Abstract
This work describes the performance variation and emission profile of a single-cylinder diesel engine connected to a hydraulic pump controlling the engine speed and load. This hydraulic system makes it possible to measure the engine power output with simplicity and low cost. By using energy analysis, the engine performance was evaluated using four different fuels: mineral diesel; biodiesel; a diesel–biodiesel blend composed of mineral diesel (90%) and biodiesel (10%) in volume; and a ternary mixture composed of mineral diesel (82%), biodiesel (10%) and ethanol (8%) in volume. The biodiesel used in the tests came from waste cooking oil, and it was produced in the local pilot plant. Ethanol was used to decrease combustion temperature due to its high enthalpy of vaporization while increasing oxygen content in the mixture. Results revealed a slight increase in the emission of NOx emissions and a reduction in CO emissions with the increase in biodiesel content. The use of ethanol resulted in lower NOx (up to 6.5%) and PM emissions (up to 31.0%) but CO and HC emissions were increased. The energy efficiency and specific fuel consumption were increased when compared to mineral diesel when biodiesel content was increased. The introduction of ethanol caused an increase in fuel consumption as well as a slight reduction in energy efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biodiesel is an alternative fuel derived from vegetable oils or animal fats [1]. It has a good miscibility with diesel fuel and can be used in diesel engines with little or no constructive changes [2].
Several researchers have studied the effect of biodiesel in engine performance verifying almost the same effective power when compared to mineral diesel [3]. An increase in fuel consumption is expected when the engine is operating with biodiesel, due to reduction in lower heating value (LHV) of fuel [4, 5]. A similar or higher engine thermal efficiency has been verified when using biodiesel compared to mineral diesel [6, 7]. Exhaust emissions from diesel engines operating with biodiesel blends have shown a general trend for reduction in CO [8], total unburned hydrocarbon (THC) and particulate matter (PM) emissions [9, 10] due to the oxygen chemically bound to the biodiesel molecule.
Nitrogen from the air or fuel molecule under conditions of high temperature in the combustion chambers can react with oxygen to form compounds known as NOx (nitrogen oxides) linked to a number of illnesses of the human respiratory system [11, 12]. Most studies show an increase in NOx emissions when using biodiesel [6, 13, 14]. This can be an obstacle to future growth in the biodiesel market and its application in engines [15]. Several solutions to this problem have been used, such as exhaust gas recirculation (EGR), a change in the injection parameters, the reduction in the compression ratio and/or the intake pressure (turbocharging pressure) [16], but the addition of oxygenated compounds such as ethyl alcohol or dimethyl ether (DME) may also produce the same result [17, 18].
Ethanol is a renewable fuel and may limit undesired exhaust gas components, and it significantly changes the physicochemical parameters of the base fuels such as density, viscosity, volatility at low temperatures and the cetane number [19]. The introduction of ethanol in the fuel blend can supply the oxygen required to form CO2 reducing PM emissions. But, on the other hand, ethanol presents a poor miscibility with diesel fuel and diesel–ethanol blends present a lower cetane number, which can increase the amount of unburned hydrocarbons compared to mineral diesel [20]. Even some vegetal oils can be used in diesel engines when mixed with ethanol [21].
Properties of ethanol can increase the ignition delay due to cooling effect, high octane number and stoichiometric A/F ratio, influencing premixing and flame speed, thus resulting in a higher fraction of combustion during premixed mode [22].
Jamrozik et al. [23] investigated the effects of introduction of ethanol in a direct injection diesel engine fueled with diesel–biodiesel ethanol blends varying the ethanol fraction from 5 to 50%. In the case of full load, NOx was increased for blends using up to 40% of ethanol in volume, but after exceeding this content, the NOx emission started to decrease. According the authors, oxygen content could increase NOx emission, but for blends using more than 40% of ethanol, the cooling effect of ethanol seems to be dominant leading to combustion deterioration and thus overall reduction in NOx emission. THC emission increased for partial loads when ethanol content increased. CO emission does not show a consistent result.
