Abstract
Introduction
Widespread use of effective antiretroviral therapy (ART) in people living with human immunodeficiency virus (PLHIV) infection has changed the prognosis of the disease from a fatal and debilitating one to a chronic manageable illness. ART leads to immune restoration, but immune functional levels seen in HIV-uninfected people are never achieved. The dynamics of immune suppression, chronic immune activation, and immune senescence predispose PLHIV to certain inflammatory and infectious conditions of the cardiovascular system.
Methods
Atherosclerotic cardiovascular disease is a chronic inflammatory condition that is a rising cause of mortality and morbidity among PLHIV. Certain risk factors prevalent among PLHIV, such as intravenous drug abuse, use of ART, and susceptibility, to tuberculous disease even at near-normal immune functional levels predispose them to inflammation and infections of the different components of the heart including the pericardium, epicardium, and endocardium. Molecular imaging using radionuclide techniques has been shown to have excellent clinical utility in the evaluation of inflammation and infection.
Results
The strengths of molecular imaging techniques lie in their sensitivity for early disease detection before morphological changes occur and in therapy response assessment before significant reparative changes have occurred. Molecular imaging techniques are now becoming more widespread in clinical application with increasing availability and utilization in developing countries where the most significant burden of HIV infection is felt.
Conclusion
This review aimed to present an update on the evidence supporting the role of molecular imaging modalities with radionuclide techniques for imaging of cardiovascular inflammation and infection focusing on atherosclerotic cardiovascular disease, pericarditis, myocarditis, and endocarditis in PLHIV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Infection by the human immunodeficiency virus (HIV) remains a significant cause of mortality and morbidity globally [1]. Infection by HIV causes rapid depletion of the host immune system as the virus targets immune cells expressing the CD 4 molecule [2]. Early depletion of the gut-associated lymphoid tissue leads to systemic translocation of gut microbes causing chronic immune activation [3]. Anti-retroviral therapy (ART) is very effective in inhibiting viral replication. The effectiveness of ART is manifested in the life expectancy of PLHIV which is now approaching that of the HIV-uninfected people [4, 5]. Despite the improvement in the immune system with ART, people living with HIV (PLHIV) never achieve complete immune recovery even with complete viral suppression [6]. The cytotoxic function of CD8+ T lymphocytes, for example (a subset of lymphocytes not directly affected by HIV), never returns to normal even with complete viral suppression [7]. PLHIV, therefore, are at increased risk of acquiring infections seen in HIV-uninfected people as well as opportunistic infections that result from relative immunosuppression. PLHIV successfully treated with ART and achieved suppressed viremia have circulating cytokines that reflect persistent immune activation and dysfunction [8]. Chronic immune activation in PLHIV has been implicated as a predisposing factor to a group of non-infectious disorders which are emerging as significant causes of mortality and morbidity. These chronic metabolic non-infectious conditions include atherosclerotic cardiovascular diseases (ASCVD); accelerated aging; liver disease, bone, and metabolic disorders; non-AIDS defining cancers and neurocognitive dysfunction [9, 10]. PLHIV are, therefore, at increased risk of infection due to immune dysfunction and non-infectious inflammatory conditions such as ASCVD due to chronic immune activation. In this review, we aimed to present an update on the evidence supporting the use of molecular imaging techniques in the evaluation of cardiovascular inflammation and infection. We will focus on ASCVD, pericarditis, myocarditis, and endocarditis. 18F-Florodeoxy-d-glucose positron emission tomography with computed tomography (18F-FDG PET/CT) has been the most reported molecular imaging technique in the evaluation of cardiovascular inflammation and infection. It will form the main thrust of this review.
Atherosclerotic cardiovascular disease
Inflammation is critical to the formation and progression of ASCVD [11]. The earliest event in atheroma formation is vascular intima injury caused by factors including smoking, hypertension, and hyperglycemia. Vascular intima injury causes the accumulation of atherogenic plasma-derived cholesterol-rich lipids in the vascular intima as well as upregulation of cell adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1), and intercellular cell adhesion molecule-1 (ICAM-1), which favor recruitment of monocytes and T lymphocytes into the vessel wall [12,13,14]. Atherogenic lipid molecules are oxidized within the vascular to form oxidized pro-inflammatory lipids [12]. Monocytes mature to become macrophages in the vessel intima. Macrophages engulf the atherogenic oxidized lipids to become foam cells. Vascular smooth muscles also accumulate the atherogenic lipids, become activated, and secrete chemoattractants and chemokines that further attract circulating immune cells to the site of atheroma formation [12]. The matured atheromatous plaque typically contains lipid-rich necrotic core covered by a fibrous cap made up of a mixture of smooth muscle cells and supporting extracellular matrix. The base of the lesion often contains foam cells and T lymphocytes. Inflammation also plays a crucial role in plaque rupture, the catastrophic complication of atherosclerosis. Macrophages produce matrix metalloproteinases that can digest the fibrous cap leading plaque rupture. T cells produce interferon-\(\gamma \) which inhibits the smooth muscle proliferation causing weakening of the fibrous cap [15].
HIV infection has been identified as an additional risk factor for ASCVD. PLHIV is twice at risk of ASCVD compared with HIV-uninfected individuals. The global burden of ASCVD among PLHIV has tripled in the last two decades [16]. The mechanisms by which HIV infection increases the risk of ASCVD include direct viral invasion of the vessel wall causing arterial inflammation; chronic immune activation due to HIV infection; ART-induced metabolic disturbances such as lipid dysregulation, insulin resistance, and diabetes mellitus; and the preponderance of the traditional cardiovascular risk factors among PLHIV [17,18,19,20].
In HIV infection, there is persistent viral replication even in individuals with completely suppressed viremia. This persistent viral replication not only leads to a progressive decline in the CD4+ T-cell population but also causes immune activation and chronic systemic inflammation. Uncontrolled viral replication seen in ART-naïve patients is associated with vascular endothelial dysfunction, the precursor of ASCVD [21]. Effective ART with suppressed viremia reduces but does not eliminate immune activation or the elevated risk of acquiring ASCVD [17, 22]. Pro-atherogenic soluble factors in PLHIV encourage foam cell formation by monocyte through the reduction of cholesterol efflux from them [23]. ART, especially with protease inhibitor, is associated with proatherogenic lipid metabolism dysregulation [24].
Inflammation is crucial to the formation, progression, and rupture of the atherosclerotic plaque. Activated inflammatory cells accentuate their glucose use to cope with the high energy demand of the inflammatory process. 18F-FDG, an analog of glucose, can, therefore, be used for PET/CT imaging of arterial inflammation in ASCVD. In the arterial wall, the intensity of 18F-FDG uptake correlates with the level of macrophage invasion [25, 26]. The intensity of 18F-FDG uptake is higher in symptomatic plaque compared with asymptomatic plaque and uptake in asymptomatic patients predicts the future risk of vascular events [27,28,29]. The utility 18F-FDG PET/CT for imaging subclinical ASCVD in PLHIV has been shown in different studies [30]. Arterial 18F-FDG accumulation is significantly higher in chronic HIV-infected individuals than age, gender, and Framingham risk score-matched controls but comparable to the level of uptake seen in the arteries of older patients with established atherosclerotic disease [31]. This finding suggests that the level of arterial inflammation in PLHIV is as high as the level in older patients with established ASCVD [31].
ART improves life expectancy in PLHIV but does eliminate the risk ASCVD. In a cohort of HIV-infected individuals who obtained 18F-FDG PET/CT before commencing ART, effective ART did not lead to a significant reduction in arterial 18F-FDG uptake after 6 months of treatment [32]. Persistent elevated arterial 18F-FDG uptake suggests residual inflammation despite effective ART. Statin therapy targeting arterial inflammation is protective against ASCVD regardless of its lipid-lowering effect. In the SATURN-HIV trial, statin therapy with rosuvastatin led to a significant reduction in soluble markers of monocyte activation (sCD14 and sCD163) without significant change in circulating monocyte level demonstrating the additional benefit of statin therapy in PLHIV on regular ART [33]. Quantification of arterial 18F-FDG uptake before and after therapy provides a direct means of determining the effectiveness of therapeutic agents targeting inflammation in ASCVD risk reduction strategy [34]. A study comparing arterial 18F-FDG uptake as a surrogate marker of inflammation between HIV-infected and HIV-uninfected patients found no significant difference in arterial 18F-FDG uptake between the two groups [35]. In a sub-group analysis, however, there was significantly higher arterial wall 18F-FDG uptake in statin-naïve HIV-infected patients compared with statin-naïve HIV-uninfected controls. These results demonstrate the residual arterial inflammation in HIV-infected people despite regular ART use.
Many other agents beyond statins have been explored for their anti-inflammatory property in ASCVD risk reduction strategy [17]. The CANTOS trial recently reported a reduction in inflammation associated with a dose-dependent reduction in recurrent major cardiovascular events in HIV-uninfected individuals treated with canakinumab, a monoclonal antibody targeting interleukin-1β (IL-1β) [36]. Hsue and colleagues have reported the utility of 18F-FDG PET/CT in evaluating the anti-inflammatory effect of canakinumab in HIV-infected individuals with suppressed viremia on ART and treated with a single dose of canakinumab. At 8 weeks post-treatment, there was a significant reduction in arterial 18F-FDG uptake [37]. 18F-FDG has, therefore, emerged as a useful non-invasive biomarker for direct monitoring of arterial inflammation in the development and evaluation of drugs targeting inflammatory pathways in ASCVD.
