Abstract
The red algal genus Hypnea has a wide geographical distribution along the coast of Brazil, where it has economic and ecological importance. The relatively simple and plastic morphology, often influenced by the conditions of its habitat, complicates the identification of Hypnea species. In the past years, several studies dealing with the taxonomy of Hypnea on the coast of Brazil have changed considerably the known diversity of species in the region. Studies using molecular markers led to the description of new species, while other names were placed in synonymy. In this review, revaluation of the morphology and the addition of new sequences for COI-5P, UPA and rbcL-3P markers were used in order to better understand the diversity of Hypnea species and their range of distribution. An identification key is presented for eleven species of the genus Hypnea occurring on the coast of Brazil: H. brasiliensis P.B. Jesus, Nauer & J.M.C. Nunes, H. cervicornis J. Agardh, H. cornuta (Kützing) J. Agardh, H. cryptica P.B. Jesus & J.M.C. Nunes, H. edeniana Nauer, Cassano & M.C. Oliveira, H. flava Nauer, Cassano & M.C. Oliveira, H. pseudomusciformis Nauer, Cassano & M.C. Oliveira, H. platyclada P.B. Jesus & J.M.C. Nunes, H. spinella (C. Agardh) Kützing, H. yokoyana Nauer, Cassano & M.C. Oliveira and H. wynnei Nauer, Cassano & M.C. Oliveira. Of all taxonomic characteristics proposed by earlier studies, the only ones that have taxonomic value considering the species analyzed in this work were: (1) habit of the thallus, (2) main axis, (3) apex shape, (4) form of the branchlets and (5) tetrasporangial sori position in the branches. However, the identification of the species based only on morphological characteristics should be made with caution, once phenotypic plasticity and convergent morphologies are present in the group. The DNA barcode technique, especially the COI-5P marker, proved to be very effective in the identification and delimitation of the species, revealing scenarios that would go unnoticed by morphology alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The genus Hypnea J.V. Lamouroux (1813) is well known for its economic importance as a source of carrageenan (Saito and Oliveira 1990). The content of k-carrageenan of Hypnea pseudomusciformis Nauer, Cassano & M.C. Oliveira is of excellent quality, about 25% and 48% of its dry weight (Reis and Yoneshigue-Valentin 2000, as Hypnea musciformis (Wulfen) J.V. Lamouroux), and production of carrageenan in Brazil increased from one to 10 tons per month, between 1999 and 2004, using biomass obtained from natural beds of H. pseudomusciformis in the northeastern region of the country (Furtado 2004, as H. musciformis).
However, the genus includes 58 species accepted taxonomically which are very difficult to identify based solely on morphological characters, with a high rate of misapplied names (Masuda et al. 1997; Geraldino et al. 2009; Guiry and Guiry 2019). Due to taxonomic difficulty, regional revisions have been recommended (e.g., Masuda et al. 1997). In the past years, several studies dealing with the taxonomy of Hypnea on the coast of Brazil were published, changing considerably the known diversity of species and their distribution in the region (Nauer et al. 2014, 2015, 2016; Jesus et al. 2015, 2016, 2019a). Schenkman (1986) did the first taxonomic study of Hypnea in Brazil. Based on morphological characters, she recognized six species: Hypnea cervicornis J. Agardh, H. cornuta (Kützing) J. Agardh, H. musciformis, H. nigrescens Greville ex J. Agardh, H. spinella (C. Agardh) Kützing and H. valentiae (Turner) Montagne. The author, however, pointed out difficulties for species identification, probably resulting from phenotypic plasticity.
Based on morphology, Yoneshigue (1985), Nunes (2005), Guimarães (2006) and Jesus et al. (2013a) cited Hypnea cenomyce J. Agardh for Brazil. Also based on morphological studies, Jesus et al. (2013a) described a new species, H. platyclada Jesus & J.M.C. Nunes, a species with cylindrical stolon and stipe, but flattened upper portions and fusiform branches. Hypnea volubilis Searles was cited for Brazil in two floristic algal surveys: a survey from Laje de Santos Marine State Park, São Paulo State (Amado-Filho et al. 2006), which is the first citation of the species for the country, and a survey of Atol das Rocas (Villaça et al. 2010).
The first published molecular study for Hypnea species occurring in Brazil was made by Nauer et al. (2014), using the plastid rbcL, gene encoding the large subunit of the enzyme ribulose-1, 5-bisphosphate carboxylase-oxygenase and two DNA barcode markers, the 5′ region of the gene cox1 that encodes cytochrome c oxidase subunit 1 (COI-5P), and the universal plastid amplicon (UPA), alongside with morphological studies. The authors cited for the first time the occurrence of H. aspera Kützing in the South Atlantic Ocean and described two new species: H. edeniana Nauer, Cassano & M.C. Oliveira and H. flava Nauer, Cassano & M.C. Oliveira.
Jesus et al. (2015) made the first citation of Hypnea stellulifera (J. Agardh) Yamagishi & Masuda for Brazil, extending the occurrence of this Asiatic species in the world. Jesus (2016) affirmed that the species H. cornuta (Kützing) J. Agardh and H. stellulifera constitute a complex to be investigated. Nauer et al. (2015) analyzing specimens from Adriatic Sea, Italy, type-locality of H. musciformis, showed molecular divergences with Brazilian specimens by analyzing the COI-5P and rbcL markers, describing a new species, H. pseudomusciformis Nauer, Cassano & M.C. Oliveira. The authors also pointed out that, for Brazil, H. nigrescens and H. valentiae corresponded to morphotypes presented by H. pseudomusciformis.