Guarieiro et al. [24] tested the emissions of ternary blends of diesel, biodiesel, ethanol and vegetable oil in a bi-cylindrical 27 kW DI Diesel engine. They verified stability of ethanol–diesel blend only up to 15% anhydrous alcohol at 25 °C and a slight decrease in NOx emissions, between 1800 and 2000 rpm. Tsang et al. [25] observed reduction in NOx and PM emissions and increase in THC emissions when ethanol fumigation was tested in a four-cylinder direct injection diesel engine. Tests were conducted by Prakash et al. [26] in a single-cylinder, four-stroke diesel engine at 5.2 kW operating with vegetable oil–diesel–ethanol blends. Reductions in NOx emissions were verified, but HC and CO emissions and opacity were increased. The energy efficiency was decreased when ethanol was added in the fuel mixture.
In this work, the effects of binary and ternary blends of diesel, ethanol and biodiesel from waste cooking oil on the performance and emissions of a single-cylinder diesel engine coupled to a pump assembly used to control speed and load were investigated using energy analyses as support. The simultaneous use of ethanol and biodiesel aims to increase the total biomass-based content of the fuel while reducing NOx and PM emission.
2 Materials and methods
2.1 Pump and engine
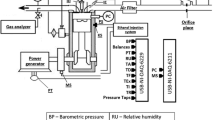
The tests were performed on a pump driven by a diesel engine that transports water between two reservoirs, as described in Fig. 1. A horizontal single-cylinder, naturally aspirated, four-stroke diesel engine (see Table 1) drives a multistage centrifugal type pump (see Table 2). The hydraulic arrangement works as a dynamometer. It is possible to evaluate the engine power output, since the efficiency of the pump is known. The instrumentation used to measure water flow rate and pressure exhibits lower uncertainty, which together with the simplicity of the system makes the assembly an interesting low-cost solution to evaluate engine performances. For each test cycle, temperatures, pressures, engine and pump speeds, exhaust gas emissions and fuel consumption were evaluated using valve V-1 to impose specific restrictions, thus controlling pump operation and thereby maintaining constant engine speed and load. Tests were carried out at the same speed for the four different fuels. In all cases, the engine operated at 1900 rpm and the pump at 3400 rpm. Two load values were tested (5.71 and 7.43 kW). The lower tank has a large diameter (D) when compared to the suction pump pipe diameter (d), D/d = 62.5. Also, the outlet pipe of the upper reservoir has adequate cross section allowing complete drainage and ensuring constant water level and load during the tests.
2.2 Instrumentation
The fuel mass flow rate was obtained by the gravimetric method using a digital scale. For each fuel, five cycles of measurements were performed with a sampling time of 20 min to determine the fuel consumption. The pump hydraulic power was obtained using inlet and outlet pressures and water flow rate, which was measured by a nonintrusive ultrasonic flowmeter. The exhaust gas emissions were determined by three gas analyzers. One of them was capable of evaluating the concentrations of CO and NOx in ppm, while the others were able to measure the opacity and concentration of unburned hydrocarbons (THC) in hexane basis. A total of 25 measurements of gas emissions and exhaust gas opacity were performed for each fuel. The main characteristics of the employed instruments are shown in Table 3.
Type K thermocouples were used to measure the inlet and outlet cooling water and engine exhaust temperature. The cooling water flow rate was kept constant at 1.00 m3/h and was measured by a rotameter for all the tests. The pump and engine speeds were measured by a digital tachometer to evaluate losses due to pulley–belt coupling.
2.3 Fuels
The waste cooking oil (WCO) was donated by local restaurants, collected at Polytechnic School and turned into biodiesel in a pilot plant with capacity of 5000 m3 per year located at the Federal University of Bahia (UFBA). The biodiesel was obtained by transesterification reaction using waste cooking oil and ethyl alcohol employing KOH as catalyst. The main properties (viscosity, lower heating value, cetane number) of the WCO biodiesel are similar to those of soybean biodiesel because a great part of the cooking oil used in Brazil is originated from that vegetable oil. The specifications for the biodiesel were determined according to standard EN-14214.
