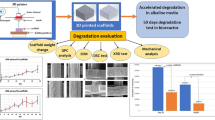
Abstract
Nowadays polylactic acid (PLA) is widely used in orthopedics surgeries as implants material due to well mechanical characterization and biomedical properties. But the PLA implants suffer from slow degradation rate when it is used in real-life scenario. In the present research work, the PLA specimens using additive manufacturing technique are fabricated and further assessed for mechanical characterization and its degradation behavior with different parameters. The change in weight of scaffolds was measured using digital weight measure, and pH value was measured using pH meter. Morphology and elemental composition of PLA scaffolds were characterized by SEM and EDS, respectively, while compressive strength is measured by the universal testing machine. Apatite formation and biocompatible nature of fabricated scaffolds were analyzed by in vitro simulated body fluid study. The outcomes of characterization exposed that scaffold with 60% infill percentage had maximum porosity, which is beneficial for the apatite formation and osseointegration. The average change in compressive strength was measured as 49.79 MPa after 14 days and 46.11 MPa after 28 days, whereas the average change in pH value was measured as 5.67 and 5.27 after 14 and 28 days of incubation period, respectively. The degradation rate of specimen 3 was 27.92% less than that of specimen 1, 35.69% less than that of specimen 5, and 87.98% more than that of specimen 9. This study concludes the positive effect of process parameters on degradation rate and biocompatible behavior of PLA implants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In tissue engineering, porous polylactic acid scaffolds are nowadays the focus of various research and development activities due to its properties like anticytotoxic nature, low density, biocompatibility, and biodegradability. These scaffolds are required for reconstruction and regeneration of damaged tissues as they act as a matrix for cell proliferation, growth, migration, differentiation, and extracellular matrix deposition (EMD) [1,2,3,4]. Polylactic acid (PLA) polymer is one of the best biocompatible polymers and widely used for biomedical applications. There are different methods available to fabricate porous structure such as solvent casting, ice particle leaching, gas foaming, solvent evaporation, laser beam machining, fused deposition modeling, solvent casting, conventional drilling, powder compaction [5,6,7,8]. It has been found that the fabrication of a polymer scaffold with traditional or conventional methods was widely popular in previous decades. The conventionally fabricated PLA scaffolds suffer from low degradation rate, poor permeability, and less interconnectivity between the pores owing to non-uniform structure [9]. It was observed that interconnectivity and porosity are essential to promote cell culture, tissue growth, loading and migration of cells, tissue growth, and flow of body fluid [10,11,12,13]. At present, a number of researchers [5, 6, 14,15,16,17,18,19,20,21] are working with non-conventional techniques and state-of-the-art fused deposition modeling (FDM) is one of them. This technique has been becoming most popular in biomedical field owing to its high flexibility of producing different shaped implants, 3D faxing, and green manufacturing nature.
It is expected that the fabrication of a polymer scaffold with different process parameters of FDM may affect the degradation rate and mechanical characterization of the scaffold. Chacon et al. [14] investigated the effect of layer thickness and build orientation on PLA specimens manufactured using fused deposition modeling process. The results revealed that part with minimum layer thickness showed the maximum tensile strength and minimum flexural strength. However, it depends upon the build orientation. In another study [22], it was found that a change in layer thickness, orientation angle, and shell thickness has a strong influence on the mechanical strength of the parts produced. Wang et al. [23] investigated the effect of porosity and pore size on pH value and weight loss of 3D-printed porous polymer scaffolds. Zhao et al. [24] mixed magnesium particles with polylactic acid to increase the degradation rate and biocompatibility of the PLA implants. It was highlighted that the degradation rate increases with an increase in the percentage of magnesium particles. The main concern for the traumatologists is the low degradation rate which reduces its applications in cases like absorbable bone fixation screw and drug delivery, where fast degradation rate is obligatory. If the degradation of the bone screw is low, it would affect the bone healing and thereby repair of damaged bone [25]. Hong et al. [26] performed a dedicated study to examine the degradation rate of pure PLA. The results displayed cracks and voids on the surface of specimens due to degradation, and the weight loss of pure polylactic acid was only 0.1% during the period of 48 weeks. Hence, the determination of suitable process parameters which will lead to the faster degradation rate of PLA specimens is the demand of the present scenario.
