matching with the corresponding points on the patient, acquired with the Pen tool, the system is able to calculate the “rigid registration matrix”. Using this matrix, it is possible to correlate the probe spatial position and US image with the related reference imaging modality (MRI). The receiver is then mounted on the US probe. Before surgery, we usually check the precision of the first registration phase, and of the system probe-receiver, matching some external anatomical landmarks on the patient with the same points on the volume surface created by the system; we usually use the midpoint of the linear US probe as the tip of a neuro-navigator pointer (Fig. 1a1).
Once the precision of the system has been verified, the navigated US probe is used as a pointer to plan the craniotomy, relying only on the MRI volume, because US imaging is not available due to bone shielding. Figure 2 summarizes the preoperative workflow.
Intraoperative procedure and data analysis
The patient is draped, and the US probe covered with a sterile plastic probe cover coupled with ultrasound gel and the craniotomy is performed under MRI navigation guidance.
After bone flap removal, but before opening the dura, brain parenchyma might shift toward the hole but does not change its shape, causing what we call brain shift without brain deformation.
At this point, a first fine-tuning registration phase is performed. The brain surface is scanned with standard B-mode US modality, and the lesion is analyzed: on the screen of the virtual navigation system, the US imaging and the correspondent preoperative MRI are displayed merged together (Fig. 1b). Thanks to this feature, it is possible to evaluate the accuracy of the first registration procedure looking for misalignment of major anatomical structures such as ventricles, arteries, mid-brain and dural structures between the MR image and the real-time US image. If a difference between the real position of a structure (assessed by US image) and the virtual position (according to the virtual navigation system) is observed, the error is measured and might be corrected in two ways (Fig. 3):
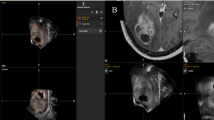
Intra-operative US (a) and corresponding preoperative MRI (b) view in a case of left temporo-parietal high-grade glioma; c and d show fusion imaging between iUS and preoperative MRI; in c, it is appreciable a misalignment (>4 mm) between the wall of the lateral ventricle in iUS (arrow head) and MRI (arrow); brain shift correction is performed; in d, US image has been freezed and the MRI has been manually adjusted to fix the error of alignment between the wall of the lateral ventricle in iUS (arrow head) and MRI (arrow)
-
1.
Freezing of the US system (both images) and dragging the MR image with the mouse to the correct position over the US image; this is possible if the same landmark is present in both modalities, allowing an in plane correction.
-
2.
Freezing only one image (US or MRI) and moving the probe until the live image display the same information as the frozen one thus matching both modalities; this is possible if no common landmark is visible, making an out of plane correction necessary.
Once the fine tuning is completed, the navigation phase starts. On the screen, the image from the US probe merged with the corresponding preoperative MRI is visualized, helping recognize the structures thanks to the continuous update and comparison (Fig. 3d). During the resection of the mass, it is possible to perform multiple US scans in order to evaluate the brain shift and the parenchyma distortion, which can be corrected and compensated (Fig. 3); the proximity of other structures (Fig. 4) and the surgical cavity (Fig. 5b). Figure 6 illustrates the intra-operative workflow.
Comparison between scans before tumor resection (a) (a1 in iUS and a2 in preoperative MRI) and after tumor resection (b) (b1 shows iUS and b2 shows fusion of iUS and preoperative MRI); after tumor removal in b2, it is noticeable the difference between preoperative MRI and iUS while before tumor removal, preoperative MRI correspond to iUS (a2)
Multiple images, cine clips and dataset (data regarding brain shift, distortion and adjustments performed) are obtained and stored to permit an offline analysis. Intraoperative qualitative analysis is performed comparing B-mode US imaging and preacquired MR images.
