Abstract
Watermelon (Citrullus lanatus) is one of the most popular fruits in Cameroon and the world at large. However, the extreme sensitivity of watermelon to parasites and climatic vagaries makes its cultivation demanding of chemical inputs that can have negative impacts on human health and the environment. In Cameroon, there is a slow improvement of fruit yield in watermelon breeding due to the lack of natural heritable genetic variation, which is a prerequisite for genetic improvement of crops. Such variation can be created through either random or targeted processes on genotypes with appropriate doses of radiation. Genetic improvement by induced mutagenesis appears today alongside hybridization as an alternative method of creating new plant varieties. However, the success of this approach is determined by the application of an appropriate and ideal dose of mutagen. The objective of this study was to evaluate the radiosensitivity of the two most cultivated watermelon varieties in Cameroon to gamma radiation from 60Co in order to determine an optimal dose or lethal dose 50 (LD50) for the induction of the genetic variability necessary for genetic improvement. Seeds of the Kaolack and Crimson sweet watermelon varieties were irradiated with five doses of gamma radiation (100, 200, 300, 400 and 600 Gy) in the laboratory of the International Atomic Energy Agency in Seibersdorf, Austria. These seeds were cultivated in a greenhouse following an utterly randomized device with three repetitions, and parameters such as the germination rate, the survival rate and the shoot length of plants were evaluated. High rates of 90% and 75% germination were obtained, respectively, for the control treatments of Kaolack and Crimson sweet, while the lowest rates were 35% at 600 Gy for Kaolack and 30% at 400 Gy for Crimson sweet. The highest survival rate of plants (96.66%) was obtained with the control seeds of the Kaolack. This variety had the lowest survival rate (45.6%) at 600 Gy dose. Statistical analysis of data obtained helped to estimate the ideal LD50 doses based on growth reduction of seedlings’ heights after gamma-ray treatment. Using a linear regression model based on parameters like plant size, the LD50 doses for Kaolack and Crimson sweet were calculated at 225.40 Gy and 221.56 Gy, respectively, and predicted between 200 and 250 Gy. These results show that the two varieties evaluated were radiosensitive as clearly expressed in the parameters evaluated, where the values decreased with the increase in the irradiation dose. The LD50 doses from this study could be safely applied as reference doses for large-scale gamma irradiation of watermelon genotypes to create desirable agronomic traits in the mutation breeding efforts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The current world’s population growth rate and food needs require that food production should be at least doubled by the year 2050 (FAO 2009; Tester and Langridge 2010; Ray et al. 2013). An important component to tackle this challenge and address pressures on global food security and nutrition is the genetic improvement of food crops (Ronald 2011) like watermelon. Watermelon [Citrullus lanatus var. lanatus (Thunb.) Matsum and Nakai] is a herbaceous fruit vegetable belonging to the Cucurbitaceae family (Schippers 2000). This family is known for its great genetic diversity (Zaini et al. 2011) and its great adaptation to tropical, subtropical regions and arid deserts (Giwa et al. 2010). C. lanatus is warm, long season, prostate and annual which has monoecious and/or andro-monoecious sexuality (Boualem et al. 2016). The fruit has a thick rind (exocarp) that has variable pigmentation with a solid or striped appearance, a fleshy mesocarp and an endocarp which varies in color from white to yellow or red (Bahari et al. 2012; Munisse et al. 2013). Esquinas-Alcazar and Gulick (1983) and Mallick and Masui (1986) proposed Central Africa and the Kalahari Desert as the center of origin for cultivated watermelon. Watermelon is one of the most important national and global fruit crops, occupying about 7% of the world area devoted to the production of fruits and vegetables (Guo et al. 2012). World watermelon production evaluated in 2011 was 104,472,354 tonnes. China ranks first with approximately 71% of world watermelon production followed by Turkey and Iran, respectively (Huh et al. 2008). In Algeria, production is 1,285,134 tonnes. In tropical Africa, some countries have significant watermelon production, such as Senegal with 224,000 tonnes, Sudan with 143,000 tonnes, Cameroon and Somalia with 28,000 tonnes each and Mauritania with 11,000 tonnes (FAO 2013).