To the best of author’s knowledge, hitherto, a few research articles are available on fabricating biomimetic PLA scaffolds with fused filament fabrication (FFF) technology and investigating the effect of infill percentage, layer thickness, and infill pattern on degradation rate and apatite formation. Therefore, the key aspect of the present experimental work is to use the capacity of FFF to fabricate scaffolds for various biomedical applications and investigate the same for different process parameters. A comprehensive study of pH value, mechanical competences, morphology, apatite formation, and degradation rate in simulated body fluid solution has been carried out.
2 Materials and methods
2.1 Selection of workpiece material
In this study, PLA was used for fabrication of scaffolds supplied by FlashForge Corporation, Type: Biodegradable plastic, Model: 3D PLA-0.6KG1.75-BLU, Ext. Temp. 180–210 °C, and Filament diameter 1.75 mm (Tolerance ± 0.03 mm). The PLA has high biocompatibility, biodegradability, and thermo-mechanical properties. It is also clinically approved by the Food and Drug Administration (FDA, USA) for some human applications such as implantable devices [27]. This is widely used in the orthopedic surgeries as bone fixation screws and pins [28]. The thermo-mechanical properties [29,30,31,32] of polylactic acid are accentuated in Table 1.
2.2 Fabrication of scaffold
Rapid prototyping technique (RPT) based on fused filament fabrication technology was used for the fabrication of designed bioscaffold structures using a nozzle deposition 3D printing system (FlashForge Creator Pro, China). The machine primarily comprises a dispensing system to extrude different quantity and types of material as per requirement. The rate of extrusion from the dispensing nozzle is controlled by a software (motion control) and the computer-aided design (CAD) program with varying process parameters such as deposition rate, level of pressure in the pump actuated pneumatically, layer thickness, and 3D geometry of the deposition pathways. A high level of accuracy and precision in the range of few microns was achieved for the position of dispensing nozzle in XYZ direction. Three different architectures (triangular, rectilinear, and honeycomb) were designed and fabricated with pattern specifications of height 25.4 mm and diameter 12.7 mm to determine the effect of pore size and distribution in different working directions such as axial and transverse. The steps followed in fabricating bioscaffolds from computer-aided design model are shown in Fig. 1. Firstly, the 3D model of the part was designed in computer-aided design software followed by conversion into stereo-lithography (.stl) file format. Once the.stl file is created, it processed by various programs to create the build file for final printing. Slicers choice the surface of the designed model and break it into numbers of two-dimensional layers. The slicer program builds the way, and the nozzle follows that path to lay down the material. A servo motor controls the speed of filament in the 3D printer according to the process parameter settings and pushes the filament through the heated nozzle. The extruder head which is a combination of the servo motor and nozzle moves in x-axis and y-axis only, and the build platform moves up and down in the z-axis with the help of a servo motor. All these three movements allow the extruder to fabricate the final part as per the designed model.
2.3 Experimental design
A Taguchi L9 orthogonal array design was used for the experimentation. Three input parameters and levels were selected on the basis of trial experimentation and literature studied [33,34,35,36,37,38,39,40,41,42]. The infill percentage was taken at three different levels (60, 80, 100%), layer thickness (0.150, 0.250, 0.350 mm), and infill pattern (triangular, rectilinear, and honeycomb). Table 2 shows the L9 orthogonal array design along with control log of experimentation.
The biospecimens were fabricated with a different set of input parameters as per L9 orthogonal array design. A total of 9 specimens were prepared, and microscopic images were taken by a high-resolution microscope (DM6M LIBS, Leica Microsystems, Germany) which are presented along with corresponding biospecimens. The distinctive feature of the different infill pattern with varying layer thickness and infill percentage is clearly visible in Fig. 2.