Discussion and conclusions
Fusion imaging between preoperative MRI and intra-operative US is a particularly useful tool since it combines benefits of two imaging modalities, overcoming the limitations of both. Neuro-navigation is based on preoperative MRI with all its positive features: spatial resolution, width of field, absence of anatomical limitations and neurosurgeons familiarity with this technique. Its main limitation relies on being based on preoperative imaging that does not reflect the real situation during surgery, not taking into account dynamic phenomena such as brain shift, tissue deformation (retractors, spatula and surgical maneuvers), tissue resection and parenchyma re-expansion. On the other hand, US imaging advantages reside in being a repeatable, real-time imaging modality. However, its limited field of view, the multiple unusual plane of insonation and its image quality, dependent on the operator abilities, make it difficult to interpret. Furthermore, most neurosurgeons did not receive a specific training in interpreting iUS images and in setting the device.
The combination of these two techniques overcomes the limitations of each single one with the result of a new type of surgery.
Fusion imaging was first introduced by radiologists for the treatment of hepatic neoplasms or lesions [13–17]. To target hepatic lesions, especially when characterized by poor sonographic signal [18], they perform an US-guided, minimal invasive (e.g., RF ablation) procedure merging the iUS imaging with CT or other modalities.
We have adopted the same technology with an approach that is somehow the opposite; virtual navigation can guide the neurosurgeon to plan the craniotomy (Fig. 1a) while fusion imaging provides orientation, and helps to understand anatomy and relationships of the lesion (Fig. 4); once these are clear, it is possible to safely rely on US images only. The conventional navigation system is in fact a standard tool in planning and performing the craniotomy [19]. However, once the bone flap has been removed, brain shift takes place, affecting neuro-navigation references and making it less accurate for fine recognition of anatomical landmarks. Indeed, even if it is possible to correct brain shift over and over again during surgery, it is not possible to fix the brain deformation that inevitably takes place through surgical manipulation; this causes the preoperative images to be inadequate in describing the real situation. In other words, preoperative MRI should help in understanding US images, always keeping in mind that only US images are really faithful to the actual intraoperative anatomy, showing in real time the degree of excision, residual mass or proximity with other structures (Figs. 4, 5).
It is evident that most neurosurgeons are not familiar with US imaging, probably because of lack of use in preoperative diagnostic process, also because of an indisputable lower quality of the images in the past. Additionally, notwithstanding the increased use of iUS in neurosurgery, only few neurosurgeons received specific training on US; furthermore, there is an inherent difficulty in understanding the images formed by US, and this leads to a longer learning curve. Fusion imaging can shorten the learning curve of the US anatomy leading, with little experience, to shift mainly toward US imaging during surgery.
Relying on our experience, we would like to emphasize a few technical aspects:
-
In regard of the registration phase, we have observed a higher level of precision during the procedure using external anatomical landmarks on subject’s eyes, ears and nose compared with the use of skin fiducial markers. In fact, the latter is affected by skin deformation and also by fiducials that might move, leading to a loss of registration accuracy. Furthermore, this procedure allows to speed up the overall registration procedure before starting the proper navigation phase with the fusion between the two imaging modalities.
-
The aim of fine-tuning procedure is to have the highest accuracy possible in the area of interest, and it is somehow acceptable to have a certain degree of inaccuracy in the representation of areas distant to the main surgical target. Anyway, it should be clear that, de facto, this operation is a manual recalibration based on the operator skills and judgment.
-
After tumor resection has been partially or completely performed, we are also aware that the two imaging modalities are not showing the same anatomical situation; therefore, the further we proceed with the surgery, the more we rely solely on US imaging, and we use the MRI as a reference for orientation (Fig. 5).
-
The size of the tip of the US probe (1 cm × 3.5 cm) needs a relatively wide craniotomy to achieve a good transparenchymal window for obtaining clear images.
Finally, we must consider the difficulty to visualize the parenchyma in case of profused bleeding or excessive use of hemostatic material, which is highly hyperechoic [20].
In our opinion, the main application of this technique is surely for intra-parenchymal tumors removal, because iUS can be of help in identifying the lesion and residual mass with great sensitivity [21, 22], but we should not underestimate its use in skull base tumors, abscesses, cysts, hematomas or aneurysms.
