Abstract
Positron emission tomography (PET) has several clinical applications in cardiovascular imaging. Myocardial perfusion imaging with PET allows accurate global and regional measurements of myocardial perfusion, myocardial blood flow and function at stress and rest in a single examination. Simultaneous assessment of function and perfusion by PET with quantitative software is currently routine practice. Combination of ejection fraction reserve with perfusion information may improve the identification of severe coronary artery disease. Myocardial viability can be estimated by quantitative comparison of fluorine-18 fluorodeoxyglucose (18F-FDG) and rest perfusion imaging. Quantitative myocardial blood flow and myocardial flow reserve measurements are becoming routinely included in clinical assessments due to the enhanced dynamic imaging capabilities of the latest PET/CT scanners. Absolute flow measurements allow evaluation of coronary microvascular dysfunction and provide additional prognostic and diagnostic information in coronary disease. Quantitative clinical software tools for computing myocardial blood flow from kinetic PET data in an automated and rapid fashion have been developed for use with several radiotracers: 13N-ammonia, 15O-water and 82Rb. The level of agreement between various software methods available for such analyses is excellent. Relative quantification of 82Rb PET myocardial perfusion tracer uptake, based on comparisons with normal perfusion databases, demonstrates high performance for the detection of obstructive coronary disease. Computerized analysis of myocardial perfusion at stress and rest reduces the variability of the assessment compared with visual analysis. New tracers, such as 18F-flurpiridaz may allow further improvements in myocardial blood flow quantification and disease detection. PET quantification can be enhanced by precise coregistration with CT angiography. In emerging clinical applications, the potential to identify vulnerable plaques by quantification of atherosclerotic plaque uptake of 18FDG and 18F-sodium fluoride tracers in carotids, aorta and coronary arteries has been demonstrated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Positron emission tomography (PET) allows accurate measurement of relative myocardial hypoperfusion, absolute myocardial blood flow and contractile function at stress and rest, in a single scanning session, allowing diagnosis of coronary artery disease (CAD) [1] and evaluation of long-term prognosis [2]. PET is considered the gold standard for measuring myocardial blood flow [3, 4] and it is also an important tool in assessment of the viability of the myocardium [5]. New cardiac PET imaging techniques are being developed to investigate plaque inflammation in the cardiovascular system [6]. The multitude of information that can be obtained from PET images necessitates the use of advanced quantitative techniques. Some parameters, such as absolute myocardial blood flow measurements, cannot be obtained by visual analysis alone. In addition, quantitative or semi-quantitative analysis of PET data, compared with visual interpretation, allows reduced inter- and intra-observer variability.
Software quantification methods for cardiac PET have evolved recently, and they allow rapid and largely automated extraction of various physiological parameters. Typically, a PET quantification software package allows integrated analysis of static, gated and dynamic datasets in a single session, facilitating inclusion of all quantification results in the clinical review. These various image datasets can be obtained from a single PET acquisition of the list-mode data—this is typically possible with the latest scanners. Static images are used to define the extent and severity of regional hypoperfusion at stress and rest. Viability imaging performed with a glucose analog—fluorine-18 flurodeoxyglucose (18F-FDG)—makes it possible to define the amount of myocardial scar (no uptake of 18F-FDG or perfusion tracer) and mismatch (uptake of 18F-FDG but no myocardial perfusion uptake). Gated PET images allow automated computation of left ventricular volumes, ejection fraction (EF) and other functional parameters, such as diastolic function. Finally, dynamic reconstruction of the data allows extraction of the time-activity curves from the blood pool and myocardium, and computation of absolute regional myocardial blood flow in ml/g/min at stress and rest and consequently myocardial flow reserve (MFR)—defined as the ratio of myocardial blood flow at stress and rest. These computations can be obtained within seconds, with minimal user interaction.
This review describes state-of-the-art PET quantification techniques, focusing particularly on applications currently in clinical use and summarizing their role in cardiovascular disease detection and management.
Relative quantification of perfusion and ischemia
Positron emission tomography myocardial perfusion is recommended for diagnosis or risk stratification of patients who have non-diagnostic results on other imaging tests [7]. Cardiac PET/CT, through analysis of perfusion changes between stress and rest (ischemia) and comparison of perfusion studies with normal limits derived from images of subjects with low likelihood of disease and normal perfusion findings, allows detection of obstructive CAD [8–10]. The relative quantification technique based on normal limits is widely used in single-photon emission computed tomography (SPECT) and has been adapted to PET imaging. Typically, an integrated score combining hypoperfusion severity and extent is derived using the polar map concept. Quantitative analysis of relative hypoperfusion has been shown to rival the accuracy of expert readers in the diagnosis of CAD by SPECT in large studies [11]. The key advantage of such quantitative analysis is its improved reproducibility compared with visual assessment [12].
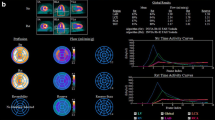
In previous reports of cardiac PET diagnostic performance for CAD detection, the average sensitivity for detecting at least one coronary artery with significant stenosis was 89 % (range 83–100 %), and the average specificity was 89 % (range 73–100 %) [13], even though it is accepted that there is no 1-to-1 relationship between obstructive disease and ischemic stenosis [14]. Most of the early PET perfusion studies were obtained with stand-alone 2D PET scanners rather than with integrated PET/CT systems and utilized visual scoring of perfusion defects in a 17-segment model, which is analogous to SPECT perfusion scoring. Although automated relative quantification was initially developed for SPECT imaging, several recent studies report the use and performance of this method in PET/CT imaging. To date, three studies have reported 82Rb PET/CT diagnostic performance with quantitative analysis based on comparison with normal limits (Fig. 1) [9, 10, 15]. Kaster et al. [15] achieved perfect 100 % sensitivity for the detection of obstructive CAD when transient ischemic dilation results were integrated with perfusion results. Figure 2 shows examples of perfusion images and quantification results based on normal limits and the direct stress-rest comparison for 82Rb. Although separate normal limits are typically required for males and females in SPECT quantification, relative PET perfusion quantification methods use combined-gender normal limits; this is possible owing to the accurate CT attenuation correction available on PET/CT scanners [9, 10, 15]. It should be noted that due to variable characteristics of normal perfusion distribution for different tracers, for example reduced isotope concentration in the lateral wall on 13N-ammonia imaging [16], relative analysis of PET perfusion will require isotope-specific normal limits.