Composed of approximately 92% of water, with moisturizing properties, watermelon has a refreshing taste that quenches thirst. It is particularly rich in phytochemicals such as flavonoids, tannins, anti-inflammatory, antiviral and antioxidant substances (Johnson et al. 2012). Lycopene (Edwards et al. 2003), Vitamins A, B6, C, carotenoids (Olson 1999) and antioxidants are some nutrients found in watermelon (Maoto et al. 2019). Watermelon is a diuretic and contains large amounts of β-carotene, a precursor of Vitamin A (Naz et al. 2014). The watermelon fruit is effective in reducing cancer, cardiovascular disorders, diabetes, blood pressure and obesity (Edwards et al. 2003; Naz et al. 2014; Lum et al. 2019). The quantitative assessment indicates that watermelon has 46% calories, 20% vitamin C and 17% vitamin A and has higher lycopene than tomato (Biswas et al. 2017). The emulsion obtained from aqueous extract of watermelon seeds is used to treat catarrhal infections, intestinal disorders, urinary tract infections and fever (Taiwo et al. 2008). Watermelon is also rich in citrulline, an effective precursor of l-arginine (Guoyao et al. 1992). Vegetable milk can be extracted from watermelon seeds which can replace or supplement cow or soy milk and could also meet the needs of vegetarians (Enzonga-Yoca et al. 2011). Syrup, a fermented drink, can be made from watermelon juice (Webster and Romshe 1951). This watermelon juice can increase blood levels of carotenoids (lycopene and β-carotene). Carotenoids are essential for humans because they have antioxidant activity and prevent free radicals from harming the body. They have protective effects against heart disease and certain cancers, such as prostate, bladder, breast, cervix, endometrium, lungs and colon cancer (Edwards et al. 2003).
Despite the importance of watermelon, little research on watermelon production has been done in Africa compared to cereals and legumes (Kuvare 2005; Davis et al. 2008). Information on cultivar performance under various technologies, environments and production systems and knowing how these systems affect the quality and phytonutrient content are necessary to ensure high fruit quality, yield and disease resistance for future breeding (Ayodele and Shittu 2013). The varieties of watermelon cultivated in Africa lack the diversity needed to cause differences in fruit yield (Gusmini and Wehner 2005). The availability of heritable variation is a prerequisite for genetic improvement of crops. Where sufficient variation does not exist naturally, it can be created through either random or targeted processes. Mutation breeding is an alternative method for the development of new plant varieties.
Mutagenesis is one method to introduce genetic variability. This approach to broaden genetic variability has been demonstrated to be efficient through the use of gamma-ray irradiation (Kumar and Shunmugavalli 2018; Olasupo et al. 2018). Mutagenesis is an abrupt, inheritable change that occurs in DNA and is not caused by segregation or genetic recombination. It can either be spontaneous or induced by mutagens (Roychowdhury and Tah 2013). The choice of which type of mutagen to use for mutation breeding is often based on past successes reported for the species and other considerations such as the availability of mutagens, costs and infrastructure (Mba 2013; Bado et al. 2015). Induced mutations are caused by the action of mutagenic agents or transposable DNA elements to create and increase variability within a population. Radiomutagenesis significantly improves the frequency of appearance of mutations and can, therefore, be used to widen genetic variability, which can be integrated into many breeding and varietal creation schemes (Guerin De Montgareuil 1984). These modifications are characterized either by gains or losses of function. The exploitation of mutants can help locate and identify affected genes and determine their biological function (Meunier 2005).
The first plant variety created by induced mutagenesis was launched in 1936, and the use of selection by induced mutation took off worldwide in the 1960s, including in Europe, Japan, India and China, regions that were much more skeptical at first. To date, more than 3000 varieties of plants in 190 plant species have been created by induced mutagenesis (Shu et al. 2012). The induced mutation has been used to produce many cultivars of improved economic value as well as development processes and resistance to plant pests and diseases. Mutations can be induced in a variety of ways, such as exposure of plant propagules, including seeds, tissues and other organs, to physical and chemical mutagens (Mba et al. 2010). Mutagens are chemicals or physical factors whose actions are random and could modify any part of the genome or gene (Morère et al. 2003). Physical mutagens are mainly electromagnetic radiation such as gamma rays, X-rays, UV light and radiation from thermal particles, such as fast neutrons, alpha and beta particles. Chemical mutagens include ethyl methanesulfonate (EMS), ethidium bromide and basic analogues such as bromouracil (van Harten 1998; Girija and Dhanavel 2009; Mba et al. 2010). Among the different mutagenic agents, gamma rays are commonly used in breeding programs to induce mutations in various plant species because of their easy availability, simple application, good penetration, high reproducibility, large mutation frequency and low disposal problems (Mba et al. 2012). Thus, more than 60% of the mutant cultivars were produced by gamma rays (Bado et al. 2015). The efficacy of a mutagenic treatment to induce genetic variation in cultivated plants depends, among other things, on the genetic makeup of the test varieties and the treatment dose (Van Harten 1998; Mba et al. 2010). In watermelon (Citrullus lanatus L.), different genotypes and doses of gamma rays have been tested by Sari and Abak (1996). Similarly, pollen irradiation tests with gamma radiation for the production of haploid embryos have been successfully carried out by Taskin et al. (2013), with the aim of creating haploid watermelon varieties. Compared to conventional breeding methods, mutation breeding is a more powerful and effective crop improvement technique, requiring only a short time to develop a new variety. Furthermore, among more current plant breeding methods, mutation breeding is a more economical and generally accepted approach than transgenic technology (GMOs) for the development of new traits (Wilde et al. 2012).