2.4 Characterization
2.4.1 Biological assessment
The PLA specimens were fabricated with a printing temperature of 210 °C. To mimic the human biological conditions, simulated body solution (SBF) was prepared and fabricated specimens were immersed in SBF solution (7.865 g of NaCl, 6.153 g of CH2OH3 CNH2, 40 ml of HCl, 0.340 g of NaHCO3, 0.305 g of MgCl2 6H2O, 0.250 g of CaCl2, 0.075 g of Na2SO4, 0.235 g K2HPO4 3H2O, 0.224 g KCL) and were placed in an incubator as shown in Fig. 3. The incubator was used to maintain the temperature of the SBF solution within the range of human body temperature. Simulated body fluid is an artificial fluid in which ion concentration is almost equivalent to human blood plasma. The SBF has the ability of apatite to form on various biomedical materials. After 14 and 28 days, biospecimens were removed from the SBF and then rinsed with deionized water to eradicate soluble inorganic salts. To reach a constant mass, specimens were kept in a vacuum chamber (air) for 2 days so as to remove any moisture content present in the specimens.
To evaluate the degradation rate of each biospecimen the “loss in weight” is measured. The fabricated biospecimens were weighted before and after immersion in SBF solution for 14 and 28 days of incubation. The weight loss of each specimen can be estimated with mathematical calculations
The pH value of SBF solution was monitored three times a week during experimentation using pH meter (Cyberscan 510, Eutech Instruments Pvt. Ltd, Singapore). The pH values were measured using pH meter, and high-resolution microscope (DM6M LIBS, Leica Microsystems, Germany) was used for the characterization of PLA scaffolds for the assessment of morphological behavior and apatite formation. The SEM micrograph and EDS spectrum (SEM; JSM-6510 Series, JEOL, Tokyo, Japan) of PLA specimens were used for metallurgical and elemental analysis.
2.4.2 Mechanical characterization
A biodegradable material should preserve its mechanical properties until it is no longer needed and then be degraded. With the same impression, the compression tests were made to determine the effect on strength of PLA scaffold after the 14 and 28 days of incubation in SBF solution. The compression tests were conducted with the universal testing machine (UTM ST Series, Tinius Olsen Pvt. Ltd, USA) having 50-kN capacity (refer to Fig. 4). The load was applied to the cross section of the specimens, and changes were observed before and after compression. The polymer-based cylindrical samples with dimensions such as 12.7 mm diameter and 25.4 mm height selected for compression testing as per ASTM-D695 standards.
3 Results and discussion
3.1 Degradation rate and pH value
Degradation rate and porosity are closely related to each other and hence determine the performance of PLA scaffolds. The possible reason could be the wall and surface area effect which results in a stronger acid-catalyzed hydrolysis [43]. The initial weight of fabricated scaffolds was taken after drying specimens at 50 °C for 1 h, and final weight was taken after 14 and 28 days to check the weight difference. The apatite formation on the surface of the polymer scaffolds verified the biocompatible nature of the polylactic acid scaffold. Figure 5 clearly shows the formation of apatite on the surface of the polymer scaffold which is also justified using energy-dispersive spectroscopy (EDS) spectrum. The EDS was used to find the elements present in the bioscaffold specimens. Figure 6 represents the elemental morphology spectrum of entire elements of the control specimen and after immersion of 28 days.
The EDS spectrum of the scaffold’s surface before immersion in SBF solution confirms the base elements C and O of an alloy which is also evidently shown in Fig. 6b. Immersion of scaffolds in SBF solution changed the chemical state of the surface of C-O alloy due to the formation of apatite. The EDS spectrum of SBF-treated surface containing carbon (C), oxygen (O2), sodium (Na), magnesium (Mg), aluminum (Al), silicon (Si), chlorine (Cl), and calcium (Ca) elements was deposited as evidently shown in Fig. 6d. It can be clearly seen that ‘Ca’ peak exists among all peaks which confirms the formation of apatite over the surface of PLA scaffolds although its amount is very less. The pure PLA considered as control specimen to check the elemental composition and highly porous structure (40% porosity) after 28 days showed the maximum amount of apatite formation on the surface of specimens.