Jesus et al. (2016), in a taxonomic review for some Hypnea species, revealed some misapplied names and a confusion with the identification of H. cervicornis. In that work, H. aspera, H. boergesenii T.Tanaka and H. flexicaulis Y.Yamagishi & M.Masuda were considered synonyms of H. cervicornis. Also, specimens previously identified as H. cervicornis in some works were described as a new species, H. brasiliensis P.B. Jesus, Nauer & J.M.C. Nunes. During a DNA barcode survey, Nauer et al. (2016) described two new species, H. wynnei Nauer, Cassano & M.C. Oliveira and H. yokoyona Nauer, Cassano & M.C. Oliveira. At last, Jesus et al. (2019a ) verified non-monophyletic lineages, in both H. cornuta and H. stellulifera species, and described one new species, H. cryptica P.B. Jesus & J.M.C. Nunes, previously referred to as H. stellulifera for the Brazilian coast. The authors reaffirmed the importance of further studies to solve other species in this complex in Hypnea.
Therefore, considering the taxonomic challenges in Hypnea species, the confusion with misapplied names and the recent description of several new species, in this work, we aim to review the genus diversity and the species range of distribution for the Brazilian coast. We generated new sequence data to complement previous studies and discuss the intraspecific and interspecific divergence rates for the three different molecular markers used. Furthermore, we discuss the usefulness of the morphological features used in the taxonomy of this genus and propose an identification key for species occurring in Brazil.
2 Materials and methods
We used 65 specimens collected along the coast (ranging from 2°54′14.51″S to 27°08′42.44″S in latitude) in intertidal and subtidal zones during a DNA barcode survey. The specimens were the same as those used by Nauer et al. (2014, 2015, 2016). For molecular procedures, apical regions were separated from the rest of the thallus of each sample, cleaned and stored in silica gel. The remaining of the thallus was preserved in 4% formalin in seawater for taxonomic studies. Voucher specimens were housed at the Herbarium of University of São Paulo (SPF). Herbarium abbreviation follows the online Index Herbariorum (Thiers 2019, continuously updated).
The algal material from silica gel was macerated in liquid nitrogen, and the DNA was extracted using the NucleoSpin Plant II kit (Macherey–Nagel, Düren, Germany), following the kit protocol. Amplification of each marker was performed using a PCR Master Mix (Promega Corp., Madison, WI, USA). The primers and cycles used were: GazF1 and GazR1 for COI-5P (Saunders 2005), p23SrV_f1 and p23SrV_r1 for UPA (Sherwood and Presting 2007) and F993—RrbcS for rbcL (Freshwater and Rueness 1994). The column GTX™ PCR DNA and Gel Band Purification Kit (GE Healthcare, Buckinghamshire, UK) were used for purification of PCR products and quantified using NanoDrop 2000 (Wilmington, DE, USA). The same PCR primers were used with a sequencing kit BigDye Terminator Cycle Sequencing Ready Reaction (Applied Biosystems, Foster City, USA) according to the protocol of the supplier. The samples were sequenced in a 3730 DNA Sequencer (Applied Biosystems, Carlsbad, CA, USA). The sequences obtained were aligned in the BioEdit 7.0 sequence editor (Hall 1999). Calliblepharis ciliata (Greville) Kützing (DQ442896, AF385653) was used as outgroup. Trees were constructed for the concatenated sequences by neighbor joining (NJ) using the MEGA5 program (Molecular Evolutionary Genetics Analysis, Tamura et al. 2011) with 2000 bootstrap replicates.
For morphological studies, the samples kept in 4% formalin were sectioned by hand with a razor blade and stained with 1% aniline blue acidified with 1 N HCl. Habit and diagnostic characters of each species were obtained with digital capture and image analysis, using a compound microscope with Leica DM4000 digital camera (Solms, Germany). The morphological characters analyzed were based on the taxonomic studies of the genus Hypnea by Masuda et al. (1997).
3 Results
Molecular data
– A total of 65 Hypnea sequences from Brazil for COI-5P (nine new and 56 from GenBank), 65 sequences for rbcL-3P (35 new and 30 from GenBank) and 65 sequences for UPA (42 new and 23 from GenBank) were used in the analyses (Table S1). To calculate the interspecific and intraspecific divergences in base pairs (bp) and % for the markers COI-5P, UPA and rbcL-3P, we used 197 sequences of Hypnea from Genbank and obtained in this study (Table 1).
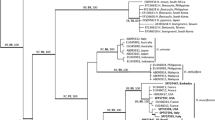
The combined results for the three molecular markers analysis indicate the occurrence of ten taxa of Hypnea for the coast of Brazil: H. brasiliensis, H. cervicornis, H. cornuta, H. cryptica, H. edeniana, H. flava, H. pseudomusciformis, H. spinella, H. wynnei, and H. yokoyana. Hypnea platyclada was recognized based on its morphology as no molecular sequences are available for this species (Table 2). Based on the results of the concatenated sequences analysis (Fig. 1), each of the ten taxa formed monophyletic clades with bootstrap support of 100. The concatenated matrix had 1275 bp, and the intraspecific divergence ranged from 0 to 1.7% (0 to 21 bp).