In our opinion, intraoperative US imaging combined with neuro-navigator represents a major innovation in neurosurgery; it is reliable, accurate, easy to use, permitting a continuous real-time feedback without interrupting surgery. Moreover, in the future, it could be possible to implement other functions/modalities in fusion imaging such as tractography (DTI) and contrast-enhanced ultrasound (CEUS).
References
Orringer DA, Golby A, Jolesz F (2012) Neuronavigation in the surgical management of brain tumors: current and future trends. Expert Rev Med Devices 9(5):491–500
Dorward NL, Alberti O, Velani B, Gerritsen FA, Harkness WF, Kitchen ND, Thomas DG (1998) Postimaging brain distortion: magnitude, correlates, and impact on neuronavigation. J Neurosurg 88(4):656–662
Nimsky C, Ganslandt O, Cerny S, Hastreiter P, Greiner G, Fahlbusch R (2000) Quantification of, visualization of, and compensation for brain shift using intraoperative magnetic resonance imaging. Neurosurgery 47(5):1070–1079 (discussion 1079–1080)
Stieglitz LH, Fichtner J, Andres R, Schucht P, Krahenbuhl AK, Raabe A, Beck J (2013) The silent loss of neuronavigation accuracy: a systematic retrospective analysis of factors influencing the mismatch of frameless stereotactic systems in cranial neurosurgery. Neurosurgery 72(5):796–807
Black PM, Moriarty T, Alexander E 3rd, Stieg P, Woodard EJ, Gleason PL, Martin CH, Kikinis R, Schwartz RB, Jolesz FA (1997) Development and implementation of intraoperative magnetic resonance imaging and its neurosurgical applications. Neurosurgery 41(4):831–842 (discussion 842–835)
Gerganov VM, Samii A, Akbarian A, Stieglitz L, Samii M, Fahlbusch R (2009) Reliability of intraoperative high-resolution 2D ultrasound as an alternative to high-field strength MR imaging for tumor resection control: a prospective comparative study. J Neurosurg 111(3):512–519
Hammoud MA, Ligon BL, elSouki R, Shi WM, Schomer DF, Sawaya R (1996) Use of intraoperative ultrasound for localizing tumors and determining the extent of resection: a comparative study with magnetic resonance imaging. J Neurosurg 84(5):737–741
Moiyadi AV (2014) Objective assessment of intraoperative ultrasound in brain tumors. Acta Neurochir (Wien) 156(4):703–704
Unsgaard G, Gronningsaeter A, Ommedal S, Nagelhus Hernes TA (2002) Brain operations guided by real-time two-dimensional ultrasound: new possibilities as a result of improved image quality. Neurosurgery 51(2):402–411 (discussion 411–402)
Pasto ME, Rifkin MD (1984) Intraoperative ultrasound examination of the brain: possible pitfalls in diagnosis and biopsy guidance. J Ultrasound Med 3(6):245–249
Lindner D, Trantakis C, Renner C, Arnold S, Schmitgen A, Schneider J, Meixensberger J (2006) Application of intraoperative 3D ultrasound during navigated tumor resection. Minim Invasive Neurosurg 49(4):197–202
Rasmussen IA Jr, Lindseth F, Rygh OM, Berntsen EM, Selbekk T, Xu J, Nagelhus Hernes TA, Harg E, Haberg A, Unsgaard G (2007) Functional neuronavigation combined with intra-operative 3D ultrasound: initial experiences during surgical resections close to eloquent brain areas and future directions in automatic brain shift compensation of preoperative data. Acta Neurochir (Wien) 149(4):365–378
Crocetti L, Lencioni R, Debeni S, See TC, Pina CD, Bartolozzi C (2008) Targeting liver lesions for radiofrequency ablation: an experimental feasibility study using a CT-US fusion imaging system. Invest Radiol 43(1):33–39