The specimen with a honeycomb pattern and 60% infilling has maximum porosity level, and it shows the high amount of formation on scaffold surface because pore size has sufficient space for cells to invade due to which cell attachment, proliferation, tissue growth occur and adequate nutrient flows across the voids of scaffold [9].
The results revealed that the weight of each specimen slightly increases after 14 days as shown in Fig. 7. It may be due to the apatite formation on the surface of specimens at the start; hence, an increase in weight was seen. After 28 days, the weight of each specimen was less which may be anticipated that degradation rate was the governing phenomenon here. The mechanism of degradation involves the diffusion of water into the polymer chain, and degradation starts from outside to interior which finally leads to the formation of ester bonds with hydrolytic chain reaction [44]. Due to the dependence on hydrolytic cleavage, PLA has more degradation rate than polymers during in vivo operation at implantation sites. This may be due to the fact that degradation rate is mediated primarily by enzymes whose activity may vary among different patients. The release of the oligomers due to which molecular weight of PLA scaffold starts decreasing, followed by the formation of the acidic environment [45]. These low weight oligomers are predominantly responsible for the solubilization of biospecimens in the medium due to which weight loss begins.
The results of the degradation study revealed that the weight loss (%) of specimen 3 was highest among all nine specimens after 28 days of the incubation period (Table 3). The degradation rate of specimen 3 was 27.92% less than that of specimen 1, 35.69% less than that of specimen 5, and 87.98% more than that of specimen 9. This highlighted that the dispersion status of PLA scaffolds fabricated with varying infill pattern, infill percentage, and layer thickness in SBF solution played a vital role in increasing degradation rate behavior of scaffolds. Hence, the degradation effect was more significant for longer incubation period; this is correlated with a change in the pH value of the SBF solution.
Specifically, the pH value drops by maximum 32.29% with respect to control value (i.e., 7.4) for specimen 3, 30.95% for specimen 5, and 29.32% for specimen 7 after 28 days of incubation as shown in Table 4. Correspondingly, the maximum percentage drop after 14 days of incubation is 29.05%, 27.03%, and 21.08%, respectively. The control value is the average pH value maintained by the human body [46]. As lesser pH value may be harmful, so authors tried to highlight the importance of this parameter by evaluating the effect of different process parameters on the pH change. The effect of decreased pH value on a bone tissue is not in the scope of the present study, and this may be taken as a future direction for further study.
Figure 8a indicates that pH value increases on increasing the infill percentage from level 1 (i.e., 60%) to level 3 (i.e., 100%) for 14 days of incubation period. Unlike the trend of infill percentage, the pH value first decreases on increasing the layer thickness from level 0.150 to 0.250 mm and then increases on a further increase of layer thickness to 0.350 mm. The average change in the pH value was 5.67% after 14 days on incubation period while 5.27% after 28 days of incubation period. A different trend was observed for 28 days of incubation period as shown in Fig. 8b, but the corresponding average change in the pH value was 5.23. Moreover, the compressive strength of specimens drops by 7.18% due to infill percentage, layer thickness, and infill pattern within 14–28 days of immersion in SBF solution.
This study highlighted the importance of process parameters during the fabrication of scaffolds which can not only improve the structural stability but also buffer the harmful effects due to pH variations. The significant drop in pH occurs when the molecular chain is not able to diffuse out of the scaffold due to its sufficiently small size.