Neighbor joining (NJ) analysis for COI-5P, UPA and rbcL-3P sequences for Hypnea species. Calliblepharis ciliata was used as outgroup. On the branches are the bootstrap values for 2000 replicates. Sequences generated in this work are in bold. Accession numbers and country of origin are included for sequences obtained from GenBank
The interspecific variation was calculated using the same matrix only with Hypnea sequences from Brazil (data not shown). For COI-5P marker, the interspecific variation ranged from 8.3 to 18.2% (69 to 130 bp). The lowest divergence found was between H. cornuta and H. cryptica, 8.3%, and the highest between H. cryptica and H. wynnei, 18.2%. For the UPA marker, the interspecific variation ranged from 1.8 to 6.9% (7 to 28 bp). The lowest divergence found was between H. spinella and H. yokoyana, 1.8%, and the highest between H. cryptica and H. wynnei, 6.9%. For the rbcL-3P marker, the interspecific variation ranged from 3.4 to 8.7% (15 to 39 bp). The lowest divergence found was between H. edeniana and H. flava, 3.4%, and the highest between H. cervicornis and H. cryptica, 8.7%. In all cases, there was no overlap of intraspecific variation with interspecific variation.
Table 3 provides a summary of the intraspecific and interspecific divergences found for the Brazilian samples of the genus Hypnea for the three markers studied. The COI-5P marker was the most variable and efficient in the separation of species, with up to 18.2% interspecific divergence. The UPA and rbcL markers have lower interspecific divergence rates, with up to 6.9% and 8.7%, respectively.
Morphological data
– The morphological characters mostly used in the taxonomic studies of the genus were summarized by Masuda et al. (1997). However, due to the high phenotypic plasticity and the presence of cryptic species, the analysis of isolated morphological characters proved to be ineffective, where for almost all morphological characters observed there are overlaps between Hypnea species. For Brazilian specimens, of all 14 characteristics proposed by Masuda et al. (1997), the only ones that have taxonomic value considering the species analyzed in this work were: (1) habit of the thallus (tufts erect or tufts densely intricate); (2) main axis (single or multiple, persistent or not); (3) apex shape (straight, bent, bifurcated, tendril); (4) form of the branchlets (spinescent, starry, etc.); and (5) tetrasporangial sori position in the branches (basal, median, apical) (Jesus et al. 2013b). Table 4 describes how those five taxonomic characteristics are found in all eleven Brazilian taxa.
Identification of key of species of the genus Hypnea for the Brazilian coast:
1a. Plant forming tufts, densely intricate … 2
1b. Plant forming tufts with upright main axes … 8
2a. Thallus entirely flattened at the apical portions … 3
2b. Thallus entirely cylindric … 4
3a. Thallus epilithic, up to 1 cm high, tetrasporangia disposed at the apical portions of the fertile branches … H. wynnei
3b. Thallus epiphytic, more than 1 cm high, tetrasporangia disposed in the mid- and/or apical portions of the thallus … H. platyclada
4a. Anastomoses present and abundant; secondary fixation disks rare or absent … 5
4b. Anastomoses absent; secondary fixation disks abundant … 7
5a. Anastomoses present throughout the extension of the thallus … H. spinella
5b. Anastomoses present in the basal portions of the thallus, free apices … 6
6a. Bent or bifurcated apices, thallus reddish-pink to brownish, up to 12 cm … H. brasiliensis
6b. Simple apices, thallus brownish-yellow, up to 3 cm … H. flava
7a. Secondary ramifications present and abundant … H. edeniana
7b. Secondary ramifications rare … H. yokoyana
8a. Star-shaped branchlets present … H. cornuta
8b. Star-shaped branchlets absent … 9
9a. Thallus yellow, with thick wall … H. cryptica
9b. Thallus brown to red or green, with thin wall … 10
10a. Apex straight and/or curved, branching with a horn-like aspect, axial cell smaller than periaxial cells … H. cervicornis
10b. Apex in the form of tendrils, straight and/or curved, branching irregular with no horn-like aspect, axial cell greater or equal to periaxial cells … H. pseudomusciformis
4 Discussion
Molecular markers
– The work of Nauer et al. (2014) was the first to use DNA barcode markers for the study of the genus in the Atlantic Ocean. The authors generated 115 COI-5P sequences with 466 bp for six species of Hypnea occurring on the southeast coast of Brazil. The intraspecific divergence ranged from 0 to 5 bp (0–0.9%), and the interspecific divergence ranged from 62 to 100 bp (10.1–16.3%). In this study, the COI-5P intraspecific divergence found was 0–14 bp (0–1.7%) and the interspecific divergence found was 69–130 bp (8.3–18.2%), closer to the values described by Nauer et al. (2014). The values are also relatively close to those proposed by Saunders (2005) for red algae and by Geraldino et al. (2009, 2010) for the Asian sequences of Hypnea. The exception is in the maximum values of intraspecific divergences (1.7% in this work and 1.3% in Geraldino et al. 2010) and interspecific differences (18.2% in this work and 13.6% in Saunders 2005).