ASCVD has a very long preclinical phase with atherogenesis starting long before clinical manifestation [38]. Our group recently demonstrated higher arterial 18F-FDG uptake in young PLHIV who otherwise had no or low-risk factors for ASCVD compared with age- and gender-matched controls [39]. Our results showed that HIV and its treatment are independent risk factors for ASCVD and that the disease process starts quite early in life among PLHIV. Demonstration of arterial inflammation at a very young age among PLHIV support the results from earlier studies reporting younger age at clinical presentation of ASCVD among PLHIV compared with the general population of HIV-uninfected people [40].
A few studies have reported non-significant differences in arterial 18F-FDG uptake between PLHIV and HIV-uninfected individuals [32, 41,42,43]. These negative reports may be a result of non-standardized imaging protocol [41], the technical challenge militating against accurate lesion comparison in test–retest study design [42], or small patient population [32, 43]. A significant challenge with 18F-18FDG PET/CT imaging of vascular inflammation lies in its inherent limited spatial resolution causing a significant underestimation of quantified vascular 18F-FDG. The average thickness of the arterial wall is less than twice the spatial resolution of most PET/CT cameras in current clinical use which causes significant underestimation due to partial volume effect [44, 45]. There is an additional need to subtract signals contributed by blood-pool activity from within the vascular lumen. To achieve the latter, target-to-background ratio (TBR) computed by obtaining the mean of multiple SUVmax measurements in the arterial wall of interest and dividing it by the SUV obtained from the lumen of an adjacent vein. TBR has a good correlation with actual arterial F-FDG uptake as shown in a kinetic modeling study [46]. There is also a good correlation between TBR taken from different vascular territory in an individual as well as good intra- and inter-observer agreements [47]. To improve the performance of 18F-FDG PET/CT for imaging of vascular inflammation in ASCDVD, the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM) recently published a set of guidelines on patient preparation, image acquisition, and reporting of 18F-FDG PET/CT [48]. When observed, these set of criteria improves quantified arterial 18F-FDG uptake measured by SUVmax and TBR [49]. Figure 1 shows the impact of observing the recommendations by the cardiovascular committee of EANM on arterial 18F-FDG uptake and background blood-pool activity clearance.
A 54-year-old female had dual-time points 18F-FDG PET/CT imaging. Early imaging acquired at 60 min post-tracer injection using the routine oncologic PET parameters shows high blood-pool background activity (right images). Delayed imaging acquired at 124 min post-tracer injecting using PET parameters dedicated for vascular inflammation imaging showed good background activity clearance with improvement in arterial wall 18F-FDG uptake (left images). Dual-time point imaging was acquired in this patient to show the improvement of arterial 18F-FDG uptake and background activity clearance obtained when uptake time is delayed and the acquisition parameters are optimized for vascular inflammation imaging
Several targets, other than glucose utilization, can be explored for arterial inflammation imaging in PLHIV [50]. Arterial 18F-FDG uptake measures metabolic activity in the tissues of the vessel wall including smooth muscles, fibroblasts, and inflammatory cells. Molecular probes targeting markers expressed specifically by inflammatory cells may be more accurate in characterizing arterial inflammation. Macrophages express CD206 molecules, the molecular target for radiolabeled tilmanocept. 99mTc-Tilmanocept is approved for sentinel lymph mapping of solid human tumors, especially early breast cancer and intermediate-thickness malignant melanoma. 99mTc-Tilmanocept injected subcutaneously accumulates more in the aortas of HIV-infected patients compared with HIV-uninfected individuals with similar Framingham risk score [51]. This higher accumulation of 99mTc reflects arterial inflammation in HIV-infected individuals compared with uninfected individuals. This study utilized 99mTc-labeled tilmanocept administered by subcutaneous injection for SPECT/CT imaging. Radiolabeling of tilmanocept with PET radionuclide such as gallium-68 has been described [52, 53]. Intravenously administered tilmanocept labeled with a PET radionuclide may offer a more effective technique for characterizing arterial inflammation in PLHIV.
In summary, 18F-FDG PET/CT is a useful imaging tool for measuring arterial inflammation as a risk for ASCVD in PLHIV. Its use as a non-invasive technique for direct monitoring of changes in arterial inflammation in response to anti-inflammatory therapy is likely to expand, as investigations into this form of treatment become more widespread. The PET system significantly under-estimate arterial 18F-FDG uptake due to the limited diameter of most vessels. This limitation emphasizes the need for the application of standardized imaging parameters which significantly improves PET quantification of arterial 18F-FDG uptake.
Pericarditis
Pericarditis is a common complication seen in PLHIV worldwide [54]. The pericardial disease has a variable manifestation from asymptomatic small pericardial effusion to cardiac tamponade [55]. In developed countries, the cause of pericardial effusion is often idiopathic [55, 56]. In tuberculosis-endemic regions of the world such as Africa, Mycobacterium tuberculosis is responsible for 86–100% of pericarditis in PLHIV [57]. Immune suppression seen in HIV infection causes impairment in cellular immunity leading to a heightened risk of acquisition of new and reactivation of previous tuberculosis (TB) [58]. There is a 10% lifetime risk of TB disease in individuals without HIV infection compared with a 10% annual risk of TB disease in PLHIV [59, 60]. The annual risk of TB disease in advanced immunosuppression of HIV infection increases to 30% [61]. Other infectious causes of pericarditis in PLHIV include bacteria (such as Staphylococcus aureus, Streptococcus pneumoniae, Listeria monocytogenes, Proteus species, Chlamydia trachomatis, Klebsiella species, and Pseudomonas aeruginosa); viruses (such as HIV, Herpes simples I/II, and Cytomegalovirus); fungi (Histoplasma capsulatum, Cryptococcus neoformans, and Candida species); and protozoa (such as Toxoplasma gondii) [56].
The lungs are the commonest organs of primary TB disease. HIV infection is a significant risk factor for the extra-pulmonary spread of TB [62]. TB spreads to involve the pericardium homogenously, from chest nodes contiguous to the pericardium, or via direct spread from infected foci in the lungs or pleura. The homogeneous route is the most common route of spread to the pericardium in PLHIV [63]. TB pericarditis progress through four stages including (1) dry stage associated with chest pain, pericardial friction rub, and widespread ST elevation without effusion; (2) effusive stage of moderate to large effusion with signs and symptoms of heart failure or pericardial tamponade; (3) adsorptive phase seen as thickened pericardium on imaging with associated thick fibrinous fluid around the heart; and (4) constrictive phase characterized by signs, symptoms, and imaging features of constrictive pericarditis without residual pericardial fluid [54]. The effusive phase is the commonest stage at which patients present at the hospital for care [64]. Figure 2 shows the images of a patient with restrictive cardiac disease caused by pericardial calcification from a previous episode of TB pericarditis.
A 62-year-old male with symptoms of heart failure and right pleural effusion. Adequate assessment of cardiac function could not be performed on echocardiography due to poor acoustic window. 18F-FDG PET/CT done shows dense pericardial calcification with no associated significant metabolic activity confirming the late sequela of a previous episode of tuberculous pericarditis. These images emphasize the utility of 18F-FDG PET as a tool to differentiate between an active tuberculous pericarditis requiring anti-tuberculous medication with anti-inflammatory therapy using corticosteroid versus the sequela of a previous infection requiring a different approach of therapy such as pericardial decortication
The definitive diagnosis of TB pericarditis rests on demonstrating the tubercle bacilli in pericardial fluid or pericardial tissue biopsy sample. Alternatively, histologically examination of pericardial biopsy specimen looking for the typical caseous necrosis due to TB may help establish the diagnosis. The currently available techniques for microscopy and culture to demonstrate acid and alcohol fast tubercle bacilli have disappointingly low sensitivity [63]. Culture, with better sensitivity, takes 2–6 weeks to confirm a positive microbial growth. Due to this low yield of the available tests for definitive diagnosis, majority of patients are treated empirically [64]. Ancillary tests using imaging and biochemical evaluations have proven useful in the management of TB pericarditis [65].
18F-FDG PET/CT is a useful supportive imaging modality for the diagnosis of pericarditis [66]. 18F-FDG accumulates in the inflamed pericardium in a manner proportional to the level of inflammation (Fig. 3). Tuberculosis causes intense inflammation leading to a high level of 18F-FDG uptake in the pericardium of patients with acute TB pericarditis. In a study comparing patterns of 18F-FDG PET/CT findings in patients with acute TB pericarditis versus idiopathic pericarditis, Dong and colleagues found significantly higher 18F-FDG uptake in the pericardium of patients with acute TB pericarditis versus idiopathic pericarditis (mean SUVmax of pericardium: 13.5 for TB pericarditis versus 3.0 for idiopathic pericarditis, p = 0.002) [67]. In TB pericarditis, the pericardium was thicker (5.1 mm versus 3.4 mm, p = 0.004) and there was associated lymphadenopathy which demonstrated significantly higher 18F-FDG uptake (mean SUVmax of lymph nodes: 5.3 versus 2.8 for TB pericarditis and idiopathic pericarditis, respectively, p < 0.001) compared with patients with idiopathic pericarditis. This study showed the potential utility of 18F-FDG PET/CT in differentiating between the two common causes of pericarditis with distinctively different treatments. Also, the study showed associated mediastinal and supraclavicular lymph node involvement with intense 18F-FDG uptake in TB pericarditis. Biopsy of these nodes for histological and/or microbiological assessment for TB involvement may provide an easier way to access tissue for disease confirmation especially since the analysis of pericardial fluid or pericardial biopsy specimen may fail to confirm the presence of the disease [63].