3.2 Compressive strength
Herein, due to the importance of compressive strength of PLA scaffold’s material in orthopedic applications, in vitro compressive strength was measured using a universal testing machine at room temperature. The specimens with different porosity levels before and after compression of the specimen with an incubation period of 28 days are shown in Fig. 9. Specimen 3 has 60% infill percentage which means 40% porosity as porosity is directly related to the infill percentage. Similarly, specimen 5 has a medium porosity (i.e., 40% infill percentage), and specimen 9 has low porosity (i.e., 100% infill percentage). The test was carried out at the maximum load-bearing capacity with permanent deformation of the scaffold. The highest peak value of the load graph was considered as maximum load, and hence, the compressive strength was calculated with respect to the surface area of the specimen. The compressive strength of specimen 3 drops by 22.2%, specimen 5 by 18.1%, and specimen 9 by 6.1%. This may be due to the low porosity level of specimen 9 since low porosity leads to higher modulus and strength. So, specimens with high compressive strength caused a slow degradation rate owing to its low porosity [47]. Moreover, due to the increase in layer thickness, the number of layers required for the fabrication was significantly reduced. Table 5 shows values of compressive strength at different time incubation periods, i.e., 14 and 28 days.
Figure 10a clearly shows that after 14 days of incubation period, the compressive strength of the scaffolds keeps on decreasing on increasing the infill percentage which may happen due to the decrease in the porosity of the scaffolds with an increase in infill percentage, whereas the compressive strength decreases in changing the level of infill pattern from level 1 (i.e., rectilinear pattern) to level 3 (i.e., honeycomb pattern). The average change in the compressive strength due to infill percentage was 49.79 MPa, due to layer thickness, and the infill pattern was 49.8 MPa, after 14 days of incubation. The same trend was observed for 28 days of incubation period as shown in Fig. 10b, but the corresponding average change in the compressive strength was 46.11 MPa due to infill percentage and 46.11 MPa due to layer thickness and infill pattern, which highlights the importance of infill percentage, and layer thickness, and infill pattern on behavior of compressive strength of PLA scaffolds. Moreover, the compressive strength of specimens drops by 7.39% due to infill percentage, 7.41% due to layer thickness, and infill pattern within 14–28 days of immersion in SBF solution.
3.3 Treated surface morphology
Figure 11b displays the formation of calcium on the surface of scaffold fabricated by 60, 80, and 100% infill percentage with three different infill patterns (triangular, rectilinear, and honeycomb). A highly porous layer was obtained on the surface of PLA scaffold at 60% infill percentage. At higher magnification (4000 ×), a layer of apatite formation is clearly observed in Fig. 11b. As the percentage of infill increased to 80%, a very less calcium has been obtained on the surface of PLA scaffold due to the decrease in the porosity level. If the infill percentage increased to 100%, a very dense and a few voids have been obtained on the scaffold surface, as can be seen in Fig. 11d. Specifically, the porous structure helps the bone tissue for infiltration and growth compared to the dense structure. The scaffold is hence a porous structure which acts as a template for bone tissue formation. Highly porous structure helps to grow the bone cells and regeneration of bone for faster recovery.
4 Conclusion
The present in vitro study made a remarkable assessment of polylactic acid scaffolds on the basis of their mechanical (compressive strength and morphological changes) and biological behavior (degradation rate, pH value, and apatite formation). The three different process parameters such as infill percentage, layer thickness, and infill pattern change up to three different levels to examine the effects. This study concludes the following key points while fabricating PLA scaffolds with the additive manufacturing process.
-
1.
Degradation study showed that weight of specimens was firstly increased and then decreased, and it is only due to apatite formation weight and degradation rate of the specimen. The maximum change in the weight after 28 days was 5 mg.
-
2.
pH value of the solution represents the degradation of the different specimens. The pH value was decreased with the time period. The maximum percentage change in the pH value with respect to the control value was 17.56% after 7 days, 29.05% after 14 days, 31.62% after 21 days, and 32.29% after 28 days.
-
3.