Nauer et al. (2014) also sequenced the UPA marker for studies of the genus Hypnea for the coast of Brazil. The authors obtained 33 sequences for the marker, representing six species found in the southeast region, with intraspecific divergence of 0–1 bp (0–0.3%), and the interspecific divergence found was 9–16 bp (2.5–4.4%). In this work, the intraspecific divergence obtained for UPA was 0–3 bp (0–0.8%) and the interspecific divergence found was 7–28 bp (1.8–6.9%). The difference found in the values of intra- and interspecific divergence of this work in relation to those presented by Nauer et al. (2014) is probably due to the number of sequences (65 and 33, respectively) and species (ten and six, respectively) analyzed. The relatively small difference between the maximum intraspecific divergence and the minimum interspecific divergence can be considered a limiting factor of the UPA marker, indicating low efficiency in the separation of closely related species (Clarkston and Saunders 2010).
Saunders and Moore (2013) proposed the utility of the 3′ region of rbcL marker (rbcL-3P) as a DNA barcode, reducing the size and effort of sequencing the full marker. In relation to the interspecific divergence within Hypnea, the variation was from 48 to 92 bp (3.6–6.8%) (Nauer et al. 2014). Geraldino et al. (2010), in their study of the Hypnea for Asia, used 42 sequences of 1356 bp representing 23 species of the genus. The interspecific divergence found for the marker was 18–93 bp (1.3–6.8%), indicating that this marker is a better alternative to COI-5P than UPA marker to identify species.
Despite the observed differences, all three markers used in this study presented no overlap between intraspecific and interspecific variations, i.e., the barcode gap, and were efficient to separate the different species of Hypnea occurring in Brazil. The COI-5P presented the highest barcode gap and was easily amplified using standard primers proposed for red algae (Saunders 2005). Therefore, the use of COI-5P, the standard DNA barcode proposed by the International Barcode of Life consortia (http://www.boldsystems.org/), is highly recommended for Hypnea species identification.
Taxonomy of Brazilian Hypnea species
– The anatomical characters, useful in distinguishing the species of the genus, were initially proposed by Kützing (1868), Okamura (1909) and Setchell (1924). However, the first extensive anatomical study was performed by Tanaka (1941), who used the cross section of the thallus in his analyses, considering internal characteristics such as the main axis cells and the presence of lenticular thickening in the walls of the medullar cells to describe species of Hypnea in Japan (Jesus et al. 2013b). The morphological characters mostly used over time in the taxonomic studies of the genus were summarized by Masuda et al. (1997). Studies with Hypnea emphasize that morphological characters undergo variation in relation to the environmental conditions and habitat of the specimen (Yamagishi and Masuda 2000; Guimarães 2006; Lucio 2006; Geraldino et al. 2010; Jesus et al. 2013b; Nauer et al. 2015). The color of the thallus is highly variable for some species, but has been more homogeneous for others. Species such as H. cryptica, H. edeniana, H. flava, H. yokoyana and H. wynnei presented uniform color of the thallus, and variation occurred only in relation to the tonality (lighter red or darker red, for example). Other species such as H. brasiliensis, H. cervicornis, H. pseudomusciformis and H. spinella showed great variation in the color of the thallus, being yellow, brown, green or red, besides the variation in the tonality (light or dark), depending on the habitat.
As for the habit and height of the thallus, the species of Brazilian coast can be divided into two groups (Fig. 2), one presenting a non-intricate tufted thallus and the other having a densely intricate thallus. In the first group are species such as Hypnea brasiliensis (Fig. 2a), H. cervicornis (Fig. 2b), H. cornuta, H. cryptica (Fig. 2c) and H. pseudomusciformis (Fig. 2f). In these species, specimens larger than 10 cm in height are relatively common. Some morphological variations of H. pseudomusciformis may form tufts, but the branches are not intricate and do not form tangles, since there is no occurrence of anastomoses or presence of secondary spinning disks in these species (Nauer et al. 2014, 2015). The second group is formed by species such as H. edeniana (Fig. 2d), H. flava (Fig. 2e), H. platyclada, H. spinella (Fig. 2g), H. yokoyana (Fig. 2h) and H. wynnei (Fig. 2i). These species have a considerably smaller size than the species of the first group, being common thalli of 2 or 3 cm in height. The appearance of the thallus in tangled tufts is due to the presence of numerous anastomoses and/or secondary fixation disks among the branches of these algae (Nauer et al. 2014, 2016; Jesus et al. 2016).
The texture of the algae varied from fleshy to cartilaginous. The species Hypnea spinella and H. cryptica presented the most cartilaginous texture, which can be explained by the fact that the external cuticle of the cell wall of these algae is noticeably thicker than the other species, a characteristic observed in anatomical sections. However, other species, such as H. cornuta, present thicker external cuticle but a delicate texture (Jesus et al. 2019a). The main axis is present in all species, with the difference being only one or multiple and whether it is persistent to the apex of the branch or highly branched. The species H. cryptica (Fig. 2c) has the most visible persistent main axis between species and the main axis of H. pseudomusciformis (Fig. 2f) has proved to be the most difficult to observe due to the amount of branching, but with careful examination it is possible to observe it through the diameter of the main axis, which is relatively larger than the diameter of the lateral branches (Nauer et al. 2015).