Relative quantification accuracy. Results from three recent manuscripts by Santana et al. [9] (n = 76), Nakazato et al. [10] (n = 167), and Kaster et al. [15] (n = 110), validating the use of relative quantification with normal limits in myocardial perfusion PET studies for detection of coronary artery disease by three separate software tools (color figure online)
An example of relative quantification for myocardial perfusion PET. Co-registered stress and rest images with contours are shown on the left. The change images (CHANGE) in the middle show the ischemic region in the septal wall. Polar maps (right) show corresponding significant defect at stress. The quantification results were stress total perfusion deficit (TPD) = 10 %, rest TPD = 4 %, ischemia by change analysis = 5 %. Ejection fraction was normal. This patient had significant >70 % lesion in the proximal left anterior descending territory as confirmed by angiographic examination (color figure online)
Quantification of heart contractile function
The quantitative analysis of myocardial contractile function has been extensively validated for myocardial SPECT [17], but such quantitative methods can be also applied to PET. A typical contemporary protocol for PET imaging includes list-mode data acquisition at stress and rest. This allows retrospective reformatting and re-binning with cardiac gating and subsequently derivation of gated data. An excellent correlation of MRI and PET 18F-FDG imaging techniques for quantification of EF as well as systolic and diastolic volumes has been reported [18, 19]. Compared with SPECT images, PET images exhibit higher resolution and better definition of the mitral valve plane due to accurate attenuation correction [10]. This allows more accurate detection of the myocardium and improved tracking of the mitral valve plane. High PET image resolution and 18F-FDG uptake in the right ventricle may also allow quantification of right ventricular function by PET [20].
PET contractile function analysis can be used in conjunction with perfusion analysis to improve patient risk stratification. In a study including 1441 patients, stress EF measured from 82Rb PET provided an independent and incremental prognostic value compared with perfusion measures [21]. Functional measurements from PET may also include phase and dyssynchrony analysis. Although these techniques have not been extensively validated with PET, measurements of histogram bandwidth and phase standard deviation from 15O-water and 18F-FDG-PET scans were found to be very well correlated with SPECT [22]. Recently, in an analysis of 486 patients, left ventricular mechanical dyssynchrony was found to be an independent predictor of all-cause mortality in ischemic cardiomyopathy [23]. Furthermore, an analysis of dyssynchrony obtained from gated 18F-FDG-PET imaging was shown to identify responders to cardiac resynchronization therapy [24]. Another quantitative parameter related to contractile function, first described and validated for SPECT, is the transient ischemic dilation of the left ventricle. This measure has been found to improve the identification of patients with severe CAD by SPECT [25]. Rischpler et al. [26] demonstrated the use of the transient ischemic dilation ratio in 82Rb PET imaging as an independent predictor of mortality.
A key advantage of PET perfusion imaging over SPECT perfusion imaging (wherein images are typically acquired at least 15 min after injection) is the possibility of performing immediate imaging of heart contractile function during stress. Thus, with PET imaging, it is possible to assess the true peak stress function. In contrast, with SPECT, given that scans are typically performed 15–45 min after stress, only post-ischemic changes due to myocardial stunning can be observed. Therefore, PET imaging allows quantification of the EF reserve—defined as the change in EF between stress and rest. In 510 patients, Dorbala et al. [27] showed that the EF reserve obtained with 82Rb PET imaging was the only independent predictor of left main and triple-vessel disease. A very high negative predictive value (97 %) for excluding severe left main and triple-vessel disease was demonstrated if the EF reserve was higher than 5 %. The same group has also shown that left ventricular EF reserve has incremental prognostic value over clinical variables and resting EF obtained from 82Rb PET [28]. Recently, the use of EF reserve quantification was evaluated for the regadenoson stress agent and 82Rb PET imaging. The mean left ventricular EF reserve was shown to be significantly higher in patients with normal myocardial perfusion imaging results (6.5 ± 5.4 %) than in those with mild (4.3 ± 5.1 %, p = 0.03) and moderate-to-severe reversible defects (−0.2 ± 8.4 %, p = 0.001). Also, the mean left ventricular EF reserve was significantly higher in patients with a low likelihood of CAD (7.2 ± 4.5 %, p < 0.0001) [29].
Myocardial blood flow and myocardial flow reserve quantification
Myocardial blood flow and MFR measurements are becoming routinely included in clinical assessments due to the enhanced dynamic imaging capabilities of the latest PET/CT scanners. PET allows non-invasive measurements in units of milliliters of blood per minute, per gram of myocardium. These absolute measurements have been validated against invasive flow estimates obtained by microsphere techniques in numerous studies. PET is currently the most validated imaging technique for the quantitative evaluation of myocardial blood flow.
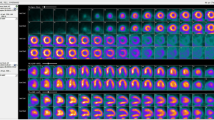
New software tools are now becoming available that will allow more widespread quantification of myocardial blood flow at stress and rest. These tools include kinetic models and algorithms for computing myocardial blood flow and MFR from kinetic PET data obtained using different radiotracers: 13N ammonia [30, 31], 15O-water [32] and 82Rb [33]. Current software tools utilizing these algorithms allow completely automated or semi-automated measurements of stress and rest flow. Figure 3 shows screenshots of the three implementations of recent clinical software for computation of myocardial blood flow and MFR. The input function is typically obtained from the first-pass blood pool analysis in the left ventricle or left atrium. Segmentation of the left ventricle and determination of the blood pool region can be accomplished automatically. Patient motion correction can also be applied to improve the robustness of the analysis. The computations are rapid, typically being accomplished within seconds. Both global and regional (or segmental) myocardial blood flow and MFR measurements are obtained, by kinetic modeling techniques, assuming the appropriate compartmental model, which is specific to a given radiotracer. The inter-scan reproducibility of software methods for absolute flow measurements has been reported to be in the order of 15 % [34]. The quantitative agreement between different software methods available for such analyses is also very good, as recently evaluated for 82Rb [35] and 13N-ammonia [36] models (Fig. 4). Clinically, 82Rb PET blood flow measurements have the widest application, since 82Rb does not require a cyclotron on site. Although the 82Rb is not a tracer with ideal imaging properties—for example it has a lower extraction fraction than other tracers—the 82Rb flow measurements have been well validated and calibrated to both 13N-ammonia [33] and 15O-water [37] measurements.