The class S-500 diesel and the anhydrous ethyl alcohol (purity of 99.5%) were kindly donated by PetroBahia (petroleum distributor of Bahia). Table 4 shows the main properties of the raw materials used for the fuel compositions. All the tests were carried out with the same batch of fuel, therefore making sure the fuel specification was always the same.
Four different fuel compositions were tested. The tests started with mineral diesel (D100). After that, the following were tested: D90B10 (90% diesel + 10% biodiesel v/v), D82B10E8 (82% diesel + 10% biodiesel + 8% ethanol v/v) and neat B100 (100% biodiesel).
2.4 Energy analysis
The tests took place at ambient temperature of 29 ± 3 °C, while the relative humidity was constant at 62 ± 4%. The engine was heated for 30 min, and lubricating oil was replaced for each fuel tested before each cycle. The combustion reaction is described in Eq. (1).
The x, y and z coefficients are the molar ratios of the diesel, biodiesel and ethanol for each fuel composition in one mol of fuel (x + y + z = 1), while the other coefficients (a, b, c, d, e, f, g, h and i) were obtained using the measured data and the mass balance of each element.
For the energy analysis, some assumptions were made:
-
The engine operates at steady state;
-
The control volume includes only the engine;
-
The blends are ideal solutions;
-
The kinetic and potential energy effects were not taken into account;
-
The atmospheric air composition was assumed as 21% oxygen and 79% nitrogen on a molar basis;
-
Since the air stream is very close to the standard reference state, the energy input accompanying it was ignored;
-
The SO2 emissions were not considered.
The engine energy flows crossing the control surface are presented in Fig. 2.
If the pressure loss and the kinetic and potential energy variations of water are not taken into account, the engine power is determined through the ratio between the hydraulic power and the pump efficiency, as described in Eq. (2):
in which \(\dot{W}_{\text{eng}}\) is the engine power (kW); \(\dot{W}_{\text{p}}\) is the hydraulic power supplied by the pump (kW); \(\dot{\forall }_{{}}\) is the water flow rate (m3 s−1); \(P_{1}\) is the water upstream pressure (Pa); \(P_{2}\) is the water downstream pressure (Pa); and \(\eta_{\text{p}}\) is the pump energy efficiency.
The pump energy efficiency was determined as the polynomial function described in Eq. 3, in which a, b and c are coefficients informed by the pump manufacturer for the speed tested:
The balance of energy flows entering and leaving the control surface can be reported as in Eq. (4):
in which \(\dot{E}_{\text{in}}\) is the energy flow rate entering into the control volume (kW); \(\dot{n}_{\text{c}}\) is the molar flow rate of equivalent fuel (diesel + biodiesel + ethanol) (kmol s−1); \(\overline{\text{LHV}}\) is the molar lower heating value of equivalent fuel (diesel + biodiesel + ethanol) (kJ kmol−1); \(\dot{Q}_{{\,{\text{CV}}}}\) is the heat flow rate crossing the engine surface (kW); \(\dot{Q}_{\text{W}}\) is the heat flow rate dissipated from cooling water (kW); and \(\dot{E}_{\text{ex}}\) is the energy flow rate contained in the exhaust gas (kW).
The heat flow rate dissipated from cooling water was determined by the product of the water mass flow rate and the difference between the water input and output enthalpies. The First Law of Thermodynamics was applied to determine the heat exchanged between the engine and the environment. The mass and energy rate balances for the single-inlet, single-exit control volume can be used to obtain the following relationship on fuel basis per mole (see Eq. 5) [27]:
in which \(\bar{h}_{\text{p}}\) is the molar combustion enthalpy in combustion gases per mole of fuel (kJ kmol−1); \(\bar{h}_{\text{R}}\) is the molar combustion enthalpy in reactants per mole of fuel (kJ kmol−1); \(n_{\text{out}}\) is the coefficient for each product in the reaction equation; \(n_{\text{in}}\) is the coefficient for each reactant in the reaction equation; \(\overline{{h_{\text{f}}^{0} }}\) is the enthalpy of formation for each compound in the combustion equation; and \(\overline{\Delta h}\) is the enthalpy change due to a change of state at a constant composition.