Species like Hypnea cervicornis, H. cornuta, H. cryptica, H. edeniana and H. flava have multiple major axes, which are branches of similar size that start from the same point at the base of the thallus. Subsequent branches have a significantly smaller size and diameter than the multiple principal axes. All species have a cylindrical axis, with the exception of H. wynnei and H. platyclada, which presented a cylindrical base and flattened upper portions. This characteristic, however, is not exclusive to those species, and there are other species of Hypnea with flat thallus, such as H. volubilis (Schneider and Searles 1991; Dawes and Mathieson 2008; Jesus et al. 2013a).
Irregular branching is present in all species of the genus and is easily observed in a first analysis, where branches can be formed in any part of the thallus, in any plane. In a more detailed analysis of some species, however, some patterns may be observed, even if these patterns are not maintained throughout the length of the thallus. In Hypnea wynnei, for example, it is more common to observe an alternate pattern of branching, but with parts of the thallus having irregular branching (Nauer et al. 2016). This pattern also occurs to a greater or lesser extent in the species H. edeniana, H. flava and H. spinella (Nauer et al. 2014). H. brasiliensis presents irregular branching pattern throughout the extension of the thallus, but the apical region commonly presents dichotomic or subdichotomic ramifications (Jesus et al. 2016). Hypnea cornuta has an alternating to spiral branching pattern, observed under stereomicroscope (Jesus et al. 2013b).
Straight apices are found in all species (Fig. 3a). In Hypnea cervicornis and H. pseudomusciformis, crooked (Fig. 3e), hook-shaped apices are common (Fig. 3b), but only in H. pseudomusciformis tendrils were observed (Fig. 3d). Tendrils, however, are not unique to this species. In Asian species, for example, the presence of tendrils in the apex of the branches is common (Masuda et al. 1997). There is a gradation in relation to the formation of hooks. Apices may be slightly curved (Fig. 3b), and others may be on a level that may resemble a tendril in formation (Fig. 3c). A specimen may present several levels of gradation of its curved apices, without uniformity. Therefore, we believe that the apices in Hypnea can be straight, curved (hooks) and to form tendrils.
Variation of the main morpho-anatomical characters in Hypnea. a–e Variety of apices from H. pseudomusciformis. a Straight or acute apex. b Recumbent apex. c Apex in the shape of a hook. d Apex in the shape of a tendril. e Bifurcated apex. f–h Variety of branchlets. f Spinescent, from H. pseudomusciformis. g Forked, from H. brasiliensis. h Starlets, from H. cryptica. i–k Variation in the position of tetrasporangial sori. i Tetrasporangia at the base of the branch, from H. cervicornis. j Tetrasporangia in the middle portion of the branch, from H. pseudomusciformis. k Tetrasporangia at the apex of the branches, from H. wynnei. l Cystocarps, from H. brasiliensis. m, n Transverse section of the thallus of two specimens of Hypnea evidencing the basic types of axial organization, defined by Bodard (1968). mH. pseudomusciformis.nH. spinella. o Lenticular thickening in the cells of the spinal cord. p, q Transverse section of tetrasporangial sori, both from H. pseudomusciformis. p Incompletely circling the radius. q Completely circling the radius
Almost all analyzed species, with the exception of Hypnea cryptica, exhibit spinescent branchlets along the entire length of the thallus (Fig. 3f). The shape of the spinous branchlets varies and may be in the form of small thorns, which can be curved upward, as in H. cervicornis and H. edeniana, or in the form of tiny branches, as in other species. The spinous branchlets in H. pseudomusciformis are larger and more abundant compared to other species and may resemble small hairs covering the thallus. In addition to spinous branchlets, H. brasiliensis also has bifurcated branchlets (Fig. 3g), a characteristic that helps to distinguish this species from the others. Spinous branchlets may also be present in H. cornuta, but this species also has star-shaped branches (Fig. 3h), which may detach from the thallus (Jesus et al. 2019a).
By using anatomical characteristics of the genus, species of Hypnea were divided into two groups in relation to the axial cell size compared to the periaxial cells, a feature observed in the transverse section of the thallus (Bodard 1968). In the first group, the axial cell is significantly smaller than the periaxial cells (Fig. 3n), while in the second group the axial cell is larger or equal to the periaxial cells (Fig. 3m). However, morphological variations of Hypnea pseudomusciformis proved that both characteristics could be found within the same species (Nauer et al. 2015). The number of periaxial cells varied uniformly among the species, being a characteristic that overlaps and is inefficient in the separation of the species.
Another feature considered in the taxonomy and observed in the cross section of the thallus is the lenticular thickening (Fig. 3o), which occurs in the cell wall of the medullary cells, mainly in the periaxial cells, and is visible even without the aid of dyes. Although they are used in the characterization of Hypnea species, Ogawa and Lewmanomont (1981) state that the presence of these thickenings depends on differences in ecological conditions in the various environments where they occur. The presence or absence of lenticular thickening on the walls of the medullary, periaxial and axial cells was also not useful in distinguishing the species. Ogawa and Lewmanomont (1981) argue that this variation may be related to habitat and environmental conditions. In addition, the presence of lenticular thickening may vary in specimens of the same species under the same conditions (Jesus et al. 2013b).