A 21-year-old HIV-infected male on antiretroviral therapy for 11 months with clinical symptoms suggestive of tuberculous disease (new-onset weight loss, fever, and night sweat) without associated cough. 18F-FDG PET/CT was obtained to evaluate for extra-pulmonary tuberculosis. Images show intense 18F-FDG uptake in thickened pericardium as well as in right supraclavicular nodes and conglomerate of anterior mediastinal nodes (arrows). Histological examination of the biopsy specimen of the right supraclavicular node confirmed tuberculosis. Symptoms resolved on treatment for extra-pulmonary tuberculosis (tuberculous pericarditis and lymphadenitis). In this case, 18F-FDG PET/CT findings support the clinical suspicion of extra-pulmonary TB, TB pericarditis in this case. Imaging findings also help guide biopsy for histological/microbiological confirmation from a peripheral site and avoiding a more invasive pericardial biopsy with its attendant complications
Among the infectious causes of pericarditis, TB appears to be the most severe with a mortality of up to 40% even with effective treatment in symptomatic PLHIV [68]. The intense 18F-FDG uptake seen in TB pericarditis is not specific to it. While TB is responsible for the majority of cases of pericarditis in PLHIV especially in TB-endemic regions of the world, infectious, inflammatory non-infectious, and neoplastic conditions which cause trapping of 18F-FDG in the pericardium are responsible for a minority of cases [69]. Neoplastic conditions that involve the pericardium, such as Kaposi sarcoma and lymphoma, also cause intense 18F-FDG uptake in the involved pericardium and associated lymphadenopathy [70]. These conditions, while less common causes of pericarditis than TB, need to be excluded. A neoplastic condition causing pericarditis may have associated mass lesion on imaging [70]. 18F-FDG PET/CT imaging offers supportive clues in the diagnosis of pericarditis, including prominent metabolically active mediastinal and supraclavicular nodes easily accessible for biopsy [71], and whole-body imaging that allows for detection of disease at other sites especially characteristic lung lesions of pulmonary TB.
Anti-tuberculous treatment (ATT) is the mainstay of treatment of TB. A subset of patients has residual TB disease after completing a standard course of ATT [72, 73]. The clinical utility of 18F-FDG PET/CT for assessing disease severity [74, 75], predict response to ATTT [76,77,78], and evaluate therapy response in the management of TB have been widely reported [79,80,81]. Successful ATT leads to a corresponding reduction in PET signal. In multiple case reports, there is a normalization of 18F-FDG PET signal in the pericardium in response to ATT and steroid therapy in patients treated for TB pericarditis [71, 82].
In summary, 18F-FDG PET/CT is useful in the diagnosis of pericarditis. Pattern of disease involvement seen on 18F-FDG PET/CT may help differentiate between TB pericarditis and idiopathic pericarditis, two common causes of pericarditis. 18F-FDG PET signal returns to normal after successful ATT. This makes 18F-FDG PET/CT useful for therapy response assessment. There are several etiological factors responsible for pericarditis in PLHIV, 18F-FDG PET/CT imaging can, unfortunately, not discriminate between these causes accurately enough to obviate the need for tissue diagnosis/microbiological confirmation.
Myocarditis
Myocarditis is characterized by inflammation of the heart muscle causing lymphocyte infiltration and necrosis of myocytes [83]. In the pre-ART era, myocarditis was seen in 52% of HIV-infected patients in an autopsy series [84]. The successful roll-out of ART has led to a significant reduction in opportunistic infection-related myocarditis. HIV infects the myocytes in patchy distributions [85]. In addition to HIV, several other opportunistic viruses, fungi, bacteria, and protozoa are responsible for myocarditis in PLHIV [86]. On histological assessment, there appears to be no difference in the morphology of myocarditis caused by different organisms or myocarditis in HIV-infected and HIV-uninfected people [87]. In patients with TB pericarditis, there is associated TB myocarditis in 53% of them, making Mycobacterium tuberculosis an important cause of myopericarditis in PLHIV [88].
Prompt and accurate diagnosis of myocarditis is challenging because symptoms and laboratory biomarkers are non-specific. Definitive diagnosis rest on demonstrating typical appearance on histological evaluation of endomyocardial biopsy (EMB) specimen. EMB is rarely done in the diagnostic evaluation of patients with suspected myocarditis. In a large study of 22,299 patients hospitalized with myocarditis, EMB was performed in 798 patients (3.6%) [89]. After adjusting for baseline characteristics and comorbidities, EMB was significantly associated with higher in-hospital mortality; longer hospital stay; and higher incidence of cardiac tamponade, ventricular tachycardia, acute renal failure, and cardiogenic shock [89]. The longer hospital-stay and higher in-hospital mortality reported in this study may be due to selection bias where patients with more severe disease had EMB compared with less sick patients who did not. The sensitivity of EMB in the diagnostic evaluation of myocarditis is low due to the patchy distribution of the disease. Imaging, therefore, plays a vital role in the diagnosis of the disease.
Cardiac magnetic resonance (CMR) is the diagnostic imaging of choice for the evaluation of myocarditis. In the acute setting, CMR assesses the hallmarks of acute inflammation including edema on T2-weighted imaging, hyperemia on early gadolinium enhancement, and myocytes necrosis on delayed gadolinium enhancement [90]. The use of each of these imaging features is not sufficiently sensitive for the diagnosis of acute myocarditis. The Lake Louise criteria (LLC) combine the three CMR features for the diagnosis of acute myocarditis. The imaging features used in LLC, while useful in differentiating healthy heart from diseases myocardium, are not specific for acute myocarditis. LCC has a lower diagnostic sensitivity for chronic myocarditis and acute myocarditis complicated by heart failure [91]. CMR is susceptible to image artifacts, and several methodological and technical problems may be encountered during image acquisition that may impact on its diagnostic performance [92]. Given these limitations of CMR, complementary imaging may be necessary for the evaluation of patients with suspected myocarditis.
18F-FDG PET/CT is a useful imaging modality in inflammation imaging. It is, however, important to emphasize here a limitation of 18F-FDG PET imaging of cardiac inflammation an infection, which is the intense physiologic uptake of F-FDG by the myocardium. The myocardium uses different metabolic substrates including glucose at different diseased and health states. It, therefore, traps 18F-FDG significantly. Intense uptake of 18F-FDG by the myocardium makes the study uninterpretable when imaging is performed for infection or inflammation indications. There is, therefore, a need for myocardial suppression procedure before 18F-FDG PET imaging of the heart. The most common method for myocardial suppression of glucose utilization (and by extension 18F-FDG utilization) is prolonged fasting for up to 16 h and dietary modification (high fat, low carbohydrate, and protein permitted diet) 1 day before the study [93]. Pharmacologic intervention using unfractionated heparin may also be explored for myocardial suppression of glucose utilization. Recently, Clément et al. [94] reported that a 1-week ketogenic diet provided a further decrease in myocardial 18F-FDG uptake and increase lesion detectability in an animal model of autoimmune myocarditis. This approach may have limited clinical applicability especially in the symptomatic patients where a prompt diagnosis is desired.
In a longitudinal study of a murine model of autoimmune myocarditis, Werner et al. showed a high 18F-FDG PET signal in the acute phase of myocarditis which showed a good correlation with the level of macrophages present at the site of myocardial inflammation [95]. 18F-FDG PET signal decreased at the subacute phase of the disease. This preclinical study showed that 18F-FDG uptake in acute myocarditis is proportional to the level of inflammation in the affected myocardium. There are limited studies in the literature that have reported the role of 18F-FDG PET/CT in the evaluation of myocarditis. Studies on 18F-FDG PET/CT imaging in PLHIV are even rarer. Pathological changes seen in myocarditis are similar between HIV-infected and HIV-uninfected people [87]. Experience reported on the use of 18F-FDG PET/CT in HIV-uninfected people may, therefore, be extrapolated to PLHIV. In a group of patients with different forms of myocarditis including acute and chronic forms of the disease and using EMB as the diagnostic gold standard, Ozawa et al. [96] reported a perfect sensitivity, specificity, positive predictive value, and negative predictive value of 100% each for 18F-FDG PET/CT in the detection of myocarditis if performed within the first 14 days of onset of symptoms. The diagnostic performance of 18F-FDG PET/CT dropped if imaging was performed beyond this time. These findings are in agreement with the preclinical study reporting the highest intensity of 18F-FDG PET signal in the acute phase of myocarditis with a signal reduction in the subacute phase of the disease [95]. Others have reported cases of myocarditis where 18F-FDG PET/CT imaging contributed to the diagnosis of the disease [97,98,99]. Following successful treatment or resolution of myocarditis, 18F-FDG PET signals return to normal [100]. This shows that 18F-FDG PET/CT may be useful as a tool for therapy response assessment.
The application of hybrid imaging with PET/MR is gaining popularity in clinical usage. The excellent soft tissue resolution and the well-characterized features of myocarditis available from CMR combined with the metabolic information available from 18F-FDG PET may offer superior diagnostic performance than either modality alone. Evidence is emerging, supporting the use of hybrid 18F-FDG PET/MR in the evaluation of patients with myocarditis [90, 101]. Initial cases reported on the utility of 18F-FDG PET/MR in the evaluation of patients with myocarditis have provided encouraging results with good concordance between the two imaging modalities [102,103,104]. In a prospective comparison of 18F-FDG PET and CMR in 55 patients with suspected myocarditis, 23 patients had CMR features suggestive of myocarditis while 18 patients had pathologic 18F-FDG uptake [105]. Six patients with pathologic CMR findings did not have corresponding 18F-FDG uptake while only one patient had pathologic 18F-FDG uptake without corresponding pathologic CMR finding. There was at least a good spatial agreement between pathologic findings on CMR finding versus pathologic 18F-FDG uptake. Using CMR findings as the reference gold standard, 18F-FDG PET had a sensitivity of 74%, a specificity of 97%, and a diagnostic accuracy of 87% [105].