The change in the process parameters significantly affected the compressive strength. The maximum percentage decrease in compressive strength was 12.8% and 22.2% for specimen 3 after 14 days and 28 days, respectively, while the minimum percentage drop in compressive strength was 3.02% and 6.1% for specimen 9 after 14 and 28 days, respectively.
-
4.
It was found that specimen 1 with 60% infill percentage, 0.150 mm layer thickness, and triangular infill pattern showed the maximum degradation rate and specimen number 9 with 100% infill percentage, 0.350 mm layer thickness, and rectilinear pattern showed the low degradation rate. So, the results outlined that the degradation rate is directly dependent upon the porosity of the specimen.
-
5.
Treated surface morphology results highlighted that the maximum apatite was formed on specimens with 60% infill percentage and represents the maximum bone cell growth in the porous structure. Hence, PLA scaffolds may be used as an implant material instead of titanium-based implants for healing small fractures due to efficient osseointegration and vascularization. However, for large fractures and the joints with high load bearing such as ankle, knees, and hip joints, a titanium implants can be preferred. Hence, polylactic acid polymer can be used for next-generation graft material.
References
Gershlak JR, Hernandez S, Fontana G et al (2017) Crossing kingdoms: using decellularized plants as perfusable tissue engineering scaffolds. Biomaterials. https://doi.org/10.1016/j.biomaterials.2017.02.011
Hasan A, Memic A, Annabi N et al (2014) Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater 10(1):11–25
Collins MN, Birkinshaw C (2013) Hyaluronic acid based scaffolds for tissue engineering—a review. Carbohydr Polym 92(2):1262–1279
Stratton S, Shelke NB, Hoshino K et al (2016) Bioactive polymeric scaffolds for tissue engineering. Bioact Mater. https://doi.org/10.1016/j.bioactmat.2016.11.001
Singh S, Singh R (2017) Some investigations on surface roughness of aluminium metal composite primed by fused deposition modeling-assisted investment casting using reinforced filament. J Braz Soc Mech Sci Eng 39:471–479. https://doi.org/10.1007/s40430-016-0524-8
Magalhães LC, Volpato N, Luersen MA (2014) Evaluation of stiffness and strength in fused deposition sandwich specimens. J Braz Soc Mech Sci Eng 36:449–459. https://doi.org/10.1007/s40430-013-0111-1
Valipour P, Ghasemi SE (2016) Erratum to: Numerical investigation of MHD water-based nanofluids flow in porous medium caused by shrinking permeable sheet (J Braz. Soc. Mech. Sci. Eng., (2016), 38, (859–868), 10.1007/s40430-014-0303-3). J Braz Soc Mech Sci Eng 38:2189
Singh S, Ramakrishna S (2017) Biomedical applications of additive manufacturing: present and future. Curr Opin Biomed Eng. https://doi.org/10.1016/j.cobme.2017.05.006
Salim S, Ariani MD (2015) In vitro and in vivo evaluation of carbonate apatite-collagen scaffolds with some cytokines for bone tissue engineering. J Indian Prosthodont Soc 15(4):349–355. https://doi.org/10.4103/0972-4052.171821
Sobral JM, Caridade SG, Sousa RA et al (2011) Three-dimensional plotted scaffolds with controlled pore size gradients: effect of scaffold geometry on mechanical performance and cell seeding efficiency. Acta Biomater. https://doi.org/10.1016/j.actbio.2010.11.003
Guan J, Fujimoto KL, Sacks MS, Wagner WR (2005) Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials. https://doi.org/10.1016/j.biomaterials.2004.10.018
Do A-V, Khorsand B, Geary SM, Salem AK (2015) 3D printing of scaffolds for tissue regeneration applications. Adv Healthc Mater. https://doi.org/10.1002/adhm.201500168