Enthalpy change was determined by Eq. (6) [27]:
in which \(\overline{h} \,(T)\) is the enthalpy at the temperature of the considered reactant or product (kJ kmol−1) and \(\overline{h} \,(T_{\text{REF}} )\) is the enthalpy at the reference temperature (kJ kmol−1). Formation enthalpies of the fuels were determined from complete combustion reaction. The energy in the exhaust gas (\(\dot{E}_{\text{ex}}\)) was determined by Eq. (4).
The engine energy efficiency (η), defined as the ratio of useful energy produced to the energy of the fuel consumed, was determined according to Eq. (7):
3 Results and discussion
3.1 Exhaust gas temperature
The exhaust gas temperatures for each fuel are shown in Fig. 3. An increase in the exhaust gas temperature accompanying an increase in biodiesel content can be seen. The oxygen in the biodiesel molecule can improve the combustion process increasing the temperature in the combustion chamber. Also, as Lapuerta et al. [28] point out, the biodiesel presents a higher bulk modulus which produces quicker responses to injector opening; thus, biodiesel is injected faster than diesel for the same engine injection timing (21° BTDC). This increases the pressure peak and the maximum temperature in the combustion chamber. In addition, the higher viscosity reduces leakages in the pump leading to an increase in the injection line pressure. However, Di et al. [2] found different results reporting a decrease in exhaust gas temperature as biodiesel content increased. The use of ethanol produced a reduction in the exhaust gas temperature probably due to its high latent heat of vaporization, which can result in a reduction in temperature inside the combustion chamber, thereby reducing the exhaust gas temperature [29]. Chahuan et al. [30] also experienced reduction in exhaust gas temperature due to the addition of ethanol in diesel blends.
3.2 Emissions profile
A slight increase in the NOx emissions with an increase in biodiesel content can be seen in Fig. 4. According to Lapuerta et al. [28], the advanced injection time may slightly advance the combustion in the combustion chamber producing a higher maximum pressure, increasing the temperature in the combustion chamber, thereby increasing the NOx emissions (Zeldovich mechanism). As can be seen in Fig. 4, the addition of ethanol (ternary blend) caused a reduction in NOx emissions, producing the lowest NOx emissions (reduction of up to 6.5% and 22% compared to mineral diesel and neat biodiesel, respectively). As Tutak [31] suggested, the formation NOx is affected by the peak flame temperature and the content of nitrogen and oxygen available in the combustion chamber. The reduction can be partly attributed to the cooling effect produced by the high latent heat of evaporation of ethanol as reported by Zhu et al. [15]. Kandasamy et al. [32] found similar results for tests with an ethanol–biodiesel blend at low and medium engine speeds justifying these results to high latent heat of vaporization and lower heating value of ethanol. This reduction in NOx emission of diesel–biodiesel blends by adding ethanol was also verified by Sachin et al. [33] The addition of a fuel with a very low cetane number is expected to significantly increase ignition delay producing the peak pressure at a later stage, resulting in a decrease in the combustion chamber pressure and temperature as discussed by Park et al. [34]. Sahin and Durgun [35] reported a slight increase in NO emission until 4% of ethanol fraction, but after that it starts to decrease because of reduction in the combustion chamber temperature.
The incomplete combustion of a hydrocarbon produces CO. Thus, its formation is strongly dependent on the air/fuel ratio. The increase in load causes a decrease in air/fuel ratio, but usually it also produces higher combustion chamber temperatures, therefore increasing flame velocity and reducing the CO emissions. The ethanol introduction can reduce exhaust gas temperature, due to the high value of heat of evaporation of ethanol, resulting in higher CO emissions, but the higher oxygen content of ethanol may produce more complete combustion and reduce CO formation [23]. Figure 5 shows that CO emissions decreased as the load was increased. A slight reduction in the CO emissions with an increase in the biodiesel content can be seen. As Di et al. [2] comment, this occurs due to the presence of oxygen in the molecule of biodiesel, which leads to a more efficient combustion process and a better conversion of CO in CO2.