Results from different implementations of the absolute flow quantification methods demonstrating regional and segmental quantification of rest and stress myocardial blood flow and myocardial flow reserve. Cedars Sinai QPET (top left) PMOD software (top right) and Siemens syngoMBF flow analysis (bottom) are shown (color figure online)
Agreement for the software packages on measurements of myocardial blood flow and myocardial flow reserve. 3D scatterplots of syngoMBF vs FlowQuant vs QPET values of rest and stress myocardial blood flow and myocardial flow reserve for global left ventricle (a, b) and regional vascular territories (c–h). R 2 is the total variance described by 3D unit basis vector (V) line of best fit. SEE is the standard deviation of residual errors from regression line of best fit for each of the three programs. Reproduced with permission from deKemp et al. [35] (color figure online)
Absolute flow measurements and MFR estimation allow evaluation of several clinically important cardiovascular conditions. Flow measurements at stress and rest provide an integrated measure of the ischemic burden due to microvascular and macrovascular disease [38]. Myocardial flow measurements at stress may potentially allow identification of endothelial dysfunction in early coronary disease [38]. In addition, resting myocardial flow heterogeneity and in particular gradient of flow reduction from the base to the apex of the left ventricle has been demonstrated to be a potential early marker of sub-clinical CAD [39]. The presence of a normal MFR can also confirm adequate pharmacological response in the absence of disease [40]. On the other hand, an MFR value <1 could be a hallmark of coronary steal, which is a dangerous clinical condition [41, 42]. Therefore, these quantitative measurements provide powerful additional prognostic and diagnostic information, which could be utilized clinically. Figure 5 shows an example of severely reduced MFR in all the territories observed in microvascular disease. Relative quantification shows only a small abnormality in this case. Patients in whom this condition is diagnosed by quantification of MFR could benefit from aggressive lipid-lowering therapy [43].
Example of severely reduced myocardial flow reserve (1.17–1.37) and reduced myocardial stress flow (0.83–0.77 ml/g/min) in all vascular territories in a patient with syndrome X (left). This patient had a borderline abnormal relative perfusion scan with mildly decreased uptake at stress in the part of the inferior wall (right) (color figure online)
Currently, diagnoses of significant, obstructive CAD by PET are typically achieved by visual or quantitative assessment of the relative hypoperfusion at stress and rest rather than by absolute flow quantification. Nevertheless, myocardial flow measurements have also been shown to be useful in the diagnosis of CAD, especially in the detection of multivessel CAD. It has been reported that quantification of 82Rb net retention, to measure the stress-rest blood flow difference in the myocardium, defined a greater extent of disease than did standard quantification based on comparisons of the relative stress-rest perfusion difference [44]. Ziadi et al. [45] studied the incremental value of absolute MFR over standard relative quantification in 120 patients undergoing rest/stress 82Rb imaging. They found that the MFR defined by PET was an independent predictor of triple-vessel CAD and provided added value to relative quantification by summed stress scoring. In a related study by Fiechter et al. [46], absolute measurements of MFR were found to increase the per-patient diagnostic accuracy of 13N-ammonia from 79 to 92 % in 73 patients. Figure 6 shows an example of a PET 82Rb scan with angiographically confirmed triple-vessel disease in which only two territories are identified as abnormal by relative quantification. These preliminary studies reporting increased diagnostic accuracy for detection of coronary disease with absolute flow measurements when combined with relative quantification will need to be confirmed in larger cohorts, since reduced absolute MFR measurements could be caused by factors other than CAD, or could be related to non-obstructive disease [38], thus reducing the specificity for the detection of the obstructive disease.
Example of added value of myocardial flow reserve (MFR) over ischemia quantification for diagnosis from 82Rb PET. Relative quantification of total perfusion deficit at stress and rest shows stress and ischemic abnormalities in left anterior descending and left circumflex territories but normal right coronary artery territory (a), while MFR is definitely abnormal in all vascular territories (0.76–1.04), with no flow reserve (MFR = 1.04) in the right coronary region (b). This case was angiographically confirmed as triple vessel disease (color figure online)
Quantification of MFR by PET has also recently been established as an important prognostic tool. MFR was shown to improve patient risk assessment compared with relative perfusion (summed stress scoring), and EF quantification alone [2]. Murthy et al., in a study of 2,782 patients, showed that the incorporation of MFR into cardiac death risk assessment resulted in correct reclassification of almost 35 % of intermediate-risk patients and a significant increase in c-index (from 0.82 to 0.84). Figure 7 shows the incremental prognostic value of MFR over ischemia measures. Based on this evidence, quantitative absolute myocardial flow measurements can be used to improve patient mortality risk stratification in addition to other PET measures, such as relative perfusion abnormality or EF.