Despite rigorous preparation for myocardial suppression, some patients may still have physiologic 18F-FDG uptake in the myocardium compromising image interpretation [105]. This has led to an attempt at investigating other radionuclide probes that target inflammation but have no physiologic uptake in the normal myocardium for use in molecular imaging of cardiac inflammation and infection. 11C-methionine is trapped by macrophages and can be used for inflammation imaging [106]. In a murine model of autoimmune myocarditis, Maya and colleagues show that the level of 11C-methionine correlates well with the level of macrophages in lesions [107]. There was a good correlation between the signal intensity of 11C-methionine and 18F-FDG. Exploration of this and other radiotracers without physiologic myocardial uptake (especially peptides labeled to radionuclide with wider availability than 11C) is warranted for clinical translation in radionuclide imaging of myocarditis.
In summary, molecular imaging with 18F-FDG PET/CT may be helpful in the diagnosis of myocarditis especially in the acute phase of the disease. With the increasing availability of PET/MR in routine clinical practice, this hybrid imaging technique may provide a better diagnostic accuracy in the assessment of myocarditis than either technique used alone. The need for myocardial suppression to prevent the physiologic uptake of 18F-FDG in the heart muscle is a limitation to the use of 18F-FDG PET/CT in the evaluation of myocarditis.
Infective endocarditis
Infective endocarditis (IE) is a relatively rare disease but one associated with high morbidity and mortality. There has been a steady decline in the incidence of IE among PLHIV in the ART era [108,109,110]. HIV itself has not been consistently shown to be an independent risk factor for IE. Certain factors prevalent among PLHIV increase their chances of acquiring infection of the native heart tissues or that of cardiac-related devices. In the developed countries, intravenous drug use is the single most important factor predisposing to IE among PLHIV with a higher incidence of IE seen in injection drug users (IDUs) who are HIV-infected compared with IDUs who are HIV negative [111]. Intravenous drug use is a less common mode of transmission of HIV in Sub-Saharan Africa compared with developed countries. Studies performed in developing countries where HIV prevalence is high have not shown HIV to be an independent risk factor for IE. In a cohort of 92 patients evaluated for probable IE in South Africa, only one patient was HIV positive [112]. In another study from the Democratic Republic of Congo, only 1.2% of 83 consecutive HIV-infected patients with heart disease had IE [113]. In the developing countries, the main risk factors for IE are rheumatic valvular heart disease, congenital heart disease, the presence of prosthetic valves, and history of infective endocarditis [112]. Skin abscess and bacteremia, which are common among PLHIV, may be additional risk factors for IE in them [114, 115].
The diagnosis of IE is not different between HIV-infected and HIV-uninfected patients. The diagnosis of IE is according to the modified Duke criteria which are based on suggestive clinical findings, echocardiography, and positive blood culture to isolate the causative organism [116]. The clinical presentation of IE does not appear to be different between HIV-infected and HIV-uninfected people [117, 118]. PLHIV is more likely to have IE which involves right-sided heart valves or a combination of right-sided and left-sided heart valves in contrast to HIV-uninfected people who have predominantly left-sided valvular involvement [117, 118]. Nonbacterial thrombotic endocarditis (also known as marantic endocarditis) has been described in patients with advanced HIV infection [119]. Marantic endocarditis is characterized by friable vegetations on the heart valves without infection and a high likelihood of distant embolization [119]. The absence of bacteremia and, consequently, a negative blood culture may impact on the diagnostic yield of the Duke criteria for the diagnosis of endocarditis in this setting. Despite this concern, no significant difference was reported in the diagnostic performance of the Duke criteria in HIV-infected IDUs compared with HIV-uninfected IDUs [120]. Among PLHIV, low CD4 counts < 50 cells/mm3 and high viral load (HIV RNA > 100,000 copies/mL), which connote severe immune suppression and high viremia, are predictive of poor IE treatment outcome [121].
In its most recent guidelines, the European Society of Cardiology included abnormal tracer uptake on radiolabeled leucocyte single-photon emission tomography and computed tomography (SPECT/CT) or 18F-FDG PET/CT as major criteria in its modification of the Duke criteria for the diagnosis of IE in patients with suspected prosthetic valve endocarditis [116]. Both radiolabeled leucocyte SPECT/CT and 18F-FDG PET/CT are useful adjuncts to echocardiography and blood culture in the diagnosis of prosthetic valve endocarditis (PVE), but less so in native valve endocarditis (NVE). To our knowledge, no studies have been done exclusively in PLHIV on the use of radionuclide techniques in the diagnosis of IE. Lessons learned from studies recruiting patients regardless of their HIV serostatus can safely be applied to the HIV-infected population. Comprehensive reviews of the diagnostic performances of radiolabeled leucocyte SPECT/CT and 18F-FDG PET/CT have been recently published [122, 123]. In the most recent meta-analysis, the pooled sensitivity of radiolabeled leucocytes scintigraphy and 18F-FDG PET/CT in patients with IE were 80% (95% CI 67–94) and 71% (95% CI 56–87), respectively [122]. The respective pooled specificity for radiolabeled leucocytes scintigraphy and 18F-FDG PET/CT in patients with suspected IE were 100% (95% CI 98–101) and 89% (95% CI 84–95). In patients with suspected cardiac implantable electronic device-related infection, the pooled sensitivity, and specificity were 85% (95% CI 77–93) and 91% (95% CI 87–96) for 18F-FDG PET/CT, respectively [122].
The application of radiolabeled leucocytes SPECT/CT is premised on leucocyte migration to the site of infection. This imaging technique provides a highly specific means of imaging cardiac and cardiac device-related infections as radiolabeled leucocytes do not accumulate to a significant extent at the site of sterile inflammation seen in the early postoperative period and as a physiologic reaction induced by prosthetic materials. Radiolabeling of leucocytes is technically demanding, requires highly skilled technicians, and involved the handling of blood with the attendant risk of staff exposure to blood-borne pathogens. In radiolabeled leucocytes SPECT/CT, imaging is acquired at 2 or 3-time-points over 24 for 99mTc-HMPAO-lebeled leucocytes and up to 48 h for 111In-oxine-labeled leucocytes scintigraphy.
18F-FDG PET/CT is a more commonly used radionuclide technique for IE imaging [124]. It does not involve blood handling, and imaging is completed within 2 hours. The better resolution of the PET system may make 18F-FDG PET/CT more sensitive for IE compared with radiolabeled leucocytes SPECT/CT. The most recent meta-analysis has, however, not confirmed this assumption [122]. 18F-FDG accumulates at the site of sterile inflammation, a drawback that may limit the utility of 18F-FDG PET/CT for IE imaging within 3 months after surgery. Prosthetic cardiac devices induce sterile inflammation causing increased physiologic 18F-FDG uptake around them [125]. A recent study did not find a significant reduction in physiologic 18F-FDG uptake around the prosthetic heart valve over 1-year postoperatively [126]. The study, however, proposed normality criteria to guide image interpretation.
Rheumatic heart disease (RHD) has since ceased to be of a public health concern in the developed countries but continues to be a leading cause of acquired heart disease contributing significantly to cardiac mortality and morbidity in developing countries [127, 128]. Rheumatic heart disease results from poorly treated group A Streptococcal pharyngitis in childhood that leads to autoimmune response characterized by damage of the heart valves and myocardium. RHD has a high predilection for the mitral valve causing mitral regurgitation and less commonly, mitral stenosis. Most recent studies in Africa show a low incidence of RHD among PLHIV [129,130,131], but when present may serve as a risk factor for IE [112]. 18F-FDG PET/CT is useful for unraveling the cause of fever in immunodeficiency states [132, 133]. Fever is the most frequent symptom of IE [116, 124]. In immunosuppressed patients presenting with fever of unknown origin where the cause of fever remains unknown after standard diagnostic work-up, 18F-FDG PET/CT helps identify IE occurring on the background of RHD, Fig. 4 [134]. A comparative study has reported the diagnostic performance of 18F-FDG PET/CT in 19 patients with acute RHD, 17 patients with chronic RHD, and 12 healthy controls. Diffuse 18F-FDG in the myocardium was seen in all but five patients with acute RHD [135]. The significance of this diffuse myocardial uptake is unknown, as no details regarding image interpretation were presented in the study. Further study regarding the role of 18F-FDG PET/CT in RHD is, therefore, needed.
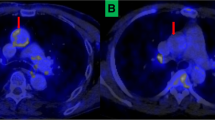
Images of a newly diagnosed HIV-infected female who had 18F-FDG PET/CT to identify the cause of fever of unknown origin. Images showed intense 18F-FDG uptake in the mitral valve and surrounding myocardium. The patient subsequently succumbed to her disease. Post-mortem analysis confirmed infective endocarditis on the background of rheumatic heart disease. These images demonstrate the potential utility of 18F-FDG PET/CT in the evaluation of endocarditis occurring on the background of rheumatic heart disease
In summary, molecular imaging using SPECT and PET techniques is increasingly being used for the diagnosis work-up of patients with suspected IE due to its accuracy in patients in whom diagnosis cannot be confirmed using the Duke criteria. Availability, especially in the developing countries, remains a limitation militating against a more widespread use of molecular imaging in the evaluation of endocarditis among PLHIV.