The life history of Hypnea is an alternation between two diploid phases and one haploid phase (Masuda et al. 1997; Nauer et al. 2019). Gametophytes and tetrasporophytes are isomorphic. Regarding the reproductive structures, tetrasporangia are ovoid and zonated and are formed in the cortical region of branchlets with its position (basal, Fig. 3i; median, Fig. 3j; or apical, Fig. 3k) being the only characteristic that could be useful in the identification of the species. Hypnea spinella, H. platyclada and H. wynnei can have tetrasporangial sori at the apex of the branches (Jesus et al. 2013a, 2016; Nauer et al. 2016). In species such as H. cervicornis, H. cryptica and H. edeniana, the sori were more common in the basal region of the branches but also can be found in the median portion. In the other species, the position varied and was found in both the mid- and basal portions of the branches. It is common for sori to completely surround the branches where they are found (Fig. 3q), but in the species H. cervicornis and H. pseudomusciformis it is also common to find the sori on only one side of the branches, circling them incompletely (Fig. 3p, Table 4).
Cystocarps (Fig. 3l) are located in branches and branchlets of female gametophyte, and spermatangial sori are organized in the cortical region of male gametophytes branchlets, both usually visible to the naked eye (Masuda et al. 1997; Nauer et al. 2019). The absence of male and the rare occurrence of female gametophytes for H. pseudomusciformis in natural populations were reported in several studies (Schenkman 1989; Reis and Yoneshigue-Valentin 2000 as H. musciformis, and Jesus et al. 2019b). Reis and Yoneshigue-Valentin (2000, as H. musciformis) suggest a prevalence of asexual reproduction over sexual reproduction in populations of H. pseudomusciformis in Brazil. The vegetative reproduction may be an important reproductive strategy, which enables the species maintenance throughout difficult environmental conditions for growth (Nauer et al. 2019).
Distribution on Brazilian coast
– Despite eleven species cited for the Brazilian coast, only three species (Hypnea brasiliensis, H. cervicornis and H. pseudomusciformis) could be found widespread along the coast of Brazil, while other species (H. platyclada and H. wynnei) could be found only in one collection site (Fig. 4). Hypnea pseudomusciformis is the most common and abundant species in the intertidal zone of the continent, but it was not found in islands or depths below 5 m. One hypothesis is that this species is palatable and can be easily predated by herbivores such as fish, mollusks and crustaceans. There were several species (H. edeniana, H. flava, H. spinella and H. yokoyana) present in the southeast and south region that could not be found in the northeast region above Bahia state, though in the literature some of those species (such H. spinella) are cited for that region. This scenario reflects the sample efforts from previously studies and the necessity of more taxonomic studies in the northeast region, especially in the subtidal zone.
Distribution of Hypnea species along the Brazilian coast based on this study. The states of Brazil for which collections were included in this study are marked in black, from S to N: Santa Catarina (SC), São Paulo (SP), Rio de Janeiro (RJ), Espírito Santo (ES), Bahia (BA), Pernambuco (PE), Paraíba (PB), Rio Grande do Norte (RN) and Ceará (CE)
In the case of the genus Hypnea, the degree of exposure to waves can define diversity, distribution and species morphology. Hypnea species were abundant in both protected and wave-exposed shores; however, some species were only found in protected shores, probably because they have a more delicate thallus. Epilithic specimens of H. pseudomusciformis are the most common in exposed shores, whose population can form a very high biomass, covering rocks, from the upper to the lower intertidal zone. In protected coasts, Hypnea species form carpets, which can be homogeneous or mixed with other algae, and H. pseudomusciformis is commonly found as epiphytes in other algae in the subtidal zone.
Along the Brazilian coast, rocky shores are more common in the southeastern region of the country, also in northern Bahia, and reefs occur from the state of Maranhão to the state of Espírito Santo, the majority of which is sandstone consolidated by the calcium carbonate produced from the dissolution of shells (Amado-Filho et al. 2006). Hypnea species are more common in reefs near beaches, where the environment is more protected and rare or absent in reefs far from the coast, more susceptible to the action of waves.
The species H. pseudomusciformis was not found in the subtidal region below 5 m depth, but is commonly found in the intertidal region and as epiphytes on other algae (mainly Sargassum spp.) being able to form very dense populations with a high biomass. Hypnea brasiliensis and H. cervicornis were also common species in reefs.
In the subtidal zone, collections were limited to islands and were made with the use of autonomous diving equipment. Most species of Hypnea were not found in this region. Hypnea brasiliensis species was the most common in this environment, growing entangled with other tiny algae forming a carpet on the rock. Hypnea wynnei was found only in this environment and was not found in the intertidal zone. These species seem to inhabit only shallow water, since no specimen was collected below 8 meters in depth.
Of the 16 Hypnea species previously cited, we molecularly confirmed the occurrence of ten: H. brasiliensis, H. cervicornis, H. cornuta, H. cryptica, H. edeniana, H. flava, H. pseudomusciformis, H. spinella, H. yokoyana and H. wynnei. The identification of the species based on morphological characteristics was imprecise, mainly due to the phenotypic plasticity present in the group, besides the existence of species with convergent morphologies. The DNA barcode technique, especially the COI-5P marker, proved to be very useful and essential in the identification and delimitation of the species, revealing scenarios that would go unnoticed by morphology. However, careful morphological work can still be useful for species identification, especially when considering their range of distribution.