Radiosensitivity and determination of the optimal dose of radiation are terms describing a relative measure of the amount of recognizable effects of radiation exposure on the irradiated material (Owoseni et al. 2007). Optimizing the dose of radiation is the first step in induced mutation breeding where its predictable value guides the researcher in the choice of the ideal dose depending on the plant materials and desired outcome. The LD50 is an important parameter to measure the short-term poisoning potential (acute toxicity) of treatment and widely used to determine the optimum mutation frequency with least possible unintended damage (Owoseni et al. 2007). According to Mba et al. (2010), the dose of mutagen considered as optimal or LD50 is that which reaches the optimal mutation frequency with the least possible damage.
The cultivation of watermelon is of great interest but faces several problems. Its extreme sensitivity to parasites and climatic hazards makes its cultivation demanding of chemical inputs that can have negative impacts on human health and the environment. Its richness in water exposes the crop in drought conditions to water stress which considerably limits plant production. Improvement by induced mutation has many advantages, such as the expansion of genetic resources, the creation of local mutant gene banks, the development of new varieties and the integration of new combinations of genes which are not found in nature (Mba 2013). Radiosensitivity test is a relative measure that indicates the quantity of recognizable effects of radiation on the irradiated objects (Morishita et al. 2003). Before any selection by induced mutagenesis, a radiosensitivity test is thus recommended. This provides a simple method for measuring mutagenic effects and determining the optimal radiation dose for mutation induction. The lethal doses 50 (LD50) usually create maximum variability with minimum numbers of undesirable mutants. The objective of this study was to assess the sensitivity to gamma radiation of two varieties of watermelon in order to determine the ideal dose LD50 for genetic improvement.
2 Materials and methods
Plant material and study site
– This study was conducted in the experimental greenhouse of the Institute of Agricultural Research for Development (IRAD), Cameroon, from March to April 2019. Two commercial varieties of watermelon with better agro-morphological performance were used for this experiment: (a) Crimson sweet fruit of light green color mottled with dark green, and with red and juicy flesh, and (b) Kaolack characterized by a high germination rate having the round fruit marbled of light green color and with red and juicy flesh (Fig. 1).
Seed irradiation
– The study used seeds of two varieties of watermelon (Kaolack and Crimson sweet)). Dry, healthy and quiescent seeds were prepared for irradiation and were sent from Cameroon to the International Atomic Energy Agency (IAEA), Agriculture and Biotechnology Laboratory, based in Seibersdorf, Austria. Twenty seeds per variety were gamma-irradiated in three replications using the gamma irradiation facility at the IAEA. Five doses of gamma radiation were used (0, 100, 200, 300, 400 and 600 Gy). The 0 Gy dose served as a comparative control. The seeds were packed in separate seed envelopes and placed in desiccators for three days to attain the desired moisture level of 8%. Irradiation was applied using a Cobalt 60 (60CO) source Gamma Cell Model No. 220 with 11.96 Gy/min dose rate. The various doses were used to establish the optimum irradiation level that can achieve optimum mutation frequency with the least possible and unintended damage (Mba et al. 2010).
Growing plants, experimental design, data collection and analysis
– The radiosensitivity test (the biological effects of the mutagen treatments on plants) was studied following the methods described by Mba et al. (2010) and Tshilenge-Lukanda et al. (2012). Irradiated seeds were planted in seedling trays containing a sandy loam rich in organic matter and previously sterilized in an autoclave. Trails were established under environmentally controlled greenhouse with temperatures of 22 to 35 °C and light regime kept at 12 h photoperiod. The experiment was set up in a completely randomized design with three replications. Seedlings were watered every day to ensure adequate soil moisture.
Seed germination
– Five days after sowing, the first germination was recorded, and then, the time of germination (time separating the date of sowing and the date of the first germination) was evaluated. The germination rate expressed as a percentage of germinated seed was evaluated three weeks after sowing by making the ratio between the numbers of seeds germinated by the number of seeds sown according to Olasupo et al. (2016).
The shoot length
– Between the 14th and the 20th day after sowing (DAS), the lengths of the stems were measured every day above the soil surface to the tip of the primary leaf using a ruler and expressed in centimeters (Fig. 2).
The survival rate
– The survival rate of M1 populations was evaluated at the 28th DAS by relating the number of plants alive to the number of germinated seeds sown according to the following relationship (Olasupo et al. 2016).