Croisier F, Jérôme C (2013) Chitosan-based biomaterials for tissue engineering. Eur Polym J. https://doi.org/10.1016/j.eurpolymj.2012.12.009
Chacón JM, Caminero MA, García-Plaza E, Núñez PJ (2017) Additive manufacturing of PLA structures using fused deposition modelling: effect of process parameters on mechanical properties and their optimal selection. Mater Des 124:143–157. https://doi.org/10.1016/j.matdes.2017.03.065
Revati R, Abdul Majid MS, Ridzuan MJM, Normahira M, Nasir NFM, Cheng EM (2017) Biodegradation of PLA-Pennisetum purpureum based biocomposite scaffold. J Phys Conf Ser Pap 908(1):012029
Hokmabad VR, Davaran S, Ramazani A (2017) Design and fabrication of porous biodegradable scaffolds: a strategy for tissue engineering. J Biomater Sci Polym Ed. https://doi.org/10.1080/09205063.2017.1354674
Wurm MC, Möst T, Bergauer B et al (2017) In-vitro evaluation of polylactic acid (PLA) manufactured by fused deposition modeling. J Biol Eng 11(1):29. https://doi.org/10.1186/s13036-017-0073-4
Lamazza L, Garreffa G, Laurito D et al (2016) Temperature values variability in piezoelectric implant site preparation: differences between cortical and corticocancellous bovine bone. Biomed Res Int. https://doi.org/10.1155/2016/6473680
Józwik J, Ostrowski D, Milczarczyk R, Krolczyk GM (2018) Analysis of relation between the 3D printer laser beam power and the surface morphology properties in Ti–6Al–4 V titanium alloy parts. J Braz Soc Mech Sci Eng 40:215. https://doi.org/10.1007/s40430-018-1144-2
Modi YK, Sanadhya S (2018) Design and additive manufacturing of patient-specific cranial and pelvic bone implants from computed tomography data. J Brazilian Soc Mech Sci Eng 40:503. https://doi.org/10.1007/s40430-018-1425-9
Aherwar A, Singh A, Patnaik A (2018) A study on mechanical behavior and wear performance of a metal–metal Co–30Cr biomedical alloy with different molybdenum addition and optimized using Taguchi experimental design. J Braz Soc Mech Sci Eng 40:213. https://doi.org/10.1007/s40430-018-1107-7
Basavaraj CK, Vishwas M (2016) Studies on effect of fused deposition modelling process parameters on ultimate tensile strength and dimensional accuracy of nylon. In: IOP conference series: materials science and engineering
Wang MO, Piard CM, Melchiorri A et al (2015) Evaluating changes in structure and cytotoxicity during in vitro degradation of three-dimensional printed scaffolds. Tissue Eng Part A. https://doi.org/10.1089/ten.tea.2014.0495
Zhao C, Wu H, Ni J et al (2017) Development of PLA/Mg composite for orthopedic implant: tunable degradation and enhanced mineralization. Compos Sci Technol 147:8–15. https://doi.org/10.1016/J.COMPSCITECH.2017.04.037
Hollister SJ (2005) Porous scaffold design for tissue engineering. Nat Mater 4(7):518–524
Lu H, Madbouly SA, Schrader JA et al (2014) Biodegradation behavior of poly(lactic acid) (PLA)/distiller’ s dried grains with solubles (DDGS) composites. ACS Sustain Chem Eng 2:2699–2706. https://doi.org/10.1021/sc500440q
Raquez JM, Habibi Y, Murariu M, Dubois P (2013) Polylactide (PLA)-based nanocomposites. Prog Polym Sci. https://doi.org/10.1016/j.progpolymsci.2013.05.014
Hamad K, Kaseem M, Yang HW et al (2015) Properties and medical applications of polylactic acid: a review. Express Polym Lett. https://doi.org/10.3144/expresspolymlett.2015.42
Lasprilla AJR, Martinez GAR, Lunelli BH et al (2012) Poly-lactic acid synthesis for application in biomedical devices—a review. Biotechnol Adv 30(1):321–328
Suryanegara L, Nakagaito AN, Yano H (2009) The effect of crystallization of PLA on the thermal and mechanical properties of microfibrillated cellulose-reinforced PLA composites. Compos Sci Technol. https://doi.org/10.1016/j.compscitech.2009.02.022