In this study, the addition of ethanol increases CO emissions significantly. It may be explained by deterioration of combustion caused by high latent heat of vaporization, which reduces temperature, and by the very low cetane number of ethanol. Banugopan et al. [36] found similar results in their work.
Figure 6 shows HC emissions for the blends. A slight decrease in HC emissions was observed when the load was increased. As indicated by Chahuan et al. [30], large amounts of excess air and low temperatures may lead to cold and lean regions in the combustion chamber, resulting in higher THC emissions at low loads. A slight decrease in THC emissions was verified when biodiesel content was increased. Higher cetane number and the oxygen present in biodiesel molecule may justify this result. The addition of ethanol increased THC emissions significantly. Besides the effect of lower temperature, the air/alcohol mixture can be trapped in crevices or quenched by the cylinder wall which may also lead to unburned hydrocarbon. Also, at partial loads, the cooling effect of ethanol results in poorer combustion and thus increasing THC emissions [37]. As Kandasamy et al. [32] comment, the higher oxygen content of ethanol blend is not only the influence for unburned hydrocarbon emissions. Also, the higher latent heat of vaporization and longer ignition delay of ethanol could affect the premixed combustion duration and thereby change the combustion efficiency.
Opacity is a relevant indicator of particulate matter amount in exhaust gases of diesel engines. Figure 7 shows the results for opacity. In general, the increase in engine load leads to larger quantity of fuel injected per stroke generating incomplete combustion, which promotes the formation of particulates. A reduction in opacity was observed when biodiesel amount was increased. Neat biodiesel (B100) reduced opacity in 17%. An important reduction in PM emissions for the mixture D82B10E8 was verified, especially at high load for which a reduction of up to 31% was observed when compared to mineral diesel (D100). As Saleh and Selim [38] indicated, the reduction in opacity can be explained by the enrichment of oxygen owing to ethanol and biodiesel and the absence of aromatic compounds in that blend. As discussed by Emiroglu and Sen [39], a lower C/H of ethanol in that blend can reduce the soot emissions. Also, the OH radicals of ethyl alcohol can contribute to soot precursor reduction, as mentioned by Zhu et al. [15]. The low surface tension of ethanol may also improve spray formation, which reduces PM emissions. The opacity found for the ternary mixture was even lower than that of pure biodiesel. The lower viscosity of this mixture compared to biodiesel and the fact that alcohol has a very short molecule with only two carbon atoms may justify this result [22]. According to Jeogwool et al. [40], ethanol has greater potential to reduce PM emissions because it is highly volatile and readily oxidized.
In general, NOx and PM emissions are difficult to minimize simultaneously. In this study, the addition of ethanol has broken the negative correlation between these emissions and both are being reduced at the same time.
3.3 Energy analysis
The results for specific fuel consumption (SFC) are shown in Fig. 8. There is a well-marked increase in the engine specific fuel consumption with the increase in biodiesel content due to the reduction in the lower heating value of mixture. The ternary blend (D82B10E8) led to a higher fuel consumption when compared to mineral diesel. This can be explained by the low heating value of ethanol and biodiesel, but it also may suggest a slightly smaller energy conversion efficiency [41] since the difference between SFC (4.6% on average) for D82B10E8 and mineral diesel is higher than lower heating value difference (4.0%).
The energy flows crossing the engine control volume including percentage ratios of input energy are shown in Table 5. There was no significant change in energy flow rate when biodiesel content was increased to 10%. On the other hand, pure biodiesel (B100) increased the energy content contained in exhausted gases, especially at high load due to increase in the exhaust gas temperature.
The engine efficiency operating with the blend D90B10 is similar to mineral diesel because a 10% v/v increase in biodiesel does not modify significantly the overall lower heating value of the blend. A slight increase in energy efficiency with the use of neat biodiesel was verified. Although biodiesel has a higher specific fuel consumption, it has a LHV much lower than that of mineral fuel. A higher cetane number and oxygen in biodiesel molecule may improve the combustion process, hence justifying this result [27].