Semilethal dose (LD50)
– The LD50 (led to 50% reduction in the shoot length) for each variety was estimated through the simple linear regression model by fitting the straight-line equation y = mx + c, where y is the response variable (shoot length), and x is the independent variable (irradiation dose), while m and c represent the slope and constant, respectively (Horn and Shimelis 2013; Ertan et al. 2017; Gnankambary et al. 2019).
Statistical analysis
– The data obtained were subjected to an analysis of variance (ANOVA) using the statistical software STATGRAPHICS 5.1. The LSD (least significant difference) test was used to compare and separate the means values at the significance level of (P < 0.05). Microsoft Excel 2010 software was used to calculate means, plot curves and linear regression.
3 Results
Germination of seeds
– Time of germination and plant survival data are presented in Table 1. The results reveal that 100 Gy dose had a stimulating effect on germination and caused seeds to germinate all in just 6 days for Kaolack variety and seven days for Crimson sweet variety. The control seeds germinated five days after sowing for Kaolack and six days after sowing for Crimson sweet. The seeds treated with 200, 300, 400 and 600 Gy germinated between 6 and 14 days after sowing for Kaolack and between 7 and 17 days after sowing for Crimson sweet.
Germination rate
– The mean and standard deviation of percent germination are presented in Table 1. The highest germination rates were observed in control treatment for the two varieties at 90% for Kaolack and 75% for Crimson sweet. The low germination rates are observed at 600 Gy treatment for all varieties. Results reveal a significant negative effect of gamma rays (P < 0.05) on germination. Germination percentage decreased drastically in all the varieties with increased Gy doses (Fig. 3).
Shoot length
– The observations on shoot length with respect to gamma-ray irradiation showed a significant effect on all the treatment doses compared to the control for Kaolack variety (Table 2). For Crimson sweet, a significant difference was observed only between 100 Gy treatment and the control. The maximum shoot lengths (69.8 cm) for Kaolack and (67.8 cm) for Crimson sweet were observed on controls. The lowest values: 9.9 and 14.1 cm, respectively, for Kaolack and Crimson sweet were observed for the treatment with the highest irradiation dose 600 Gy (Table 2).
Survival rate
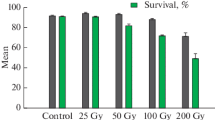
– Plant survival data are presented in Table 2. Results reveal a significant effect (P < 0.05) of gamma rays for Kaolack variety. Survival rates were greater for the control at (96.66%). This variety had the lowest survival rate (45.6%) with a dose of 600 Gy (Fig. 4).
For the Crimson sweet variety, the average survival rates varied from 86.6 to 65%, respectively, for the control and the 300 Gy dose. However, unlike the Kaolack variety, no significant difference was observed for the Crimson sweet variety concerning the survival rate (Table 2, Fig. 5).
Determination of the LD50
– Figures 6 and 7 show linear regression of seedling heights to irradiation doses. The seedling height responses of Kaolack and Crimson sweet against irradiation doses are given by the linear equations: y = − 0.124x + 77.95 and y = − 0.128x + 78.36, respectively. LD50 doses were predicted based on seedling height (Table 3). The results indicated that LD50 doses varied among varieties. It was concluded that the optimum dose LD50 for Kaolack variety was 225.40 Gy and 221.56 Gy for Crimson sweet.
4 Discussion
The present study compared the responses of two watermelon varieties using five gamma radiation doses to establish the LD50 and to determine associated effects on early growth characters. Results revealed that seed germination, seedling height and survival rate decreased substantially with increased gamma radiation doses. These results were similar to those of other workers who reported that increasing the irradiation dose decreased the seed germination and seedling growth (Tshilenge-Lukanda et al. 2013; Verma et al. 2017; Karidiatou et al. 2019). However, according to Kodym et al. (2012), sensitivity to a mutagen depends on the type of material and varies from one species to another.
The germination rate for the Kaolack variety gradually decreases from 90% for the 0 Gy control to 35% for 600 Gy, while for the Crimson sweet variety, this drop goes from 75% for the control to 37.5% for 600 Gy. Thus, for both varieties, the percentage of germination gradually decreases with the increase in the dose of irradiation. Similar results have been observed in pumpkin and squash by Ertan et al. (2017). These same observations were made, respectively, by Solanki and Sharma (1994) and Singh et al. (2007) in lentils and by Girija and Dhanavel (2009) in cowpeas. These results differ from those of Essel et al. (2016) which showed no significant effect of increasing doses of gamma irradiation on cowpea germination. The destruction of auxin, changes in the content of ascorbic acid and biochemical disturbances after mutagenic therapy may be responsible for this inhibition of germination (Shah et al. 2008).