Conclusion
Molecular imaging using radionuclide techniques is increasingly showing higher utilization for characterizing and diagnosing cardiovascular inflammation and infection in PLHIV. Molecular imaging techniques, especially with 18F-FDG PET/CT, are useful for risk prediction, disease quantification, and for measuring the impact of intervention in subclinical atherosclerotic cardiovascular disease among PLHIV. The ability to risk-stratify patients may be helpful in the future to select patients who may benefit from primary prophylaxis against ASCVD. Inflammation is becoming a critical therapy target in ASCVD. The ability of molecular imaging to directly quantify lesion or arterial inflammation makes it a suitable tool for evaluating the effectiveness of new therapeutic agents targeting inflammation in ASCVD. PET and SPECT imaging techniques are essential tools in the evaluation of cardiac infection especially in suspected infective endocarditis where the diagnosis remains inconclusive after standard diagnostic work-up. PET and SPECT techniques are of clinical value to guide tissue biopsy for histological or microbiological confirmation of cardiac inflammation and infection especially since these conditions may have patchy distribution leading to sampling error when biopsy is done blindly without guidance to the sites of disease involvement. The prompt return to normalcy of metabolic changes before morphological restoration makes molecular techniques using radionuclide modalities for therapy response assessment preventing over or under-treatment and associated consequences. More studies are necessary to define better the roles of molecular imaging techniques in cardiovascular inflammation and infection among PLHIV. This call is likely to be answered soon as SPECT and PET techniques become increasingly available in developing countries where the greater proportion of PLHIV resides.
References
UNAIDS (2019) Global HIV & AIDS statistics—2019 fact sheet. https://www.unaids.org/en/resources/fact-sheet. Accessed 9 Feb 2020
Simon V, Ho DD, Abdool Karim Q (2006) HIV/AIDS epidemiology, pathogenesis, prevention, and treatment. Lancet 368:489–504. https://doi.org/10.1016/S0140-6736(06)69157-5
Cassol E, Malfeld S, Mahasha P, van der Merwe S, Cassol S, Seebregts C et al (2010) Persistent microbial translocation and immune activation in HIV-1-infected South Africans receiving combination antiretroviral therapy. J Infect Dis 202:723–733. https://doi.org/10.1086/655229
The Collaboration of Observational HIV Epidemiological Research Europe (COHERE) in Euro-Coord, Lewden C, Bouteloup V, De Wit S, Sabin C, Mocroft A et al (2012) All-cause mortality in treated HIV-infected adults with CD4 ≥500/mm3 compared with the general population: evidence from a large European observational cohort collaboration. Int J Epidemiol 41:433–445. https://doi.org/10.1093/ije/dyr164
Sabin CA (2013) Do people with HIV infection have a normal life expectancy in the era of combination antiretroviral therapy? BMC Med 11:251. https://doi.org/10.1186/1741-7015-11-251
Yang X, Su B, Zhang X, Liu Y, Wu H, Zhang T (2020) Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: challenges of immunological non-responders. J Leukoc Bio. https://doi.org/10.1002/JLB.4MR1019-189R
Perdomo-Celis F, Velilla PA, Taborda NA, Rugeles MT (2019) An altered cytotoxic program of CD8+ T-cells in HIV-infected patients despite HAART-induced viral suppression. PLoS ONE 14:e0210540. https://doi.org/10.1371/journal.pone.0210540
Nabatanzi R, Bayigga L, Cose S, Jones SR, Joloba M, Canderan G et al (2019) Monocyte dysfunction, activation and inflammation after long-term antiretroviral therapy in an African cohort. Clin Infect Dis 220:1414–1419. https://doi.org/10.1093/infdis/jiz320
Deeks SG (2011) HIV infection, inflammation, immunosenescence, and Aging. Annu Rev Med 62:141–155. https://doi.org/10.1146/annurev-med-042909-093756
Deeks SG (2009) HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ 3338:a3172. https://doi.org/10.1136/bmj.a3172
Ross R (1999) Atherosclerosis an inflammatory disease. N Engl J Med 340:115–126
Insull W (2009) The pathology of atherosclerosis: plaque development and plaque responses to medical treatment. Am J Med 122(Suppl):S3–S14. https://doi.org/10.1016/j.amjmed.2008.10.013
Li H, Cybulsky MI, Gimbrone MA Jr, Lippy P (1993) An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononuclear leukocyte adhesion molecule, in rabbit aortic endothelium. Arterioscler Thromb 13:197–204. https://doi.org/10.1161/01.atv.13.2.197
Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M et al (2001) A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest 107:1255–1262. https://doi.org/10.1172/JCI11871
Fan J, Watanabe T (2013) Inflammatory reactions in the pathogenesis of atherosclerosis. J Atheroscler Thromb 10:63–71. https://doi.org/10.5551/jat.10.63
Shah ASV, Stelzle D, Lee KK, Beck EJ, Alam S, Clifford S et al (2018) Global burden of atherosclerotic cardiovascular disease in people living with HIV. Circulation 138:1100–1112. https://doi.org/10.1161/CIRCULATIONAHA.117.033369
Titanji B, Gavegnano C, Hsue P, Schinazi R, Marconi VC (2020) Targeting inflammation to reduce atherosclerotic cardiovascular risk in people with HIV infection. J Am Heart Assoc 9:e014873. https://doi.org/10.1161/JAHA.119.014873
Hemkens LG, Bucher HC (2014) HIV infection and cardiovascular disease. Eur Heart J 35:1373–1381. https://doi.org/10.1093/eurheartj/eht528
Nguyen KA, Peer N, de Villiers A, Mukasa B, Matsha TE, Mills EJ et al (2017) Metabolic syndrome in people living with human immunodeficiency virus: an assessment of the prevalence and the agreement between diagnostic criteria. Inj J Endocrinol 2017:1613657. https://doi.org/10.1155/2017/1613657
Njuguna B, Kiplagat J, Bloomfield GS, Pastakia SD, Vedanthan R, Koethe JR (2018) Prevalence, risk factors, and pathophysiology of dysglycemia among people living with HIV in Sub-Saharan Africa. J Diabetes Res 2018:6916497. https://doi.org/10.1155/2018/6916497
Torriani FJ, Komarow L, Parker RA, Cotter BR, Currier JS, Dubé MP et al (5152s) Endothelial function in human immunodeficiency virus-infected antiretroviral-naïve subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) study 5152s. J Am Coll Cardiol 52:569–576. https://doi.org/10.1016/j.jacc.2008.04.049
Nabatanzi R, Bayigga L, Cose S, Jones SR, Joloba M, Canderan G et al (2019) Monocyte dysfunction, activation, and inflammation after long-term antiretroviral therapy in an African cohort. J Infect Dis 220:1414–1419. https://doi.org/10.1093/infdis/jiz320
Maisa A, Hearps AC, Angelovich TA, Pereira CF, Zhou J, Shi MDY et al (2015) Monocytes from HIV-infected individuals show impaired cholesterol efflux and increased foam cell formation after transendothelial migration. AIDS 29:1445–1457. https://doi.org/10.1097/QAD.0000000000000739
Hui DY (2003) Effects of HIV protease inhibitor therapy on lipid metabolism. Prog Lipid Res 42:81–92. https://doi.org/10.1016/s0163-7827(02)00046-2
Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC et al (2006) In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol 48:1818–1824. https://doi.org/10.1016/j.jacc.2006.05.076
Graebe M, Pedersen SF, Borgwardt L, Højgaard L, Sillesen H, Kjaer A (2009) Molecular pathology in vulnerable carotid plaques: correlation with [18]-fluorodeoxyglucose positron emission tomography (FDG-PET). Eur J Vasc Endovasc Surg 37:714–721. https://doi.org/10.1016/j.ejvs.2008.11.018
Rudd JHF, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N et al (2002) Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation 105:2708–2711. https://doi.org/10.1161/01.cir.0000020548.60110.76
Rominger A, Saam T, Wolpers S, Cyran CC, Schmidt M, Foerster S et al (2009) 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med 50:1611–1620. https://doi.org/10.2967/jnumed.109.065151
Figueroa AL, Abdelbaky A, Truong QA, Corsini E, MacNabb MH, Lavender ZR et al (2013) Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV event. JACC Cardiovasc Imaging 6:1250–1259. https://doi.org/10.1016/j.jcmg.2013.08.006
Schoepf IC, Buechel RR, Kovari H, Hammoud DA, Tarr PE (2019) Subclinical atherosclerosis imaging in people living with HIV. J Clin Med 8:1125. https://doi.org/10.3390/jcm8081125
Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayyakumar J et al (2012) (2012) Arterial inflammation in patients with HIV. JAMA 308:379–386. https://doi.org/10.1001/jama.2012.6698