The addition of ethanol generated an increase in the percentage energy lost in the exhaust gas. As Belgiorno et al. [42] indicated, the combustion duration is decreased when ethanol is added; thus, the period of time necessary for heat transfer to ambient occurs is also reduced, which increases the energy in the engine exhaust gas. The significant increase in the HC and CO emissions with introduction of ethanol, even with a lower exhaust gas temperature, suggested that the use of ethanol caused a discrete reduction in engine efficiency, which is verified in Table 5.
As Labeckas et al. [41] commented, the low cetane number blend containing ethanol also produces significant cooling of the fuel sprays due to the high latent heat for evaporation, which may lead to changes in combustion characteristics (start of combustion, maximum cylinder gas pressure and temperature), increasing incomplete diffusion burning and deteriorating the combustion process. On the other hand, Hulwan and Joshi [43] found different and contradictory results, reporting decrease in energy efficiency for ethanol blends only at low loads, which suggests that different engines (or different calibrations) at other operational conditions (load and speed) may produce different results.
4 Conclusions
Biodiesel has been presented as a promising substitute for mineral diesel. Characteristics of biodegradability, low toxicity and renewability make it an important source of energy for the global matrix. Ethanol can be a valuable alternative when using biodiesel in compression ignition engines due to its high heat of vaporization, which reduces the temperature in the combustion chamber lowering NOx emissions.
This work presented tests for four different fuels in a single-cylinder engine driving a multistage centrifugal pump. The pump assembly proved to be an effective and low-cost solution to measure engine power output. As expected, biodiesel generated higher rates of NOx and the lower CO and HC emissions when compared to the mineral diesel due to the high cetane number and the presence of oxygen in its molecule. The addition of ethanol to diesel–biodiesel blend produced lower NOx emissions (6.5%), and it also reduced PM emission up to 31% compared to mineral diesel. These effects may be attributed to the cooling effect produced by the high latent heat of the ethanol, to the oxygen in the fuel, to the lower C/H ratio and to the OH radical which may reduce PM precursors. Further studies need to be carried out in order to verify the action of new low-cost compounds that are intended to improve the cetane number of ethanol blends since CO and HC emissions were increased with introduction of ethanol as a result of incomplete combustion.
The energy analysis shows that neat biodiesel presented the highest specific fuel consumption due to its lower heating value. On the other hand, the results demonstrated greater energy efficiency for biodiesel. This can be explained by the improved efficiency of combustion process due to oxygen amount in biodiesel molecule. A discrete reduction in engine efficiency was verified with the use of ethanol. The low cetane number of ethanol and its significant cooling effect of the fuel sprays caused by its high latent heat for evaporation may deteriorate the combustion process.
References
Tamilselvan P, Nallusamy N, Rajkumar S (2017) A comprehensive review on performance, combustion and emission characteristics of biodiesel fuelled diesel engines. Renew Sustain Energy Rev 79:1134–1159
Di Y, Cheung CS, Huang Z (2009) Experimental investigation on regulated and unregulated emissions of a diesel engine fueled with ultra-low sulfur diesel fuel blended with biodiesel from waste cooking oil. Sci Total Environ 407:835–846. https://doi.org/10.1016/j.scitotenv
Candeia RA, Filho JRC, Brasilino MGA, Bicudo TC, Santos IMG (2009) Influence of soybean biodiesel content on basic properties of biodiesel–diesel blends. Fuel 88:738–743