Incremental prognostic value of myocardial flow reserve (MFR) over ischemia measures. Unadjusted annualized cardiac mortality by tertiles of MFR and by categories of myocardial ischemia. The annual rate of cardiac death increased with increasing summed stress score and decreasing MFR. Importantly, lower MFR consistently identified higher-risk patients at every level of myocardial scar/ischemia, also among those with visually normal positron emission tomography scans and normal left ventricular function. Reproduced with permission from Murthy et al. [2]
The practicality of the routine clinical imaging with PET is challenged by the need for an on-site cyclotron (in the case of 13N-ammonia and 15O-water imaging), or an on-site generator (in the case of 82Rb imaging). In addition, 82Rb does not have optimal imaging characteristics, due to its long positron range and low extraction fraction. A new imaging tracer, 18F-flurpiridaz, has recently been proposed for cardiac PET imaging [47]. This tracer exhibits high image contrast in the myocardium, and prolonged retention and superior extraction compared with SPECT tracers (Tc-99m or Tl-201) or 82Rb PET. Cardiac PET imaging with 18F-flurpiridaz could be performed without a cyclotron on site, because of the relatively long half-life of this tracer. Although the longer half-life of 18F-flurpiridaz may result in higher patient doses compared with doses for 82Rb or 13N-ammonia PET, the reported doses are nevertheless less than 6.4 mSv for the full rest/stress study [48]. New myocardial blood flow quantification models have been proposed and validated for 18F-flurpiridaz. Sherif et al. [49] showed that the superior imaging characteristics of this tracer enable estimation of absolute flow values by simplified analysis of retention and standardized uptake values (SUVs). These properties of 18F-flurpiridaz open up new opportunities in the use of absolute quantification since SUV computations do not require the measurement of the myocardial input function and thus would allow a treadmill exercise regimen outside of the scanner. Such simplified techniques may lead to a wider acceptance of the quantitative models. Furthermore, the high resolution of 18F-flurpiridaz images may allow improved quantitative accuracy and may warrant the use of sophisticated techniques eliminating cardiac [50] and patient motion [51] to provide optimal quantitative results. In initial validation of 18F-flurpiridaz, its diagnostic accuracy was reported to be higher than that of SPECT with visual scoring of static images in a phase II trial [48]; however, clinical application of this tracer for myocardial blood flow quantification has not yet been reported.
Relative quantification of myocardial viability
Positron emission tomography has been considered the most sensitive method and the “gold standard” for assessment of myocardial viability [5, 52, 53]. In a simple static protocol, which has been accepted clinically, relative uptake of 18FDG is measured and compared with perfusion at rest. PET viability imaging is used to identify patients with a significant amount of viable recoverable myocardium and can predict functional recovery [54]. The amount of viable myocardium has long-term prognostic implications [55–58]. Patients with viable myocardium will be more likely to benefit from revascularization than those who do not show such viable tissue. Typically, 18F-FDG viability imaging is combined with a resting PET perfusion scan (using 82Rb or 13N-ammonia tracer) or even a resting SPECT scan. Regions which are hypoperfused at rest but are viable (have some 18F-FDG uptake) are defined by combined assessment of both scans. If a certain degree of such mismatch between perfusion and viability is detected, it indicates that the patient may benefit from a revascularization procedure. Currently the usual clinical method for detecting such viability patterns is visual assessment of the mismatch between static images of the resting perfusion defect and the static 18F-FDG-PET scan. However, visual methods for the identification of mismatch suffer from the usual problems of inter- and intra-observer variability. If a specific threshold is required for the decision on the intervention, automated quantitative techniques should be considered to ensure objective patient management. To this end, several relative quantification techniques which automatically compute the amount of mismatch and scar have been developed [17, 57, 59, 60].
In the first report on quantitative viability analysis, Beanlands et al. [59] proposed mismatch and scar scores derived from combined perfusion and viability polar maps. These parameters combine extent and severity of the defects. The Cedars-Sinai group has developed an integrated analysis of perfusion and viability in their software. Viability quantification is accomplished as follows: 3D left ventricular contours derived from resting PET or SPECT scans and 18F-FDG-PET images are co-registered in 3D to correct for rotational and positional errors between the two scans, using methods developed for alignment of stress/rest image pairs [61]. Subsequently, the contours derived from the rest perfusion data are applied to the spatially co-registered 18F-FDG images. Quantification of the viability scores (mismatch and scar regions) is performed after direct image count normalization between the perfusion and viability scans. The method of assigning mismatch and scar scores is based on the approach taken in the PARR-1 study [59], which involves automatic registration and utilization of normal limits for resting perfusion. The viability analysis is performed in polar coordinates, with computation of scar and mismatch scores for each patient’s polar map within the spatial location of the resting perfusion defect, as defined by total perfusion deficit analysis [62]. When the normalized perfusion value at a given polar map location is lower than the normalized 18F-FDG uptake, the mismatch score is calculated from the difference between the two values. The scar score is calculated relative to the normalized 18F-FDG uptake in each polar map segment. When normalized perfusion exceeds 18F-FDG uptake, the scar score is assigned a value equal to the normalized perfusion value in that segment. The total scar score in the entire polar map is then computed as the sum of all segmental scar and mismatch scores, and reported as a percentage of the total area of the left myocardium. An example of mismatch and scar quantification is shown in Fig. 8.
Viability quantification by PET. Rest perfusion images and viability images are shown (two left columns). Polar map quantification (middle column and 3D representation, right column) shows resting perfusion defect with total perfusion deficit of 43 % (top polar map) Mismatch of 15 % (middle polar map) and scar of 29 % (bottom polar map) (color figure online)
Quantitative analysis of mismatch and scar has been used in several clinical trials. The original technique developed by the Ottawa group was utilized in the PARR-1 and PARR-2 trials [59, 63]. The objective of the PARR-1 trial by Beanlands et al. [59] was to determine whether the extent of the viability (mismatch or scar) is related to the amount of recovery of left ventricular function and to develop a model predicting recovery after revascularization, based on the viability imaging results. They found that the quantitative measure of scar was a significant independent predictor of left ventricular function recovery after revascularization. In a subsequent sub-study of the PARR-2 trial, the investigators found that in 182 patients with ischemic left ventricular dysfunction, the risk of cardiac death, myocardial infarction or cardiac-related re-hospitalization was reduced when a quantitative mismatch variable was above 7 % [63]. Quantitative analysis of mismatch and scar was also recently applied by Uebleis et al. [64] in 244 patients to evaluate the prognostic significance of 18F-FDG-PET viability scanning for the prediction of patients with ischemic cardiomyopathy and left ventricular dysfunction. Their analysis showed that the patients with mismatch greater than 5 % (as established by the quantitative software) who did not undergo early revascularization had significantly higher mortality than the group who had similar mismatch (≥5 %) and did undergo the intervention. This study illustrates the role of quantitative viability imaging in the selection of patients for appropriate therapy. It would have been very difficult to derive such definite mismatch thresholds, which can be used to guide therapy, had quantitative analysis not been employed.