Zanni MV, Toribio M, Robbins GK, Burdo TH, Lu MT, Ishai AE et al (2016) Effects of antiretroviral therapy on immune function and arterial inflammation in treatment-naïve patients with human immunodeficiency virus infection. JAMA Cardiol 1:474–480. https://doi.org/10.1001/jamacardio.2016.0846
Funderburg NT, Jiang Y, Debanne SM, Storer N, Danielle L, Clagett B et al (2014) Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clin Infect Dis 58:588–595. https://doi.org/10.1093/cid/cit748
Pirro M, Simental-Mendía LE, Bianconi V, Watts GF, Banach M, Sahebkar A (2019) Effect os statin therapy on arterial inflammation based on 18F-FDG PET/CT: a systematic review and meta-analysis of interventional studies. J Clin Med 8:118. https://doi.org/10.3390/jcm8010118
Tawakol A, Ishai A, Li D, Pakx RAP, Hur S, Kaiser Y et al (2017) Association of arterial and lymph node inflammation with distinct inflammatory pathways in human immunodeficiency virus infection. JAMA Cardiol 2:163–171. https://doi.org/10.1001/jamacardio.2016.4728
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C et al (2017) Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 377:1119–1131. https://doi.org/10.1056/NEJMoa1707914
Hsue PY, Li D, Ma Y, Ishai A, Manion M, Nahrendorf M et al (2018) IL-1β inhibition reduces atherosclerotic inflammation in HIV infection. J Am Coll Cardiol 72:2809–2810. https://doi.org/10.1016/j.jacc.2018.09.038
Tuzcu EM, Kapadia SR, Tutar E, Ziada KM, Hobbs RE, McCarthy PM et al (2001) High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: evidence from intravascular ultrasound. Circulation 103:2705–2710. https://doi.org/10.1161/01.cir.103.22.2705
Lawal IO, Ankrah AO, Popoola GO, Lengana T, Sathekge MM (2019) Arterial inflammation in young patients with human immunodeficiency virus infection: A cross-sectional study using F-18 FDG PET/CT. J Nucl Cardiol 26:1258–1265. https://doi.org/10.1007/s12350-018-1207-x
Boccara F, Lang S, Meuleman C, Ederhy S, Mary-Krause M, Costagliola D et al (2013) HIV and coronary heart disease: time for a better understanding. J Am Coll Cardiol 61:511–523. https://doi.org/10.1016/j.jacc.2012.06.063
Knudsen A, Hag AMF, Loft A, von Benzon E, Keller SH, Møller HJ et al (2015) HIV infection and arterial inflammation assessed by 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET): a prospective cross-sectional study. J Nucl Cardiol 22:372–380. https://doi.org/10.1007/s12350-014-0032-0
Lo J, Lu MT, Ihenachor EJ, Wei J, Looby SE, Fitch KV et al (2015) Effects of statin therapy on coronary artery plaque volume and high risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomized double-blind placebo-controlled trial. Lancet HIV 2:e52–e63. https://doi.org/10.1016/S2352-3018(14)00032-0
Yarasheski KE, Laciny E, Overton ET, Reeds DN, Harrod M, Baldwin S et al (2012) 18FDG PET-CT imaging detects arterial inflammation and early atherosclerosis in HIV-infected adults with cardiovascular disease risk factors. J Inflamm 9:22. https://doi.org/10.1186/1476-9255-9-26
Burg S, Dupas A, Stute S, Dieudonné A, Huet P, Le Guludec D et al (2013) Partial volume effect estimation and correction in the aortic vascular wall in PET imaging. Phys Med Biol 58:7527–7542. https://doi.org/10.1088/0031-9155/58/21/7527
Huet P, Burg S, Le Guludec D, Hyafil F, Buvat I (2015) Variability and uncertainty of 18F-FDG PET imaging protocols for assessing inflammation in atherosclerosis: suggestions for improvement. J Nucl Med 56:552–559. https://doi.org/10.2967/jnumed.114.142596
Toczek J, Wu J, Hillmer AT, Han J, Esterlis I, Cosgrove KP et al (2020) Accuracy of arterial [18F]-Fluorodeoxyglucose uptake quantification: A kinetic modeling study. J Nucl Cardiol. https://doi.org/10.1007/s12350-020-02055-x(Epub ahead of print on 10 February 2020)
Rudd JHF, Myers KS, Bansilal S, Machac J, Pinto CA, Tong C et al (2008) Atherosclerosis inflammation imaging with 18F-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med 49:871–878. https://doi.org/10.2967/jnumed.107.050294
Bucerius J, Hyafil F, Verberne HJ, Slart RHJA, Lindner O, Sciagra R et al (2016) Position paper of the Cardiovascular Committee of the European Association of Nuclear medicine (EANM) on PET imaging of atherosclerosis. Eur J Nucl Med Mol Imaging 43:780–792. https://doi.org/10.1007/s00259-015-3259-3
Lawal IO, Mokoala KG, Popoola GO, Lengana T, Ankrah AO, Stoltz AC et al (2019) Impact of optimized PET imaging conditions on 18F-FDG uptake quantification in patients with apparently normal aortas. J Nucl Cardiol. https://doi.org/10.1007/s12350-019-01833-6(Epub ahead of print on 06 August 2019)
Lawal IO, Ankrah AO, Stoltz AC, Sathekge MM (2019) Radionuclide imaging of inflammation in atherosclerotic vascular disease among people living with HIV infection: current practice and future perspective. Eur J Hybrid Imaging 3:5. https://doi.org/10.1186/s41824-019-0053-7
Zanni MV, Toribio M, Wilks MQ, Lu MT, Burdo TH, Walker J et al (2017) Application of a novel CD206+ macrophage-specific arterial imaging strategy in HIV-infected individuals. J Infect Dis 215:1264–1269. https://doi.org/10.1093/infdis/jix095
Stroup SP, Kane CJ, Farchschchi-Heydari S, James CM, Davis CH, Wallace AM et al (2012) Preoperative sentinel lymph node mapping of the prostate using PET/CT fusion imaging and Ga-68-labeled tilmanocept in an animal model. Clin Exp Metastasis 29:673–680. https://doi.org/10.1007/s10585-012-9498-9
Qin Z, Hoh CK, Hall DJ, Vera DR (2015) A tri-modal molecular imaging agent for sentinel lymph node mapping. Nucl Med Biol 42:917–922. https://doi.org/10.1016/j.nucmedbio.2015.07.011
Ntsekhe M, Mayosi BM (2013) Tuberculous pericarditis with and without HIV. Heart Fail Rev 18:367–373. https://doi.org/10.1007/s10741-012-9310-6
Heidenreich PA, Eisenberg MJ, Kee LL, Somelofski CA, Hollander H, Schiller NB et al (1995) Pericardial effusion in AIDS: incidence and survival. Circulation 92:3229–3234. https://doi.org/10.1161/01.CIR.92.11.3229
Thienemann F, Sliwa K, Rockstroh JK (2013) HIV and the heart: the impact of antiretroviral therapy: a global perspective. Eur Heart J 34:3538–3546. https://doi.org/10.1093/eurheartj/eht388
Ntsekhe M, Hakim J (2005) Impact of human immunodeficiency virus infection on cardiovascular disease in Africa. Circulation 112:3602–3607. https://doi.org/10.1161/CIRCULATIONAHA.105.549220
Pawlowski A, Jansson M, Sköld M, Rottenberg ME, Källenius G (2012) Tuberculosis and HIV co-infection. PLoS Pathog 8:e1002464. https://doi.org/10.1371/journal.ppat.1002464
Selwyn PA, Hartel D, Lewis VA, Schoenbaum EE, Vermund SH et al (1989) A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med 320:545–550. https://doi.org/10.1056/NEJM198903023200901
Aaron L, Saadoun D, Calatroni I, Launay O, Mémain N, Vincent V et al (2004) Tuberculosis in HIV-infected patients: a comprehensive review. Clin Microbiol Infect 10:388–398. https://doi.org/10.1111/j.1469-0691.2004.00758.x
Maartens G, Wilkinson RJ (2007) Tuberculosis. Lancet 370:2030–2043. https://doi.org/10.1016/S140-6736(07)61262-8
Gounden S, Perumal R, Magula NP (2018) Extrapulmonary tuberculosis in the setting of HIV hyperendemicity at a tertiary hospital in Durban, South Africa. South Afr J Infect Dis 33:57–64. https://doi.org/10.1080/23120053.2017.1403207
Isiguzo G, De Bruyn E, Howlett P, Ntsekhe M (2020) Diagnosis and management of tuberculous pericarditis: what is new? Curr Cardiol Rep 22:2. https://doi.org/10.1007/s11886-020-1254-1
Mayosi BM, Wiysonge CS, Ntsekhe M, Volmink JA, Gumedze F, Maartens G et al (2006) Clinical characteristics and initial management of patients with tuberculous pericarditis in the HIV era: the investigation of the management of pericarditis in Africa (IMPI Africa) registry. BMC Infect Dis 6:2. https://doi.org/10.1186/1471-2334-6-2
Alajaji W, Xu B, Sripariwuth A, Menon V, Kumar A, Schleicher M et al (2018) Noninvasive multimodality imaging for the diagnosis of constrictive pericarditis: a contemporary review. Circ Cardiovasc Imaging 11:e007878. https://doi.org/10.1161/CIRCIMAGING.118.007878
Lawal I, Sathekge M (2016) F-18 FDG PET/CT imaging of cardiac and vascular inflammation and infection. Brit Med Bullet 120:55–74. https://doi.org/10.1093/bmb/ldw035
Dong A, Dong H, Wang Y, Cheng C, Zuo C, Lu J (2013) 18F-FDG PET/CT in differentiating acute tuberculous from idiopathic pericarditis: preliminary study. Clin Nucl Med 38:e160–e165. https://doi.org/10.1097/RLU.0b013e31827a2537
Mayosi BM, Wiysonge CS, Ntsekhe M, Gumedze F, Volmink JA, Maartens G et al (2008) Mortality in patients treated for tuberculous pericarditis in Sub-Saharan Africa. S Afr Med J 98:36–40