The growth parameter based on seedling height was observed for the two varieties with the maximum values of 69.8 ± 2.8 cm for the Kaolack variety against 67.8 ± 6.1 cm for the Crimson sweet variety, respectively. The lowest values: 9.9 ± 1.6 and 14.1 ± 3.2 cm, respectively, for Kaolack and Crimson sweet were observed in the batches with the highest irradiation dose 600 Gy. There is a reduction in seedling height with an increase in the irradiation dose. These results are in agreement with the previous studies reported on rice varieties in Sierra Leone, on beans (Bajaj et al. 1970), corn (Marcu et al. 2013), eggplant (Ulukapi et al. 2015), chilli (Sikder et al. 2013), squash and pumpkin (Ertan et al. 2017). Reduction of this trait with increasing gamma-ray dose may be due to the insufficient water and nutrient uptake as a result of the severe effect of high irradiation dose on root growth and development (Ulukapi et al. 2015).
The highest percentage of survivors (96.66%) is obtained with the control treatment for the Kaolack variety. This same variety has the lowest survival rate (45.6%) with the 600 Gy dose. This reduction in survival rate is also observed in the Crimson sweet variety and varies from 86.6 to 65%. Manju and Gopimony (2009) reported that the reduction in plant survival is an index of post-germination mortality resulting from the irradiation effect on the physiological and cytological mechanisms of plants. The lower percentage of survival after the treatment with the gamma rays may be attributed to a drop in auxin level resulting in poor establishment and chromosomal aberrations caused by the mutagenic treatments (Mahure et al. 2010).
According to Maluszynski et al. (2003), radiosensitivity test between LD30 and LD50 is preferable to obtain the desired and optimum traits. The data collected for shoot length were used to determine the LD50. The LD50 doses of Kaolack and Crimson sweet plants were 225.40 Gy and 221.56 Gy, respectively. This indicated that varieties could respond differently to gamma radiation. This observation is similar to the findings of Tabasum et al. (2011). They revealed that the response of genotypes to various doses of gamma irradiation was different, and the highest dosage caused a reduction in physiological processes. However, Ukai (1983) suggested an LD50 of 400 Gy for Cucurbita spp. Ertan et al. (2017) estimated the LD50 of 173 Gy in pumpkins based on the length of the shoots. Olasupo et al. (2016) presented a wide variation of LD 50% ranging from 326 to 1053 Gy for seed germination and from 148.8 to 620.2 Gy for seedling survival in cowpea.
In conclusion, determination of the optimum dose of a mutagen in mutation breeding studies is critical for the development of lines with desirable agronomic traits. The results of the present study indicate that investigations of growth parameters (such as root and shoot lengths) are more effective than those of seed traits (germination and emergence) for assessing irradiation history. The results also show that the difference in radiosensitivity between the two varieties is not very significant. The LD50 or doses of mutagen, which can reach an optimal mutation frequency with the least possible damage to the genome, were estimated between 200 Gy and 250 Gy for the two varieties. The use of this technique may speed up naked seed watermelon breeding programs for obtaining new, improved cultivars or hybrids with high yield potential and better resistance to diseases. As we envisage pursuing this research, these optimum mutagen doses determined for the different watermelon varieties could be useful in watermelon varietal improvement programs in Cameroun.