Absolute quantification of myocardial metabolism
Imbalances in cardiac metabolism are related to several cardiovascular disorders. PET techniques, by radiolabeling and measuring the kinetics of metabolic substrates, allow in vivo quantification of glucose uptake, oxygen consumption and fatty acid oxidation in the heart. The relative quantification of a radiotracer can be accomplished by static viability imaging as described earlier, but quantification of glucose metabolism in absolute units is possible only through dynamic quantitative 18F-FDG imaging [58, 65, 66]. Tracer kinetic compartmental models have been developed for the analysis of 18F-FDG metabolism and allow computation of regional glucose utilization rates in µmol/min/g both at stress and at rest [66]. The mismatch between blood flow and glucose utilization could be used to establish the presence of “metabolic ischemia”. Such quantitative imaging methods could perhaps play a role in the therapeutic management of patients with cardiomyopathy. The use of 18F-FDG for metabolic imaging and quantification is clinically feasible, since it does not require a cyclotron on site and 18F-FDG is a routinely available tracer.
In addition, oxidative metabolism of the myocardium can be assessed by 11C-palmitate [67] or 11C-acetate tracers [68, 69] tracers (although these tracers require a cyclotron on site). Quantitative kinetic models for 11C-acetate have been developed to derive, non-invasively, the regional myocardial clearance rates of 11CO2 (turnover rate constant in 1/min) from the myocardium [68, 69]. Based on invasive comparisons in canine studies, these values could be calibrated to absolute units (µmol/min/g) reflecting the myocardial oxygen consumption. 11C-acetate imaging has been shown in human studies to identify hibernating myocardium potentially better than viability studies using 18F-FDG [70]. 11C-palmitate tracer kinetic modeling is more complex than 11C-acetate modeling and it allows in vivo evaluation of the reduction of fatty acid oxidation in the ischemic myocardium [67, 71, 72]. The regional relative uptake and time-activity curves can be derived to estimate the clearance half times and the retention fraction of the 11C-palmitate from the myocardium [71]. This technique could potentially be used to evaluate the consequences of the ischemic injury. Nevertheless, these sophisticated quantitative techniques for evaluation of myocardial oxidative metabolism originally established and validated primarily in animal models have, largely, not been used clinically to date.
PET quantification and hybrid imaging
Virtually all PET scanners are offered currently by vendors in hybrid PET/CT configuration. Many of these systems feature high-end (64-slice or higher) CT components, capable of state-of-the-art CT coronary angiography (CTA). Several groups have investigated the feasibility of obtaining improved diagnostic performance by augmenting quantitative PET analysis with anatomical information from coronary CTA. Kajander et al. [73] studied the quantitative combined analysis of 15O-water PET blood flow and coronary CTA. They demonstrated that the combination of PET and coronary CTA achieves 95 % sensitivity and 100 % specificity, allowing higher diagnostic accuracy than either CTA or PET alone. In a similar study, Danad et al. [74] found that the diagnostic accuracy of quantitative 15O-water PET/CTA was superior to that of either 15O-water PET or CTA alone for the detection of clinically significant CAD.
Combined quantitative analysis of PET and CTA requires perfect alignment of the PET and CT scans. This cannot be guaranteed simply by ensuring that the patient does not move on the bed, since CTA and PET are typically acquired with different breathing patterns. The alignment can be performed interactively; however, such manual intervention is likely to introduce inter-observer variability into the hybrid imaging results. Nakazato et al. [75] validated a fully automated technique for the alignment of PET and CTA. They found that despite CTA and PET scans being performed without the patient leaving the table, misalignments between PET and CTA were typically between 14 and 27 mm. The automated software successfully corrected these misalignments, allowing combined 3D visualization of coronary vessel anatomy and hypoperfusion. Subsequently, the quantitative results were modified in 11 out of 32 studies based on the co-registration with CTA [75]. Such 3D fusion of the anatomical and functional scans may allow more precise left ventricular contouring and vascular territory adjustment for subsequent quantitative analysis.
Vulnerable plaque quantification
Positron emission tomography imaging could also be utilized to characterize atherosclerotic plaque deposits within vessel walls. Elevated macrophage content and associated histological evidence of inflammation are the major hallmarks of ruptured arterial plaques. Among the tracer compounds for imaging macrophage accumulation, 18F-FDG is approved for human scanning and is used extensively for the detection and staging of tumors, and for imaging of the glucose utilization in viable myocardium [76] (see previous sections). 18F-FDG is currently the most validated PET compound for vulnerable plaque imaging [77]. High-end PET/CT with 64-slice (or higher) CT allows precise anatomical delineation of the plaque and vessel anatomy by CTA. PET studies of plaque inflammation in the carotid vessels [78] and in the aorta [79] have been reported. Thanks to the increased PET imaging resolution of the new hybrid PET/CT scanners, it may also be possible to image coronary arteries. Increased 18F-FDG signal along the course of the coronary arteries in patients with known CAD has been reported, but without direct confirmation that plaques existed at the sites of increased uptake [80]. Higher 18F-FDG signal at culprit plaque sites was shown in patients with acute coronary syndrome [81]. Another preliminary study with 64-slice PET/CT also found increased 18F-FDG uptake at culprit sites of acute myocardial infarction after coronary stenting [82]. The other PET tracer that can potentially depict coronary plaque biology and is approved for human scanning is 18F-sodium fluoride. Preliminary hybrid PET/CT imaging in coronaries with 18F-sodium fluoride has been performed [83]. It has also been shown, in a longitudinal 18F-FDG-PET/CT study, that focal arterial inflammation precedes subsequent calcification in the same location [84].
The quantification of vascular atherosclerotic plaques from PET images presents new challenges. In cases of plaque inflammation, small hot spots need to be differentiated from uptake in other adjacent structures. In the monitoring of the disease progression, the level of focal activity may need to be compared in patients with suspected inflammation versus controls, or versus previous studies. The analysis techniques are similar to the methods used in oncological imaging for tracer uptake quantification in tumors. Typically, SUVs or target-to-background ratios are used in the analysis of the vascular plaque uptake [82, 83, 85, 86]. Small plaque sizes and the need for partial volume correction, especially in the coronary vessels, may make quantification of SUVs problematic. New PET reconstruction techniques, which employ resolution recovery principles, can aid in the correction of the tracer activity localized in small lesions [87]. The regions of interest are most robustly guided by coregistered CTA, which provides the anatomical definition of the plaque boundaries. Although it is possible to judge tracer uptake in the vasculature by visual grading, the results are highly variable. Quantification of target-to-background ratios or SUVs allows detection of subtle, but significantly increases the tracer concentration in inflamed plaques. In an example from our study of coronary plaque imaging with PET, we show the quantification of 18F-FDG atherosclerotic plaque uptake in two cases with acute coronary syndrome (Fig. 9).