Kim MS, Kim EK, Choi JY, Oh JK, Chang SA (2019) Clinical utility of [18F]FDG-PET /CT in pericardial disease. Curr Cardiol Rep 21:107. https://doi.org/10.1007/s11886-019-1193-x
James OG, Christensen JD, Wong TZ, Borges-Neto S, Koweek LM (2011) Utility of FDG PET/CT in inflammatory cardiovascular disease. Radiographics 31:1271–1286. https://doi.org/10.1148/rg.315105222
Testempassi E, Kubota K, Morooka M, Ito K, Masuda-Miyata Y, Yamashita H et al (2010) Constrictive tuberculous pericarditis diagnosed using 18F-fluorodeoxyglucose positron emission tomography: a report of two cases. Ann Nucl Med 24:421–425. https://doi.org/10.1007/s12149-010-0365-y
Lawal I, Fourie B, Mathebula M, Moagi I, Lengana T, Moeketsi N et al (2019) FDG-PET/CT as a non-invasive biomarker for assessing adequacy of treatment and predicting relapse in patients treated for pulmonary tuberculosis. J Nucl Med. https://doi.org/10.2967/jnumed.119.233783(Epub ahead of print on August 26, 2019)
Malherbe ST, Shenai S, Ronacher K, Loxton AG, Dolganov G, Kriel M et al (2016) Persisting positron tomography lesion activity and Mycobacterium tuberculosis mRNA after tuberculosis cure. Nat Med 22:1094–1100. https://doi.org/10.1038/nm.4177
Malherbe ST, Chen RY, Dupont P, Kant I, Kriel M, Loxton AG et al (2020) Quantitative 18F-FDG PET-CT scan characteristics correlate with tuberculosis treatment response. EJNMMI Res 10:8. https://doi.org/10.1186/s13550-020-0591-9
Malherbe ST, Dupont P, Kant I, Ahlers P, Kriel M, Loxton AG et al (2018) A semi-automatic technique to quantify complex tuberculous lung lesions on 18F-fluorodeoxyglucose positron emission tomography/computerized tomography images. EJNMMI Res 8:55. https://doi.org/10.1186/s13550-018-0411-7
Ankrah AO, van der Werf TS, de Vries EF, Dierckx RA, Sathekge MM, Glaudemans AW (2016) PET/CT imaging of Mycobacterium tuberculosis infection. Clin Transl Imaging 4:131–144. https://doi.org/10.1007/s40336-016-0164-0
Sathekge M, Maes A, Kgomo M, Stoltz A, Van de Wiele C (2011) Use of 18F-FDG PET to predict response to first-line tuberculostatics in HIV-associated tuberculosis. J Nucl Med 52:880–885. https://doi.org/10.2967/jnumed.110.083709
Sathekge M, Maes A, D’Asseler Y, Vorster M, Gongxeka H, Van de Wiele C (2012) Tuberculous lymphadenitis: FDG PET and CT findings in responsive and nonresponsive disease. Eur J Nucl Med Mol Imaging 39:1184–1190. https://doi.org/10.1007/s00259-012-2115-y
Lawal I, Zeevaart J, Ebenhan T, Ankrah A, Vorster M, Kruger HG, Govender T et al (2017) Metabolic imaging of infection. J Nucl Med 58:1727–1732. https://doi.org/10.2967/jnumed.117.191635
Sathekge MM, Ankrah AO, Lawal I, Vorster M (2018) Monitoring response to therapy. Semin Nucl Med 48:166–181. https://doi.org/10.1053/j.semnuclmed.2017.10.004
Dureja S, Sen IB, Acharya S (2014) Potential role of F-18 FDG PET-CT as an imaging biomarker for the noninvasive evaluation in uncomplicated skeletal tuberculosis: a prospective clinical observational study. Eur Spine J 23:2449–2454. https://doi.org/10.1007/s00586-014-3483.8
Ozmen O, Koksal D, Ozcan A, Tatci E, Gokcek A (2014) Decreased metabolic uptake in tuberculous pericarditis indicating response to antituberculous therapy on FDG PET/CT. Clin Nucl Med 39:917–919. https://doi.org/10.1097/RLU.0000000000000443
Pham TV, Torres M (2015) Human immunodeficiency virus infection-related heart disease. Emerg Med Clin N Am 33:613–622. https://doi.org/10.1016/j.em.2015.04.009
Anderson DW, Virmani R, Reilly JM, O’Leary T, Cunnion RE, Robinowitz M et al (1988) Prevalent myocarditis at necropsy in the acquired immunodeficiency syndrome. J Am Coll Cardiol 11:792–799. https://doi.org/10.1016/0735-1097(88)90213-6
Barbaro G (2001) Cardiovascular manifestations of HIV infection. J R Soc Med 94:348–390. https://doi.org/10.1177/014107680109400804
Bruno R, Socchi P, Filice G (2003) Overview on the incidence and the characteristics of HIV-related opportunistic infections and neoplasms of the heart: impact of highly active antiretroviral therapy. AIDS 17(Suppl 1):S83–S87. https://doi.org/10.1097/00002030-200304001-00012
Herskowitz A, Wu TC, Willoughby SB, Vlahov D, Ansari AA, Beschorner WE et al (1994) Myocarditis and cardiotropic viral infection associated with severe left ventricular dysfunction in late-stage infection with human immunodeficiency virus. J Am Coll Cardiol 24:1025–1032. https://doi.org/10.1016/0735-1097(94)90865-6
Syed FF, Ntsekhe M, Gumedze F, Badri M, Mayosi BM (2014) Myopericarditis in tuberculous pericardial effusion: prevalence, predictors and outcome. Heart 100:135–139. https://doi.org/10.1136/heartjnl-2013-304786
Elbadawi A, Elgendy IY, Ha LD, Mentias A, Ogunbayo GO, Tahir MW et al (2018) National trends and outcomes of endomyocardial biopsy for patients with myocarditis: from the National inpatient sample database. J Cardiac Fail 24:337–341. https://doi.org/10.1016/j.cardfail.2018.03.013
Chen W, Jeudy J (2019) Assessment of myocarditis: cardiac MR, PET/CT, or PET/MR? Curr Cardiol Rep 21:76. https://doi.org/10.1007/s11886-019-1158-0
Lurz P, Luecke C, Eitel I, Föhrenbach F, Frank C, Grothoff M et al (2016) Comprehensive cardiac magnetic resonance imaging in patients with suspected myocarditis: the MyoRacer Trial. J Am Coll Cardiol 67:1800–1811. https://doi.org/10.1016/j.jacc.2016.02.013
Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT et al (2009) Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol 53:1475–1487. https://doi.org/10.1016/j.jacc.2009.02.007
Chareonthaitawee P, Beanlands RS, Chen W, Dorbala S, Miller EJ, Murthy VL et al (2017) Joint SNMMI-ASNC expert consensus document on the role of 18F-FDG PET/CT in cardiac sarcoid detection and therapy monitoring. J Nucl Med 58:1341–1353. https://doi.org/10.2967/jnumed.117.196287
Clément A, Boutley H, Poussier S, Pieson J, Lhuillier M, Kolodziej A et al (2018) A 1-week extension of a ketogenic diet provides a further decrease in myocardial 18F-FDG uptake and a high detectability of myocarditis with FDG-PET. J Nucl Cardiol. https://doi.org/10.1007/s12350-018-1404-7(Epub ahead of print on August 20, 2018)
Werner RA, Wakabayashi H, Bauer J, Schütz C, Zechmeister C, Hayakawa N et al (2019) Longitudinal 18F-FDG PET imaging in a model of autoimmune myocarditis. Eur Heart J Cardiovasc Imaging 20:467–474. https://doi.org/10.1093/ehjci/jey119
Ozawa K, Funabashi N, Daimon M, Takaoka H, Takano H, Uehara M et al (2013) Determination of optimum periods between onset of suspected acute myocarditis and 18F-fluorodeoxyglucose positron emission tomography in the diagnosis of inflammatory left ventricular myocardium. Int J Cardiol 169:196–200. https://doi.org/10.1016/j.ijcard.2013.08.098
Takano H, Nakagawa K, Ishio N, Daimon M, Daimon M, Kobayashi Y et al (2008) Active myocarditis in a patient with chronic active Epstein-Barr virus infection. Int J Cardiol 130:e11–13. https://doi.org/10.1016/j.ijcard.2007.07.040
Tanimura M, Dohi K, Imanaka-Yoshida K, Omori T, Moriwaki K, Nakamori S et al (2017) Fulminant myocarditis with prolonged active lymphocytic infiltration after hemodynamic recovery. Int Heart J 58:294–297. https://doi.org/10.1536/ihj.16-225
Piriou N, Sassier J, Pallardy A, Serfaty JM, Trochu JN (2015) Utility of cardiac FDG-PET imaging coupled to magnetic resonance for the management of an acute myocarditis with non-informative endomyocardial biopsy. Eur Heart J Cardiovasc Imaging 16:574. https://doi.org/10.1093/ehjci/jeu319
Moeriwaki K, Dohi K, Omori T, Tanimura M, Sugiura E, Nakamori S et al (2017) A survival case of fulminant right-side dominant eosinophilic myocarditis. Int Heart J 58:459–462. https://doi.org/10.1536/ihj.16-338
Rischpler C, Woodard PK (2019) PET/MR imaging in cardiovascular imaging. PET Clin 14:233–244. https://doi.org/10.1016/j.cpet.2018.12.005
Abgral R, Dweck MR, Trivieri MG, Robson PM, Karakatsanis N, Mani V et al (2017) Clinical utility of combined FDG-PET/MR to assess myocardial disease. JACC Cardiovasc Imaging 10:594–597. https://doi.org/10.1016/j.jcmg.2016.02.029
von Olshausen G, Hyafil F, Langwieser N, Laugwitz KL, Schwaiger M, Ibrahim T (2014) Detection of acute inflammatory myocarditis in Epstein Barr virus infection using hybrid 18F-fluoro-deoxyglucose-positron emission tomography/magnetic resonance imaging. Circulation 130:925–926. https://doi.org/10.1161/CIRCULATIONAHA.114.011000