References
Agardh JG (1851) Species genera et ordines algarum, seu descriptiones succinctae specierum, generum et ordinum, quibus algarum regnum constituitur. Volumen secundum: algas florideas complectens. Part 2, fasc. 1. C.W.K. Gleerup, Lund, pp 337–506
Amado-Filho GM, Horta PA, Brasileiro PS, Barros-Barreto MB, Fujii MT (2006) Subtidal benthic marine algae of the Marine State Park of Laje de Santos (Sao Paulo, Brazil). Braz J Ocean 54:225–234
Bodard M (1968) Les Hypnea au Sénégal (Hypnéacées, Gigartinales). Bull l’inst Fondam d’Afr Noire 3:811–829
Clarkston BE, Saunders GW (2010) A comparison of two DNA barcode markers for species discrimination in the red algal family Kallymeniaceae (Gigartinales, Florideophyceae), with a description of Euthora timburtonii sp. nov. Botany 88:119–131
Dawes CJ, Mathieson AC (2008) The seaweeds of Florida. University Press of Florida, Gainesville
Freshwater DW, Rueness J (1994) Phylogenetic relationships of some European Gelidium (Gelidiales, Rhodophyta) species based on rbcL nucleotide sequence analysis. Phycologia 33:187–194
Furtado MR (2004) Desequilíbrio climático abre mercado para novos hidrocolóides. Quím e Deriv 430:1–4
Geraldino PJL, Yang EC, Kim MS, Boo SM (2009) Systematics of Hypnea asiatica sp. nov. (Hypneaceae, Rhodophyta) based on morphology and nrDNA SSU, plastid rbcL, and mitochondrial cox1. Taxon 58:606–616
Geraldino PJL, Riosmena-Rodriguez R, Liao LM, Boo SM (2010) Phylogenetic relationships within the genus Hypnea (Gigartinales, Rhodophya), with a description of H. caespitosa sp. nov. J Phycol 46:336–345
Guimarães SMPB (2006) Checklist of Rhodophyta from the State of Espírito Santo. Bol Inst Bot 17:143–194
Guiry MD, Guiry GM (2019) AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org; searched on 10 June 2019
Hall TA (1999) BioEdit: a user-friendly biological alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Jesus PB (2016) Estudos biossistemáticos em espécies do gênero Hypnea J.V. Lamouroux (Gigartinales, Rhodophyta). Universidade Estadual de Feira de Santana, Feira de Santana
Jesus PB, Guimarães SMPB, Nunes JMC (2013a) Hypnea platyclada, a new species of red alga (Rhodophyta, Cystocloniaceae) from Brazil. Phytotaxa 85:26–34
Jesus PB, Schnadelbach AS, Nunes JMC (2013b) O gênero Hypnea (Cystocloniaceae, Rhodophyta) no litoral do estado da Bahia, Brasil. Sitientibus Sér Ciênc Biol 13:1–21
Jesus PB, Silva MS, Lyra GM, Nunes JMC, Schnadelbach AS (2015) Extension of the distribution range of Hypnea stellulifera (Cystocloniaceae, Rhodophyta) to the South Atlantic: morphological and molecular evidence. Aquat Bot 123:26–36
Jesus PB, Nauer F, Lyra GM, Cassano V, Oliveira MC, Nunes JMC, Schnadelbach AS (2016) Species delimitation and phylogenetic analyses of some cosmopolitan species of Hypnea (Rhodophyta) reveal synonyms and misapplied names to H. cervicornis, including a new species from Brazil. J Phycol 52:1–44
Jesus PB, Costa AL, Nunes JMC, Manghisi A, Genovese G, Morabito M, Schnadelbach AS (2019a) Species delimitation methods reveal cryptic diversity in the Hypnea cornuta complex (Cystocloniaceae, Rhodophyta). Eur J Phycol 54:135–153
Jesus PB, Pestana EMS, Affe HMJ, Rocha DSB, Caires TA, Nunes JMC, Schnadelbach AS (2019b) Reproductive morphology and phenological aspects of one morphological variant of Hypnea pseudomusciformis (Gigartinales, Rhodophyta). Acta Bot Bras 33:67–77
Joly AB, Ferreira MM, Pinheiro-Vieira F, Yoneshigue-Braga Y (1968) Additions to the American South Atlantic marine algae. Arq. Est. Biol. Mar. Univ. Fed. Ceará 8:177–181
Kützing FT (1868) Tabulae phycologicae; oder, Abbildungen der Tange, Vol. 18. Gedruckt auf kosten des Verfassers (in com- mission bei W. Köhne), Nordhausen, Germany, 35 pp
Lamouroux JV (1813) Essai sur les genres de la famille des Thalassiophytes non articulées. Annales du Muséum d’Histoire Naturelle, Paris 20: 21–47, 115–139, 267–293, pls 7–13
Lucio AM (2006) El género Hypnea (Rhodophyta) en las costas del Océano Atlántico. Departamento de Biología Vegetal de la Universidad Complutense de Madrid, Tese de doutorado - Faculdad de Ciencias Biológicas, Madrid
Masuda M, Yamagishi Y, Chiang Y-M, Lewmanomont K, Xia B (1997) Overview of Hypnea (Rhodophyta, Hypneaceae). In: Abbott IA (ed) Taxonomy of economic seaweeds, vol 6. California Sea Grant College System, La Jolla
Nauer F, Guimarães NR, Cassano V, Yokoya NS, Oliveira MC (2014) Hypnea species (Gigartinales, Rhodophyta) from the southeastern coast of Brazil based on molecular studies complemented with morphological analyses, including descriptions of Hypnea edeniana sp. nov. and H. flava sp. nov. Eur J Phycol 49:550–575