PET 18F-FDG coronary uptake quantification. Examples of increased 18F-FDG uptake at stent site in patients with acute ST elevation myocardial infarction (STEMI). a A 54-year-old man imaged after percutaneous coronary stenting of proximal left anterior descending artery (LAD) for STEMI. Quantification of maximum target-to background ratio (maxTBR) at stent site was 2.1 (white circles). b A 49-year-old man imaged after stenting of proximal right coronary artery (RCA) for STEMI. maxTBR at stent site was 2.1. Reproduced with permission from Cheng et al. [82] (color figure online)
Future directions
As hybrid PET/CT scanners become more ubiquitous and image analysis becomes more automated, we will likely see some integration of the quantitative CT and or CTA results with PET results, since visual hybrid analysis of the data from these two modalities has been shown to have incremental diagnostic value [73]. Additionally, a calcium scoring scan, which can be readily obtained on any PET/CT scanner, could perhaps be used in conjunction with the PET images for enhanced diagnosis or prognosis. For example, Bybee et al. [88] studied patients with no history of prior CAD and normal PET perfusion scans and found a high prevalence of significant subclinical atherosclerosis in patients who had elevated calcium scores. Thus, it is possible that quantitative combination of multiple parameters obtained from both CT or CTA and multiple PET parameters could be developed to derive integrated quantitative scores expressing the risk of disease, as has been demonstrated for SPECT imaging [89].
Concerns about radiation dose will influence the use of hybrid PET/CT or PET/CTA scanning. In efforts to reduce the dose and scan time, software methods have been proposed for PET attenuation correction using CT calcium scoring scans [90], eliminating the need for CT attenuation map scans. On the other hand, it is also potentially possible to obtain calcium scores from the attenuation maps [91] and use them as an additional independent marker of the disease. Low-dose protocols have been proposed for hybrid PET/CTA imaging [92]. It has been reported that hyperemic (stress) blood flow alone (without rest scan) may be sufficient for detection of obstructive CAD [74]. Therefore, combining physiological and anatomical imaging could be accomplished with PET stress-only/CTA imaging, which could significantly reduce the overall dose burden and scan duration [92, 93]. Furthermore, prospective gating and other dose-reducing techniques allow acquisition of high quality coronary CTA scans with doses lower than 1 mSv [94]. These novel CT acquisition techniques may facilitate more routine use of hybrid PET/CTA imaging and hybrid quantification. Finally, cardiac PET/MR has entered the clinical arena allowing further reduction of the overall radiation dose and may also offer some new clinical applications such as imaging of cardiac sarcoidosis [95].
It is also likely that cardiac respiratory gating [96, 97] and advanced PET reconstruction techniques, available with the new equipment, will increase resolution and allow better definition of motion for PET images. Further, it may allow improvement of the accuracy of the quantification methods by the analysis of the motion-frozen data [98]. Consequently, very high quality, high-resolution PET images free of patient and respiratory and cardiac motion could perhaps enable quantification of endocardial and epicardial perfusion and absolute myocardial blood flow. Such high-resolution quantification will, very likely, also be required for vulnerable plaque quantification in the coronary arteries to avoid partial volume and motion effects.
Summary
Positron emission tomography cardiovascular imaging is a powerful clinical and research tool. It allows determination of the extent and severity of CAD, identification of early coronary disease by absolute myocardial flow measurements, detection of viable myocardium, determination of myocardial metabolism, measurement of cardiac function, and potential differentiation of vulnerable plaques from stable plaques. Additional synergistic possibilities exist when it is coupled with CTA. A multitude of clinically relevant cardiovascular imaging parameters can be obtained from PET imaging. Some of these variables are inherently quantitative and it is not possible to use visual analysis for their determination (such as absolute myocardial blood flow obtained from dynamic data, phase analysis or ejection fraction and systolic/diastolic volumes obtained from gated data). Other variables such as relative myocardial hypoperfusion, myocardial viability or tracer plaque uptake have traditionally been assessed by visual means, but quantitative methods allow improved reproducibility and potentially also improved accuracy. The quantification of PET images allows objective determination of the disease likelihood and appropriate risk stratification. Virtually all quantitative functional and perfusion parameters can be obtained rapidly (typically in a matter of seconds) from reconstructed tomographic data with minimal user interaction. For these reasons, the use of advanced objective quantification methods in cardiovascular PET is becoming an integral part of clinical examinations and represents a primary advantage over other modalities.