Inkinen S, Hakkarainen M, Albertsson AC, Södergård A (2011) From lactic acid to poly(lactic acid) (PLA): characterization and analysis of PLA and its precursors. Biomacromolecules 12(3):523–532
Farah S, Anderson DG, Langer R (2016) Physical and mechanical properties of PLA, and their functions in widespread applications—a comprehensive review. Adv Drug Deliv Rev 107:367–392
Zein I, Hutmacher DW, Tan KC, Teoh SH (2002) Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials. https://doi.org/10.1016/S0142-9612(01)00232-0
Wei G, Ma PX (2004) Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials. https://doi.org/10.1016/j.biomaterials.2003.12.005
Pham QP, Sharma U, Mikos AG (2006) Electrospun poly(ε-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules. https://doi.org/10.1021/bm060680j
Ghasemi-Mobarakeh L (2015) Structural properties of scaffolds: crucial parameters towards stem cells differentiation. World J Stem Cells. https://doi.org/10.4252/wjsc.v7.i4.728
De Ciurana J, Serenó L, Vallès È (2013) Selecting process parameters in RepRap additive manufacturing system for PLA scaffolds manufacture. In: Procedia CIRP
Van Vlierberghe S, Dubruel P, Schacht E (2011) Biopolymer-based hydrogels as scaffolds for tissue engineering applications: a review. Biomacromolecules 12(5):1387–1408
Baker BM, Gee AO, Metter RB et al (2008) The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials. https://doi.org/10.1016/j.biomaterials.2008.01.032
Chang HI, Wang Y (2011) Cell responses to surface and architecture of tissue engineering scaffolds. In: Regenerative medicine and tissue engineering-cells and biomaterials. InTech, Croatia. https://doi.org/10.5772/21983
Inzana JA, Olvera D, Fuller SM et al (2014) 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials. https://doi.org/10.1016/j.biomaterials.2014.01.064
Haugh MG, Murphy CM, McKiernan RC et al (2011) Crosslinking and mechanical properties significantly influence cell attachment, proliferation, and migration within collagen glycosaminoglycan scaffolds. Tissue Eng Part A. https://doi.org/10.1089/ten.tea.2010.0590
Singh BN, Pramanik K (2018) Generation of bioactive nano-composite scaffold of nanobioglass/silk fibroin/carboxymethyl cellulose for bone tissue engineering. J Biomater Sci Polym Ed 29(16):2011–2034. https://doi.org/10.1080/09205063.2018.1523525
Liu H, Webster TJ (2006) Less harmful acidic degradation of poly (lactic-co-glycolic acid) bone tissue engineering scaffolds through titania nanoparticle addition. Int J Nanomed 1(4):541–545
Zhang JF, Sun X (2004) Mechanical properties of poly(lactic acid)/starch composites compatibilized by maleic anhydride. Biomacromolecules. https://doi.org/10.1021/bm0400022
Oyane A, Kim HM, Furuya T et al (2003) Preparation and assessment of revised simulated body fluids. J Biomed Mater Res, Part A. https://doi.org/10.1002/jbm.a.10482
Sung H-J, Meredith C, Johnson C, Galis ZS (2004) The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials. https://doi.org/10.1016/j.biomaterials.2004.01.066
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no potential conflict of interest among all authors.
Additional information
Technical Editor: Estevam Barbosa Las Casas, Ph.D.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, D., Babbar, A., Jain, V. et al. Synthesis, characterization, and bioactivity investigation of biomimetic biodegradable PLA scaffold fabricated by fused filament fabrication process. J Braz. Soc. Mech. Sci. Eng. 41, 121 (2019). https://doi.org/10.1007/s40430-019-1625-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40430-019-1625-y