Tüccar G, Özgür T, Aydin K (2014) Effect of diesel–microalgae biodiesel–butanol blends on performance and emissions of diesel engine. Fuel 132:47–52
Shirneshan A, Morteza A, Barat G, Ali MB, Gholamhassan N (2013) Brake specific fuel consumption of diesel engine by using biodiesel from waste cooking oil. World Sci J 1:45–52
Sanli H, CanakciM Alptekin E, Turkcan A, Ozsezen AN (2015) Effects of waste frying oil based methyl and ethyl ester biodiesel fuels on the performance, combustion and emission characteristics of a DI diesel engine. Fuel 159:179–187
Santos TB, Ferreira VP, Torres EA, Silva JAMD, Ordonez JC (2017) Energy analysis and exhaust emissions of a stationary engine fueled with diesel-biodiesel blends at variable loads. J Braz Soc Mech Sci Eng 39:3237–3247
Roy M, Wang W, Bujold J (2013) Biodiesel production and comparison of emissions of a DI diesel engine fueled by biodiesel–diesel and canola oil–diesel blends at high idling operations. Apply Energy 106:198–208. https://doi.org/10.1016/j.apenergy.2013.01.057
Tan P-q, Hu Z-y, Lou D-m, Li Z-j (2012) Exhaust emissions from a light-duty diesel engine with Jatropha biodiesel fuel. Energy 39:356–362
EPA (2002) A comprehensive analysis of biodiesel impacts on exhaust emissions, EPA 420-P-02-001; U.S. Environmental Protection Agency: Washington, DC
Guarieiro LLN, Guarieiro ALN (2013) Vehicle emissions: what will change with use of biofuel? Chapter 14, 357, 386, Intech, Chennai
Mollenhauer K, Tschoeke H (2010) Handbook of diesel engines. Springer, Berlin
Fattah IMR, Masjuki HH, Liaquat AM, Ramli R, Kalam MA, Riazuddin VN (2013) Impact of various biodiesel fuels obtained from edible and non-edible oils on engine exhaust gas and noise emissions. Renew Sustain Energy Rev 18:552–567. https://doi.org/10.1016/j.rser.2012.10.036
Saravanan et al (2012) Correlation for thermal NOx formation in compression ignition (CI) engine fuelled with diesel and biodiesel. Energy 42:401–410. https://doi.org/10.1016/j.energy.2012.03.028
Zhu L, Cheung CS, Zheng WG, Huang Z (2001) Combustion performance and emission characteristics of a diesel engine fueled with ethanol-biodiesel blend. Fuel 90:1743–1750
Martins J (2013) Motores de Combustão Interna (in Portuguese), 4th edn. Publindustria, Porto. ISBN: 978-989-723-033-2
Yilmaz N, Sanchez TM (2012) Analysis of operating a diesel engine on biodiesel-ethanol and biodiesel-methanol blends. Energy 46:126–129
Jie L, Shenghua L, Yi L, Yanju W, Guangle L, Zan Z (2010) Regulated and nonregulated emissions from a dimethyl ether powered compression ignition engine. Energy Fuels 24:2465–2469
Kuszewski H (2019) Experimental investigation of the autoignition properties of ethanol–biodiesel fuel blends. Fuel 235:1301–1308
Kumar S, Cho JH, Park J, Moon I (2013) Advances in diesel-alcohol blends and their effects on the performance and emissions of diesel engines. Renew Sustain Energy Rev 22:46–72
Hiregoudar Y, Maniunatha K, Kishore KS (2018) Experimental study on performance and emission of a turbocharged diesel engine fueled with diesel and vegetable oils blended with ethanol. Mater Today Proc 5:6288–6296
Shamun S, Belgiorno G, DiBlasio G, Beatrice C, Tunér M, Tunestål P (2018) Performance and emissions of diesel-biodiesel-ethanol blends in a light duty compression ignition engine. Appl Therm Eng 145:444–452
Jamrozik A, Tutak W, Pyrc M, Sobiepanski M (2017) Effect of diesel-biodiesel-ethanol blend on combustion, performance, and emissions characteristics on a direct injection diesel engine. Therm Sci 21(1B):591–604
Guarieiro LLN, deSouza AF, Torres EA, Andrade JB (2009) Emission profile of 18 carbonyl compounds, CO, CO2, and NOx emitted by a diesel engine fuelled with diesel and ternary blends containing diesel, ethanol and biodiesel or vegetable oils. Atmos Environ 43:2754–2761