References
Ayodele OJ, Shittu OS (2013) Cost-benefit analysis of melon (egusi) seed and seed-oil yield responses to phosphorus fertilizer application. Int Res J Agric Sci Soil Sci 3:152–155
Bado S, Forster BP, Nielen S, Ghanim A, Lagoda PJL, Till BJ, Laimer M (2015) Plant mutation breeding: current progress and future assessment. Plant Breed Rev 39:23–88
Bahari M, Rafii MY, Saleh GB, Latif MA (2012) Combining ability analysis in complete diallel cross of Watermelon [Citrullus lanatus (Thunb.) Matsum. and Nakai]. Sci World J 2012:1–6
Bajaj YPS, Saettler AW, Adams MW (1970) Gamma irradiation studies on seeds, seedlings and callus tissue cultures of Phaseolus vulgaris L. Rad Bot 10:119–124
Biswas R, Ghosal S, Chattopadhyay A, Datta S (2017) A comprehensive review on watermelon seed oil—An underutilized product. J Pharm 71:2250–3013
Boualem A, Lemhemdi A, Sari MA, Pignoly S, Troadec C, Choucha FA, Solmaz I, Sari N, Dogimont C, Bendahmane A (2016) The andromonoecious sex determination gene predates the separation of Cucumis and Citrullus genera. PLoS ONE 11:1–13
Davis AR, Webber CL, Perkins-Veazie P, Russo V, Lopez Galarza S, Sakata Y (2008) A review of production systems on watermelon quality. Cucurbitaceae 2008. In: Proceedings IXth EUCARPIA meeting on genetics and breeding of Cucurbitaceae, Avignon, France, 21–24 May
Edwards AI, Vinyard BT, Wiley ER, Brown ED, Collins IK, Perkins-Veazie P, Baker RA, Clevidence BA (2003) Consumption of watermelon juice increases plasma concentrations of lycopene and ß-carotene in humans. J Nutr 133:1043–1050
Enzonga-Yoca JA, Nitou JG, Allou Kippré V, Niamayoua RK, Mvoula-Tsieri M, Silou T (2011) Caractérisation chimique et évaluation de la température de conservation du lait des graines de cucurbitacées: Cucumeropsis mannii et Citrullus lanatus. J Anim Plant Sci 10:1232–1238
Ertan SK, Ahmet B, Dilek K (2017) Determination of semi-lethal (LD50) doses for mutation breeding of Turkish winter squash (Cucurbita maxima Duch.) and pumpkin (Cucurbita moschata Duch.). Fresenius Environ Bull 26:3209–3216
Esquinas-Alcazar JT, Gulick PJ (1983) Genetic resources of Cucurbitaceae. A global report. International Board for Plant Genetic Resources, Rome, p 101
Essel E, Asante IK, Odamtten G (2016) Mutagenic effect of gamma irradiation on seed germination and yield components of cowpea. J Ghana Sci Assoc 17:53–59
FAO (2009) FAO’s Director-General on how to feed the World in 2050. Popul Dev Rev 35:837–839
FAO (2013) Food and agriculture organisation of the United Nations: Economic and Social Department: the statistics division. http://faostat.fao.org/site/339/default.aspx. Accessed 12 Nov 2013
Girija M, Dhanavel D (2009) Mutagenic effectiveness and efficiency of gamma rays, ethyl methane sulphonate and their combined treatments in cowpea (Vigna unguiculata L. Walp). Glob J Mol Sci 4:68–75
Giwa S, Abdullah LC, Adam NM (2010) Investigating Egusi (Citrullus colocynthis L.) seed oil as potential biodiesel feedstock. Energies 3:607–618
Gnankambary K, Teyouré BJ, Nerbéwendé S, Mahamadou S, Djibril Y, Tinga JO (2019) Assessment of radio-sensitivity for three cowpea genotypes to gamma irradiation. Int J Genet Mol Biol 11:29–33
Guerin De Montgareuil P (1984) Radioagronomie in Echos. Groupe CEA, France, pp 56–57
Guo S, Zhang J, Sun H (2012) The draft genome of watermelon (Citrullus lanatus) and resequencing of 20 diverse accessions. Nat Genet 45:51–58
Guoyao W, Collins JK, Perkins-Veazie P and Siddiq M (1992) Dietary supplementation with melon pomace juice enhances arginine availability and ameliorates the metabolic syndrome in Zucker diabetic fatty rats. J Nutr 45:34–54
Gusmini G, Wehner TC (2005) Foundations of yield improvement in watermelon. Crop Sci 45:141–146
Horn LN, Shimelis H (2013) Investigation on radio-sensitivity of gamma irradiation on selected cowpea (Vigna unguiculata L. Walp.) genotypes. Sci Res and Ess 8:1991–1997
Huh YC, Solmaz I, Sarı N (2008) Morpholojical characterization of Korean and Turkish watermelon germplasm. In: Pitrat M (ed) Cucurbitaceae 2008, Proceedings of the IXth EUCARPIA meeting on genetics and breeding of Cucurbitaceae, INRA, Avignon, France, 21–24 May 2008
Johnson JT, Iwang EU, Hemen JT, Odey MO, Efion EE, Eteng OE (2012) Evaluation of anti-nutrient contents of watermelon Citrullus lanatus. Annal Biol Res 3:5145–5150