Nensa F, Poeppel TD, Krings P, Schlosser T (2014) Multiparametric assessment of myocarditis using simultaneous positron emission tomography/magnetic resonance imaging. Eur Heart J 35:2173. https://doi.org/10.1093/eurheartj/ehu086
Nensa F, Kloth J, Tezgah E, Poeppel TD, Heusch P, Goebel J et al (2018) Feasibility of FDG-PET in myocarditis: comparison to CMR using integrated PET/MRI. J Nucl Cardiol 25:785–794. https://doi.org/10.1007/s12350-016-0616-y
Oka S, Okudaira H, Ono M, Schuster DM, Goodman MM, Kawai K et al (2014) Differences in transport mechanisms of trans-1-amino-3-[18F]fluorocyclobutanecarboxylic acid in inflammation, prostate cancer, and glioma cells: comparison with L-[methyl-11C]methionine and 2-deoxy-2-[18f]fluoro-D-glucose. Mol Imaging Biol 16:322–329. https://doi.org/10.1007/s11307-013-0693-0
Maya Y, Werner RA, Schütz C, Wakabayashi H, Samnick S, Lapa C et al (2016) 11C-methionine PET of myocardial inflammation in a rat model of experimental autoimmune myocarditis. J Nucl Med 57:1985–1990. https://doi.org/10.2967/jnumed.116.174045
Muñoz-Moreno MF, Ryan P, Alvaro-Meca A, Valencia J, Tamayo E, Resino S (2019) National temporal trend analysis of infective endocarditis among patients infected with HIV in Spain (1997–2014): a retrospective study. J Clin Med 8:1167. https://doi.org/10.3390/jcm8081167
Bor DH, Woolhandler S, Nardin R, Brusch J, Himmelstein DU (2013) Infective endocarditis in the U.S., 1998–2009: a nationwide study. PLoS ONE 8:e60033. https://doi.org/10.1371/journal.pone.0060033
Polanco A, Itagaki S, Chiang Y, Chikwe J (2014) Changing prevalence, profile, and outcomes of patients with HIV undergoing cardiac surgery in the United States. Am Heart J 167:363–368. https://doi.org/10.1016/j.ahj.2013.09.021
Wilson LE, Thomas DL, Astemborski J, Freedman TL, Vlahov D (2002) Prospective study of infective endocarditis among injection drug users. J Infect Dis 185:1761–1766. https://doi.org/10.1086/340827
Koegelenberg CF, Doubell AF, Orth H, Reuter H (2003) Infective endocarditis in the Western Cape Province of South Africa: a three-year prospective study. QJM 93:217–225. https://doi.org/10.1093/qjmed/hcg028
Longo-Mbenza B, Tonduangu K, Muvova D, Phuati MB, Seghers KV, Kestelot H (1995) A clinical study of cardiac manifestations related to acquired immunodeficiency syndrome (AIDS) in Kinshasa [in French]. Arch Mal Coeur Vaiss 88:1437–1443
Spijkerman IJB, van Ameijden EJC, Mientjes GHC, Coutinho RA, van den Hoek A (1996) Human immunodeficiency virus infection and other risk factors for skin abscess and endocarditis among injection drug users. J Clin Epidemiol 49:1149–1154
Furuno JP, Johnson JK, Schweizer ML, Uche A, Stine OC, Shurland SM et al (2011) Community-associated methicillin-resistant Staphylococcus aureus bacteremia and endocarditis among HIV patients: a cohort study. BMC Infect Dis 11:298. https://doi.org/10.1186/1471-2334-11-298
Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Zotti FD et al (2015) 2015 ESC guidelines for the management of infective endocarditis. Eur Heart J 36:3075–3123. https://doi.org/10.1093/eurheartj/ehv319
Nel SH, Naidoo DP (2014) An echocardiographic study of infective endocarditis, with special reference to patients with HIV. Cardiovasc J Afr 25:50–57. https://doi.org/10.5830/CVJA-2013-084
Tsabedze N, Vachiat A, Zachariah D, Manga P (2018) A new face of cardiac emergencies: human immunodeficiency virus-related cardiac disease. Cardiol Clin 36:161–170. https://doi.org/10.1016/j.ccl.2017.09.005
Cammarosano C, Lewis W (1985) Cardiac lesions in acquired immune deficiency syndrome (AIDS). J Am Coll Cardiol. https://doi.org/10.1016/S0735-1097(85)80397-1
Cecchi E, Imazio M, Tidu M, Forno D, De Rosa FG, Dal Conte I et al (2007) Infective endocarditis in drug addicts: role of HIV infection and the diagnostic accuracy of Duke criteria. J Cardiovasc Med 8:169–175. https://doi.org/10.2459/01.JCM.0000260824.14596.86
Gebo KA, Burkey MD, Lucas GM, Moore RD, Wilson LE (2006) Incidence of, risk factors for, clinical presentation, and 1-year outcomes of infective endocarditis in an urban HIV cohort. J Acquir Immune Defic Syndr 43:426–432. https://doi.org/10.1097/01.qai.0000243120.67529.78
Cantoni V, Sollini M, Green R, Berchiolli R, Lazzeri E, Mannarino T et al (2018) Comprehensive meta-analysis on [18F] FDG PET/CT and radiolabeled leucocyte SPECT-SPECT/CT imaging in infectious endocarditis and cardiovascular electronic device infections. Clin Transl Imaging 6:3–18. https://doi.org/10.1007/s40336-018-0265-z
Gomes A, Glaudemans AWJM, Touw DJ, van Melle JP, Willems TP, Maass AH et al (2017) Diagnostic value of imaging in infective endocarditis: a systematic review. Lancet Infect Dis 17:e1–e14. https://doi.org/10.1016/S1473-3099(16)30141-4
Habib G, Erba PA, Iung B, Donal E, Cosyns B, Leroche C et al (2019) Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: a prospective cohort study. Eur Heart J 40:3222–3233. https://doi.org/10.1093/eurheartj/ehz620
Mathieu C, Mikaïl N, Benali K, Iung B, Duval X, Nataf P et al (2017) Characterization of 18F-fluorodeoxyglucose uptake pattern in noninfected prosthetic heart valves. Circ Cardiovasc Imaging 10:e005585. https://doi.org/10.1161/CIRCIMAGING.116.005585
Roque A, Pizzi MN, Fernández-Hidalgo N, Permanyer E, Cuellar-Calabria H, Romero-Farina G et al (2020) Morpho-metabolic post-surgical patterns of non-infected prosthetic heart valves by [18F]FDG PET/CTA: “normality” is a possible diagnosis. Eur Heart J Cardiovasc Imaging 21:24–33. https://doi.org/10.1093/ehjci/jez222
Essop MR, Nkomo VT (2005) Rheumatic and nonrheumatic valvular heart disease: epidemiology, management, and prevention in Africa. Circulation 112:3584–3591. https://doi.org/10.1161/CIRCULATIONAHA.105.539775
Curry C, Zuhlke L, Mocumbi A, Kennedy N (2018) Acquired heart disease in low-income and middle-income countries. Arch Dis Child 103:73–77. https://doi.org/10.1136/archdischild-2016-312521
Manafe N, Ngale A, Biquiza N, Zimba I, Majid N, Mocumbi AO (2019) Need for active cardiovascular screening in HIV-infected children under antiretroviral therapy in Africa. Cardiovasc Diagn Ther 9:68–72. https://doi.org/10.21037/cdt.2018.09.18
Hovis IW, Namuyonga J, Kisitu GP, Ndagire E, Okello E, Longenecker CT et al (2019) Decreased prevalence of rheumatic heart disease confirmed among HIV-positive youth. Pediatr Infect Dis J 38:406–409. https://doi.org/10.1097/INF.0000000000002161
Gleason B, Mirembe G, Namuyonga J, Okello E, Lwabi P, Lubega I et al (2016) Prevalence of latent rheumatic heart disease among HIV-infected children in Kampala, Uganda. J Acquir Immune Defic Syndr 71:196–199. https://doi.org/10.1097/PHH.0000000000000419
Martin C, Castaigne C, Tondeur M, Flamen P, De Wit S (2013) Role and interpretation of fluorodeoxyglucose-positron emission tomography/computed tomography in HIV-infected patients with fever of unknown origin: a prospective study. HIV Med 14:455–462. https://doi.org/10.1111/hiv.12030
Lawal IO, Popoola GO, Lengana T, Ankrah AO, Ebenhan T, Sathekge MM (2019) Diagnostic utility of 18F-FDG PET/CT in fever of unknown origin among patients with end-stage renal disease treated with renal replacement therapy. Hell J Nucl Med 22:70–75. https://doi.org/10.1967/s002449910962
Sathekge M, Stoltz A, Gheysens O (2015) Rheumatic fever: a forgotten but still existing cause of fever of unknown origin detected on FDG PET/CT. Clin Nucl Med 40:250–252. https://doi.org/10.1097/RLU.0000000000000619
Nagesh CM, Saxena A, Patel C, Karunanithi S, Nadig M, Malhotra A (2015) The role of 18F fluorodeoxyglucose positron emission tomography (18F-FDG-PET) in children with rheumatic carditis and chronic rheumatic heart disease. Nucl Med Rev Cent East Eur 18:25–28. https://doi.org/10.5603/NMR.2015.0006
Funding
No external funding was received in the course of this work.
Author information
Authors and Affiliations
Contributions
IO Lawal: literature search, literature review, content planning, writing, and editing. AC Stoltz: content planning, editing, and critical review. MM Sathekge: literature review, content planning, writing, editing, and critical review.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This expert review does not contain any studies with human subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lawal, I.O., Stoltz, A.C. & Sathekge, M.M. Molecular imaging of cardiovascular inflammation and infection in people living with HIV infection. Clin Transl Imaging 8, 141–155 (2020). https://doi.org/10.1007/s40336-020-00370-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-020-00370-4