Tsang KS, Zhang ZH, Cheung CS, Chan TL (2010) Reducing emissions of a diesel engine using fumigation ethanol and a diesel oxidation catalyst. Energy Fuels 24:6156–6165
Prakash T, Geo VE, Martin LJ, Nagalingam B (2018) Effect of ternary blends of bio-ethanol, diesel and castor oil on performance, emission and combustion in a CI engine. Renew Energy 122:301–309
Canakci M, Hosoz M (2006) Energy and exergy analyses of a diesel engine fuelled with various biodiesels. Energy Sour Part B 1:379–394
Lapuerta M, Armas O, Rodríguez-Fernández J (2008) Effect of biodiesel fuels on diesel engine emission. Prog Energy Combust Sci 34:198–223
Rakopoulos CD, Antonopoulos KA, Rakopoulos DC (2007) Experimental heat release analysis and emissions of a HSDI diesel engine fueled with ethanol–diesel fuel blends. Energy 32:1791–1808
Chauhan BS, Kumar N, Pal SS, Jun YD (2011) Experimental studies on fumigation of ethanol in a small capacity diesel engine. Energy 36:1030–1038
Tutak W (2014) Bioethanol E85 as a fuel for dual fuel diesel engine. Energy Convers Manag 86:39–48
Kandasamy SK, Selvaraj AS, Rajagopal TKR (2019) Experimental investigations of ethanol blended biodiesel fuel on automotive diesel engine performance, emission e durability characteristics. Renew Energy 141:411–419
Sachin MK, Abdul PS, Manida T, Nuwong C (2019) Performance and emission assessment of optimally blended biodiesel-diesel-ethanol in diesel engine generator. Appl Therm Eng 155:525–533
Park SH, Youn IM, Lee CS (2011) Influence of ethanol blends on the combustion performance and exhaust emission characteristics of a four-cylinder diesel engine at various engine loads and injection timings. Fuel 90:748–755
Sahin Z, Durgun O (2009) Prediction of the effects of ethanol-diesel fuel blends on diesel engine performance characteristics, combustion, exhaust emissions, and cost. Energy Fuels 23:1707–1717
Banugopan VN, Prabhakar S, Annamalai K, Jayaraj S, Sentilkumar P (2010) Experimental investigation on DI diesel engine fuelled by ethanol diesel blend with varying inlet air temperature. IEE 2010:128–134
Zhang ZH, Tsang KS, Cheung CS, Chan TL, Yao CD (2011) Effect of fumigation methanol and ethanol on the gaseous and particulate emissions of a direct-injection diesel engine. Atmos Environ 45:2001–2008
Saleh HE, Selim YE (2017) Improving the performance and emissions characteristics of a diesel engine fueled by jojoba methyl ester-diesel-ethanol ternary blends. Fuel 208:690–701
Emiroglu AO, Sen M (2018) Combustion, performance and emissions characteristics of various alcohol blends in a single cylinder diesel engine. Fuel 212:34–40
Jeogwool L, Sunyoup L, Seokhwan L (2018) Experimental investigation on the performance and emissions characteristics of ethanol/diesel dual-fuel combustion. Fuel 220:72–79
Labeckas G, Slavinskas S, Mazeika M, Laurinaitis K (2011) Performance and emission characteristics of diesel engine fuelled with ethanol-diesel-biodiesel blend. Eng Rural Dev 2011:26–27
Belgiorno G, Blasio GD, Shamun S, Beatrice C, Tunestal P, Tunér M (2018) Performance and emissions of diesel-gasoline-ethanol blends in a light duty compression ignition engine. Fuel 217:78–90
Hulwan DB, Joshi SV (2011) Performance, emission and combustion characteristic of a multicylinder DI diesel engine running on diesel-ethanol-biodiesel blends of high ethanol content. Appl Energy 99:5042–5055
Author information
Authors and Affiliations
Corresponding author
Additional information
Technical Editor: Fernando Marcelo Pereira, PhD.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ferreira, V.P., Torres, E.A., da Silva, J.A.M. et al. Evaluation of ethanol as additive for NOx and PM reduction in CI engines fueled with diesel–biodiesel blends using a pump assembly. J Braz. Soc. Mech. Sci. Eng. 42, 408 (2020). https://doi.org/10.1007/s40430-020-02494-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40430-020-02494-0