References
Naya M, Murthy VL, Taqueti VR et al (2014) Preserved coronary flow reserve effectively excludes high-risk coronary artery disease on angiography. J Nucl Med 55:248–255
Murthy VL, Naya M, Foster CR et al (2011) Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 124:2215–2224
Camici PG, Rimoldi OE (2009) The clinical value of myocardial blood flow measurement. J Nucl Med 50(7):1076–1087
Gould KL, Johnson NP, Bateman TM et al (2013) Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol 62:1639–1653
Wijns W, Vatner SF, Camici PG (1998) Hibernating myocardium. N Engl J Med 339:173–181
Joshi F, Rosenbaum D, Bordes S, Rudd JH (2011) Vascular imaging with positron emission tomography. J Intern Med 270:99–109
Klocke FJ, Baird MG, Lorell BH et al (2003) ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). Circulation 108:1404–1418
Sampson UK, Dorbala S, Limaye A, Kwong R, Di Carli MF (2007) Diagnostic accuracy of rubidium-82 myocardial perfusion imaging with hybrid positron emission tomography/computed tomography in the detection of coronary artery disease. J Am Coll Cardiol 49:1052–1058
Santana CA, Folks RD, Garcia EV et al (2007) Quantitative (82)Rb PET/CT: development and validation of myocardial perfusion database. J Nucl Med 48:1122–1128
Nakazato R, Berman DS, Dey D et al (2012) Automated quantitative Rb-82 3D PET/CT myocardial perfusion imaging: normal limits and correlation with invasive coronary angiography. J Nucl Cardiol 19:265–276
Arsanjani R, Xu Y, Hayes SW et al (2013) Comparison of fully automated computer analysis and visual scoring for detection of coronary artery disease from myocardial perfusion SPECT in a large population. J Nucl Med 54:221–228
Xu Y, Hayes S, Ali I et al (2010) Automatic and visual reproducibility of perfusion and function measures for myocardial perfusion SPECT. J Nucl Cardiol 17:1050–1057
Beanlands RS, Chow BJ, Dick A et al (2007) CCS/CAR/CANM/CNCS/CanSCMR joint position statement on advanced noninvasive cardiac imaging using positron emission tomography, magnetic resonance imaging and multidetector computed tomographic angiography in the diagnosis and evaluation of ischemic heart disease—executive summary. Can J Cardiol 23:107–119
Marzilli M, Merz CN, Boden WE et al (2012) Obstructive coronary atherosclerosis and ischemic heart disease: an elusive link! J Am Coll Cardiol 60:951–956
Kaster T, Mylonas I, Renaud JM, Wells GA, Beanlands RSB, deKemp RA (2012) Accuracy of low-dose rubidium-82 myocardial perfusion imaging for detection of coronary artery disease using 3D PET and normal database interpretation. J Nucl Cardiol 19:1135–1145
Klingensmith WC 3rd, Noonan C, Goldberg JH, Buchwald D, Kimball JT, Manson SM (2009) Decreased perfusion in the lateral wall of the left ventricle in PET/CT studies with 13N-ammonia: evaluation in healthy adults. J Nucl Med Technol 37:215–219
Germano G, Kavanagh PB, Slomka PJ, Van Kriekinge SD, Pollard G, Berman DS (2007) Quantitation in gated perfusion SPECT imaging: the Cedars-Sinai approach. J Nucl Cardiol 14:433–454
Schaefer WM, Lipke CS, Nowak B et al (2003) Validation of an evaluation routine for left ventricular volumes, ejection fraction and wall motion from gated cardiac FDG PET: a comparison with cardiac magnetic resonance imaging. Eur J Nucl Med Mol Imaging 30:545–553
Li Y, Wang L, Zhao SH et al (2014) Gated F-18 FDG PET for assessment of left ventricular volumes and ejection fraction using QGS and 4D-MSPECT in patients with heart failure: a comparison with cardiac MRI. PLoS ONE 9:e80227
Wang L, Zhang Y, Yan C et al (2013) Evaluation of right ventricular volume and ejection fraction by gated (18)F-FDG PET in patients with pulmonary hypertension: comparison with cardiac MRI and CT. J Nucl Cardiol 20:242–252
Lertsburapa K, Ahlberg AW, Bateman TM et al (2008) Independent and incremental prognostic value of left ventricular ejection fraction determined by stress gated rubidium 82 PET imaging in patients with known or suspected coronary artery disease. J Nucl Cardiol 15:745–753
Pazhenkottil AP, Buechel RR, Nkoulou R et al (2011) Left ventricular dyssynchrony assessment by phase analysis from gated PET-FDG scans. J Nucl Cardiol 18:920–925
AlJaroudi W, Alraies MC, Hachamovitch R et al (2012) Association of left ventricular mechanical dyssynchrony with survival benefit from revascularization: a study of gated positron emission tomography in patients with ischemic LV dysfunction and narrow QRS. Eur J Nucl Med Mol Imaging 39:1581–1591
Lehner S, Uebleis C, Schussler F et al (2013) The amount of viable and dyssynchronous myocardium is associated with response to cardiac resynchronization therapy: initial clinical results using multiparametric ECG-gated [18F]FDG PET. Eur J Nucl Med Mol Imaging 40:1876–1883
Xu Y, Arsanjani R, Clond M et al (2012) Transient ischemic dilation for coronary artery disease in quantitative analysis of same-day sestamibi myocardial perfusion SPECT. J Nucl Cardiol 19:465–473
Rischpler C, Higuchi T, Fukushima K et al (2012) Transient ischemic dilation ratio in 82Rb PET myocardial perfusion imaging: normal values and significance as a diagnostic and prognostic marker. J Nucl Med 53:723–730
Dorbala S, Vangala D, Sampson U, Limaye A, Kwong R, Di Carli MF (2007) Value of vasodilator left ventricular ejection fraction reserve in evaluating the magnitude of myocardium at risk and the extent of angiographic coronary artery disease: a 82Rb PET/CT study. J Nucl Med 48:349–358
Dorbala S, Hachamovitch R, Curillova Z et al (2009) Incremental prognostic value of gated Rb-82 positron emission tomography myocardial perfusion imaging over clinical variables and rest LVEF. JACC Cardiovasc Imaging 2:846–854
Hsiao E, Ali B, Blankstein R et al (2013) Detection of obstructive coronary artery disease using regadenoson stress and 82Rb PET/CT myocardial perfusion imaging. J Nucl Med 54:1748–1754
Choi Y, Huang SC, Hawkins RA, Kuhle WG, Dahlbom M, Hoh CK, Czernin J, Phelps ME, Schelbert HR (1993) A simplified method for quantification of myocardial blood flow using nitrogen-14 ammonia and dynamic PET. J Nucl Med 34:488–497