Jung EM, Schreyer AG, Schacherer D, Menzel C, Farkas S, Loss M, Feuerbach S, Zorger N, Fellner C (2009) New real-time image fusion technique for characterization of tumor vascularisation and tumor perfusion of liver tumors with contrast-enhanced ultrasound, spiral CT or MRI: first results. Clin Hemorheol Microcirc 43(1–2):57–69
Montali G, Solbiati L, Croce F, Ierace T, Ravetto C (1982) Fine-needle aspiration biopsy of liver focal lesions ultrasonically guided with a real-time probe. Report on 126 cases. Br J Radiol 55(658):717–723
Nakai M, Sato M, Sahara S, Takasaka I, Kawai N, Minamiguchi H, Tanihata H, Kimura M, Takeuchi N (2009) Radiofrequency ablation assisted by real-time virtual sonography and CT for hepatocellular carcinoma undetectable by conventional sonography. Cardiovasc Interv Radiol 32(1):62–69
Ross CJ, Rennert J, Schacherer D, Girlich C, Hoffstetter P, Heiss P, Jung W, Feuerbach S, Zorger N, Jung EM (2010) Image fusion with volume navigation of contrast enhanced ultrasound (CEUS) with computed tomography (CT) or magnetic resonance imaging (MRI) for post-interventional follow-up after transcatheter arterial chemoembolization (TACE) of hepatocellular carcinomas (HCC): preliminary results. Clin Hemorheol Microcirc 46(2–3):101–115
Park HJ, Lee MW, Lee MH, Hwang J, Kang TW, Lim S, Rhim H, Lim HK (2013) Fusion imaging-guided percutaneous biopsy of focal hepatic lesions with poor conspicuity on conventional sonography. J Ultrasound Med 32(9):1557–1564
Jung TY, Jung S, Kim IY, Park SJ, Kang SS, Kim SH, Lim SC (2006) Application of neuronavigation system to brain tumor surgery with clinical experience of 420 cases. Minim Invasive Neurosurg 49(4):210–215
Selbekk T, Jakola AS, Solheim O, Johansen TF, Lindseth F, Reinertsen I, Unsgard G (2013) Ultrasound imaging in neurosurgery: approaches to minimize surgically induced image artefacts for improved resection control. Acta Neurochir (Wien) 155(6):973–980
Le Roux PD, Berger MS, Wang K, Mack LA, Ojemann GA (1992) Low grade gliomas: comparison of intraoperative ultrasound characteristics with preoperative imaging studies. J Neurooncol 13(2):189–198
Woydt M, Krone A, Becker G, Schmidt K, Roggendorf W, Roosen K (1996) Correlation of intra-operative ultrasound with histopathologic findings after tumour resection in supratentorial gliomas. A method to improve gross total tumour resection. Acta Neurochir (Wien) 138(12):1391–1398
Acknowledgments
The authors would like to thank Mrs. Caroline King for her kind advice in revising the manuscript and Mr. Luca Lodigiani for his technical support. The research leading to these results has received funding from the European Union Seventh Framework Program. FP7/2007-2013 under Grant agreement n.602923.
Conflict of interest
Francesco Prada, Massimiliano Del Bene, Luca Mattei, Cecilia Casali, Assunta Filippini, Federico Legnani, Antonella Mangraviti, Andrea Saladino, Alessandro Perin, Carla Richetta, Ignazio Vetrano, Alessandro Moiraghi, Marco Saini and Francesco DiMeco have no conflict of interest to disclose.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). All patients provided written informed consent to enrollment in the study and to the inclusion in this article of information that could potentially lead to their identification.
Human and animal studies
The study was conducted in accordance with all institutional and national guidelines for the care and use of laboratory animals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prada, F., Del Bene, M., Mattei, L. et al. Fusion imaging for intra-operative ultrasound-based navigation in neurosurgery. J Ultrasound 17, 243–251 (2014). https://doi.org/10.1007/s40477-014-0111-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40477-014-0111-8