Karidiatou G, Teyouré BJ, Nerbéwendé S, Mahamadou S, Djibril Y, Tinga JO (2019) Assessment of radio-sensitivity for three cowpea genotypes to gamma irradiation. Int J Genet Mol Biol 11:29–33
Kodym A, Afza R, Forster BP, Ukai Y, Nakagawa H (2012) Methodology for physical and chemical mutagenic treatments. In: Plant mutation breeding and biotechnology, pp 169–180
Kumar R, Shunmugavalli N (2018) Assessment of gamma rays induced variability in M2 generation of Sesamum (Sesamum indicum L.). Int J Chem 6:292–296
Kuvare USK (2005) Greenhouse production of watermelon (Citrullus lanatus). Stellenbosch, South Africa, Department of Horticulture, University of Stellenbosch, MS Thesis, p 300
Lum T, Connolly M, Marx A, Beidler J, Hooshmand S, Kern M, Liu C, Hong MY (2019) Effect of fresh watermelon consumption on the acute satiety response and cardiometabolic risk factors in overweight and obese adults. Nutrients 11:595
Mahure HR, Choudhary ML, Prasad KV, Singh SK (2010) Mutation in chrysanthemum through gamma irradiation. Indian J Hort 67:356–358
Mallick MFR, Masui M (1986) Origin, distribution and taxonomy of melons. Sci Hort 28:251–261
Maluszynski M, Szarejko I, Maluszynski J (2003) Mutation techniques. In: Thomas B, Murphy DJ, Murray BG (eds) Encyclopedia of applied plant sciences. Elsevier Academic Press, San Diego, pp 186–201
Manju P, Gopimony R (2009) A new okra variety through induced mutation in interspecific hybrids of Abelmoschus Species. In: Shu QY (ed) Induced plant mutations in the genomics era. Joint FAO/IAEA Programme, Vienna, Austria, pp 91–94
Maoto M, Beswa D, Jideani AIO (2019) Watermelon as a potential fruit snack. Int J Food Prop 22:355–370
Marcu D, Damian G, Cosma C, Cristea V (2013) Gamma radiation effects on seed germination, growth and pigment content, and ESR study of induced free radicals in maize (Zea mays). J Bio Phy 39:625–634
Mba C (2013) Induced mutations unleash the potentials of plant genetic resources for food and agriculture. Agronomy 3:200–231
Mba C, Afza R, Bado S, Jaim SM (2010) Induced mutagenesis in plants using physical and chemical agents. In: Plant cell culture essential methods, pp 111–130
Mba C, Afza R, Shu QY (2012) Mutagenic radiations: X-rays, ionizing particles and ultraviolet. In: Shu Q, Forster BP, Nakaga-wa H (eds) Plant mutation breeding and biotechnology. CABI, Oxfordshire, UK, pp 83–90
Morère JL, Pujol R (2003) Dictionnaire raisonné de biologie. Frison-Roche, Paris
Meunier E (2005) Des plantes mutantes dans nos assiettes. Inf’OGM, 67, Septembre 2005, http://www.infogm.org/spip.php?Article2406. Accessed 10 May 2010
Morishita T, Yamaguchi H, Degi K, Shikazono N, Tanaka A, Abe T (2003) Dose response and mutation induction by ion beam irradiation in buckwheat. Nucl Instr Meth Phys Res B 206:565–569
Munisse P, Jensen BD, Andersen SB (2013) Genetic differentiation of watermelon landraces in Mozambique using microsatellite markers. Afr J Biotechnol 12:5513–5521
Naz A, Butt MS, Sultan MT, Qayyum MMN, Naiz RS (2014) Watermelon lycopene and allied health claims. Excli J 13:650–666
Olasupo FO, Ilori CO, Forster BP, Bado S (2016) Mutagenic effects of gamma radiation on eight accessions of cowpea (Vigna unguiculata [L.] Walp.). American J Plant Sci 7:339–351
Olasupo FO, Ilori CO, Forster BP, Bado S (2018) Selection for novel mutations induced by gamma irradiation in cowpea [Vigna unguiculata (L.) Walp.]. Int J Plant Breed Genet 12:1–12
Olson JA (1999) Carotenoids. In: Shils ME, Olson JA, Shike M, Ross AC (eds) Modern nutrition in health and disease, 9th edn. Williams & Wilkins, Baltimore, pp 525–541
Owoseni O, Okwaro H, Afza R, Bado S, Dixon A, Mba C (2007) Radiosensitivity and in vitro mutagenesis in African accessions of cassava, Manihot esculenta Crantz. Plant Mutat Rep 1:32–36
Ray DK, Mueller ND, West PC, Foley JA (2013) Yield trends are insufficient to double global crop production by 2050. PLoS ONE 8:e66428
Ronald P (2011) Plant genetics, sustainable agriculture and global food security. Genetics 188:11–20
Roychowdhury R, Tah J (2013) Mutagenesis A potential approach for crop improvement. In: Hakeem KR, Ahmad P, Ozturk M (eds) Crop improvement: new approaches and modern techniques. Springer, Boston, pp 149–187