Nauer F, Cassano V, Oliveira MC (2015) Description of Hypnea pseudomusciformis sp. nov., a new species based on molecular and morphological analyses, in the context of the H. musciformis complex (Gigartinales, Rhodophyta). J Appl Phycol 27:2405–2417
Nauer F, Cassano V, Oliveira MC (2016) Hypnea wynnei and Hypnea yokoyana (Cystocloniaceae Rhodophyta), two new species revealed by a DNA barcoding survey on the Brazilian coast. Phytotaxa 268:123–134
Nauer F, Ayres-Ostrock L, Amorim AM, Santos JP, Chow F, Plastino EM, Oliveira MC (2019) Life history, growth, and pigment content of two morphological variants of Hypnea pseudomusciformis (Gigartinales, Rhodophyta). J Appl Phycol 31:1271–1284
Nunes JMC (2005) Rodoficeas marinhas bentonicas do estado da Bahia, Brasil. Tese de Doutorado. Instituto de Biociências da Universidade de São Paulo. Departamento de Botânica. São Paulo
Ogawa H, Lewmanomont K (1981) Economic seaweeds of Thailand. I. The genus Hypnea in the vicinity of Si Racha, Chonburi Province. Kasetsart Univ Fish Res Bull 12:1–14
Okamura K (1909) Icones of Japanese Algae. Tokyo: published by the author, vol. 2
Oliveira-Filho EC (1977) Algas marinhas bentônicas do Brasil. Tese (Livre-Docência). Universidade de São Paulo, São Paulo
Reis RP, Yoneshigue-Valentin Y (2000) Phenology of Hypnea musciformis (Wulfen) Lamouroux (Rhodophyta, Gigartinales) in three populations from Rio de Janeiro State, Brazil. Bot Mar 43:299–304
Saito RS, Oliveira EC (1990) Chemical screening of Brazilian marine algae producing carrageenans. Hydrobiologia 204:585–588
Saunders GW (2005) Applying DNA barcoding to red macroalgae: a preliminary appraisal holds promise for future applications. Philos Trans R Soc B 360:1879–1888
Saunders GW, Moore TE (2013) Refinements for the amplification and sequencing of red algal DNA barcode and RedToL phylogenetic markers: a summary of current primers, profiles and strategies. Algae 28:31–43
Schenkman RPF (1986) Cultura de Hypnea (Rhodophyta) in vitro como subsídio para estudos morfológicos, reprodutivos e taxonômicos. Ph.D. thesis, Universidade de São Paulo, Brazil
Schenkman RPF (1989) Hypnea musciformis (Rhodophyta): ecological influence on growth. J Phycol 25:192–196
Schneider CW, Searles RB (1991) Seaweeds of the southeastern United States. Cape Hatteras to Cape Canaveral. Duke University Press, Durham
Setchell WA (1924) American Samoa: Part I. Vegetation of Tutuila Island. Part II. Ethnobotany of the Samoans. Part III. Vegetation of Rose Atoll. Publications of the Carnegie Institution of Washington, p 341
Sherwood AR, Presting GG (2007) Universal primers amplify a 23S rDNA plastid marker in eukaryotic algae and cyanobacteria. J Phycol 43:605–608
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary analysis using maximum likelihood, evolutionary distance, and maxi. Mol Biol Evol 28:2731–2739
Tanaka T (1941) The genus Hypnea from Japan. Sci Pap Inst Algol Res, Fac Sci, Hokkaido Univ 2: 227–250
Thiers B (2019) Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://www.nybg.org/bsci/ih/ih.html. [continuously updated]
Villaça R, Fonseca AC, Jensen VK, Knoppers B (2010) Species composition and distribution of macroalgae on Atol das Rocas, Brazil, SW Atlantic. Bot Mar 53:113–122
Yamagishi Y, Masuda M (2000) A taxonomic revision of a Hypnea charoides-valentiae complex (Rhodophyta, Gigartinales) in Japan, with a description of Hypnea flexicaulis sp. nov. Phycol Res 48:27–35
Yoneshigue Y (1985) Taxonomie et ecologie des algues marines dans la région de Cabo Frio (Rio de Janeiro, Bresil). PhD Thesis - Faculté dês Sciences de Lumin. L’Universite D’aix – Marseille I. 466 p
Acknowledgements
We are grateful to Rosário Petti and Willian Oliveira for technical support. Financial support was provided by Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP (Biota 2013-11833-3). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. The following authors acknowledge Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq for the Productivity Fellowships: PBJ CNPq (150068/2017- 4), MCO (301491/2013-5), JMCN (307368/2015-7) and VC (302549/2017-0).
Author information
Authors and Affiliations
Contributions
FN and MCO conducted field work. FN and PBJ conducted laboratory work, and gathered and analyzed data. MCO and VC supervised the study and contributed to discussion. All authors were involved in writing and discussion of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nauer, F., Jesus, P.B., Cassano, V. et al. A taxonomic review of the genus Hypnea (Gigartinales, Rhodophyta) in Brazil based on DNA barcode and morphology. Braz. J. Bot 42, 561–574 (2019). https://doi.org/10.1007/s40415-019-00544-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-019-00544-z