Sari N, Abak K (1996) Farkli Isin dozlarininveIs¸ I nlamaya alternative uygulamalarinkarpuzda haploid embryo uyartımınaetkileri. Tu rkiye II. UlusalBahçe Bitkileri Kongresi Bildirileri, Cilt II, Adana, Turkey. pp 212–215
Schippers RR (2000) African indigenous vegetables. An overview of the cultivated species. Natural resources institute/ACP-EU technical centre for agricultural and rural cooperation, Chatham, UK, pp 214
Shah TM, Mirza JI, Haq MA, Atta BM (2008) Radiosensitivity of various chickpea genotypes in M1 generation in laboratory studies. Pak J Bot 40:649–665
Shu Q, Forster BP, Nakagawa H (2012) Plant mutation breeding and biotechnology. CABI
Sikder, S, Biswas, P, Hazra, P, Akhtar, S, Chattopadhyay, A, Badigannavar, AM and D’Souza SF (2013) Induction mutation in tomato (Solanum lycopersicum L.) by gamma irradiation and EMS. Indian J Genet 73: 392–399
Singh S, Singh R, Singh N, Prasad J, Shahi J (2007) Mutagenic efficiency of gamma-rays, ethyl methane sulphonate and its combination on microsperma lentil (Lens culinaris Medik). Int J Agric Sci 3:113–118
Solanki I, Sharma B (1994) Mutagenic effectiveness and efficiency of gamma rays, ethylene imine and N-nitroso-N-ethyl urea in macrosperma lentil (Lens culinaris Medik.). The Indian J Genet Plant Breed 54:72–76
Tabasum A, Cheema AA, Hameed A, Rashid M, Ashraf M (2011) Radiosensitivity of rice genotypes to gamma radiations based on seedling traits and physiological indices. Pak J Bot 43:1211–1222
Taiwo AA, Agbotoba MO, Oyedepo JA, Shobo OA, Oluwadare I and Olawunmi MO (2008) Effects of drying methods on properties of watermelon (Citrullus lanatus) seed oil. Af J Food Agric Nutr and Dev 8:1456–1460
Taskin H, Yucel NK, Baktemur G, Comlekcioglu S, Buyukalaca S (2013) Incidence de divers genotypes et doses de rayons gamma sur l’haploidisation par la technique du pollen irradié chez la pastèque (Citrullus lanatus L.). Can J Plant Sci 93:1165–1168
Tester M, Langridge P (2010) Breeding technologies to increase crop production in a changing world. Science 327:818–822
Tshilenge-Lukanda L, Funny-Biola C, Tshiyoyi-Mpunga A, Mudibu J, Ngoie-Lubwika M, Mukendi-Tshibingu R, Kalonji-Mbuyi A (2012) Radio-sensitivity of some groundnut (Arachis hypogaea L.) genotypes to gamma irradiation: indices for use as improvement. Brit J Biotechnol 3:169–178
Tshilenge-Lukanda LA, Kalonji-Mbuyi A, Nkongolo KKC, Kizungu RV (2013) Effect of gamma irradiation on morpho-agronomic characteristics of groundnut (Arachis hypogaea L.). Am J Plant Sci 4:2186–2192
Ulukapi K, Ozdemir B, Onus N (2015) Determination of proper gamma radiation dose in mutation breeding in eggplant (Solanum melongena L.). In: Advances in Environmental and Agricultural Science, pp 149–153
Van Harten AM (1998) Mutation breeding: theory and practical applications. Cambridge University Press, Cambridge, p 330
Verma AK, Sharma S, Kakani RK, Meena RD, Choudhary S (2017) Gamma radiation effects seed germination, plant growth and yield attributing characters of fennel (Foeniculum vulgare Mill.). Int J Current Microbiol Appl Sci 6:2448–2458
Webster JE, Romshe FA (1951) Watermelon syrup: its composition and composition of the juice from which it was made. Am Soc Hort Sci 57:302–304
Wilde HD, Chen Y, Jiang P, Bhattacharya A (2012) Targeted mutation breeding of horticultural plants. Emir J Food Agric 24:31–41
Ukai Y (1983) Mutation breeding. Gamma Field Symposia N° 20 Suppl. Yokendo, Tokyo
Zaini NAM, Anwar F, Hamid AA, Saari N (2011) Kundur [Benincasa hispida (Thunb.) Cogn.]: a potential source for valuable nutrients and functional foods. Food Res Int 44:2368–2376
Author information
Authors and Affiliations
Contributions
FPE, BJM and GNN conceived and designed the experiments. MNA, OAS and NHB performed the experiments and drafted the manuscript. BJM and MR analyzed the data. MAA helped perform the analysis with constructive discussion. MT reviewed and edited the manuscript. All authors read and approved the submitted manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Paul Ernest, F., Hortense Noëlle, M., Godswill, NN. et al. Radiosensitivity of two varieties of watermelon (Citrullus lanatus) to different doses of gamma irradiation. Braz. J. Bot 43, 897–905 (2020). https://doi.org/10.1007/s40415-020-00659-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-020-00659-8