Choi Y, Huang SC, Hawkins RA, Kim JY, Kim BT, Hoh CK, Chen K, Phelps ME, Schelbert HR (1999) Quantification of myocardial blood flow using N-13-ammonia and PET: comparison of tracer models. J Nucl Med 40:1045–1055
Katoh C, Morita K, Shiga T, Kubo N, Nakada K, Tamaki N (2004) Improvement of algorithm for quantification of regional myocardial blood flow using 15O-water with PET. J Nucl Med 45:1908–1916
Lortie M, Beanlands RS, Yoshinaga K, Klein R, Dasilva JN, DeKemp RA (2007) Quantification of myocardial blood flow with 82Rb dynamic PET imaging. Eur J Nucl Med 34:1765–1774
El Fakhri G, Kardan A, Sitek A et al (2009) Reproducibility and accuracy of quantitative myocardial blood flow assessment with (82)Rb PET: comparison with (13)N-ammonia PET. J Nucl Med 50:1062–1071
deKemp RA, Declerck J, Klein R et al (2013) Multisoftware reproducibility study of stress and rest myocardial blood flow assessed with 3D dynamic PET/CT and a 1-tissue-compartment model of 82Rb kinetics. J Nucl Med 54:571–577
Slomka PJ, Alexanderson E, Jacome R et al (2012) Comparison of clinical tools for measurements of regional stress and rest myocardial blood flow assessed with 13N-ammonia PET/CT. J Nucl Med 53:171–181
Prior JO, Allenbach G, Valenta I et al (2012) Quantification of myocardial blood flow with 82Rb positron emission tomography: clinical validation with 15O-water. Eur J Nucl Med Mol Imaging 39:1037–1047
Schelbert HR (2010) Anatomy and physiology of coronary blood flow. J Nucl Cardiol 17:545–554
Johnson NP, Gould KL (2005) Clinical evaluation of a new concept: resting myocardial perfusion heterogeneity quantified by markovian analysis of PET identifies coronary microvascular dysfunction and early atherosclerosis in 1,034 subjects. J Nucl Med 46:1427–1437
Kaufmann PA, Rimoldi OE, Gnecchi-Ruscone T, Luscher TF, Camici PG (2007) Systemic nitric oxide synthase inhibition improves coronary flow reserve to adenosine in patients with significant stenoses. Am J Physiol Heart Circ Physiol 293:H2178–H2182
Van Tosh A, Votaw JR, Reichek N, Palestro CJ, Nichols KJ (2013) The relationship between ischemia-induced left ventricular dysfunction, coronary flow reserve, and coronary steal on regadenoson stress-gated (82)Rb PET myocardial perfusion imaging. J Nucl Cardiol 20:1060–1068
Heijne M, Raijmakers PG, Harms HJ et al (2010) Coronary steal: revealing the diagnosis with quantitative cardiac PET/CT. J Nucl Cardiol 17:1118–1121
Baller D, Notohamiprodjo G, Gleichmann U, Holzinger J, Weise R, Lehmann J (1999) Improvement in coronary flow reserve determined by positron emission tomography after 6 months of cholesterol-lowering therapy in patients with early stages of coronary atherosclerosis. Circulation 99:2871–2875
Parkash R, deKemp RA, Ruddy TD et al (2004) Potential utility of rubidium 82 PET quantification in patients with 3-vessel coronary artery disease. J Nucl Cardiol 11:440–449
Ziadi MC, Dekemp RA, Williams K et al (2012) Does quantification of myocardial flow reserve using rubidium-82 positron emission tomography facilitate detection of multivessel coronary artery disease? J Nucl Cardiol 19:670–680
Fiechter M, Ghadri JR, Gebhard C et al (2012) Diagnostic value of 13N-ammonia myocardial perfusion PET: added value of myocardial flow reserve. J Nucl Med 53:1230–1234
Maddahi J (2012) Properties of an ideal PET perfusion tracer: new PET tracer cases and data. J Nucl Cardiol 19(Suppl 1):S30–S37
Berman DS, Maddahi J, Tamarappoo BK et al (2013) Phase II safety and clinical comparison with single-photon emission computed tomography myocardial perfusion imaging for detection of coronary artery disease: flurpiridaz F 18 positron emission tomography. J Am Coll Cardiol 61:469–477
Sherif HM, Nekolla SG, Saraste A et al (2011) Simplified quantification of myocardial flow reserve with flurpiridaz F 18: validation with microspheres in a pig model. J Nucl Med 52:617–624
Berman DS, Germano G, Slomka PJ (2012) Improvement in PET myocardial perfusion image quality and quantification with flurpiridaz F 18. J Nucl Cardiol 19(Suppl 1):S38–S45
Woo J, Tamarappoo B, Dey D et al (2011) Automatic 3D registration of dynamic stress and rest (82)Rb and flurpiridaz F 18 myocardial perfusion PET data for patient motion detection and correction. Med Phys 38:6313–6326
Schinkel AF, Bax JJ, Poldermans D, Elhendy A, Ferrari R, Rahimtoola SH (2007) Hibernating myocardium: diagnosis and patient outcomes. Curr Probl Cardiol 32:375–410
Klein C, Nekolla SG, Bengel FM et al (2002) Assessment of myocardial viability with contrast-enhanced magnetic resonance imaging: comparison with positron emission tomography. Circulation 105:162–167
Slart RH, Bax JJ, van Veldhuisen DJ et al (2006) Prediction of functional recovery after revascularization in patients with coronary artery disease and left ventricular dysfunction by gated FDG-PET. J Nucl Cardiol 13:210–219
Desideri A, Cortigiani L, Christen AI et al (2005) The extent of perfusion-F18-fluorodeoxyglucose positron emission tomography mismatch determines mortality in medically treated patients with chronic ischemic left ventricular dysfunction. J Am Coll Cardiol 46:1264–1269
Zhang X, Liu XJ, Hu S et al (2008) Long-term survival of patients with viable and nonviable aneurysms assessed by 99mTc-MIBI SPECT and 18F-FDG PET: a comparative study of medical and surgical treatment. J Nucl Med 49:1288–1298
Santana CA, Faber TL, Soler-Peter M et al (2008) Prognostic performance of quantitative PET tools for stratification of patients with ischemic cardiomyopathy undergoing myocardial viability assessment. Nucl Med Commun 29:970–981
Anagnostopoulos C, Georgakopoulos A, Pianou N, Nekolla SG (2013) Assessment of myocardial perfusion and viability by positron emission tomography. Int J Cardiol 167:1737–1749
Beanlands RSB, Ruddy TD, deKemp RA et al (2002) Positron emission tomography and recovery following revascularization (PARR-1): the importance of scar and the development of a prediction rule for the degree of recovery of left ventricular function. J Am Coll Cardiol 40:1735–1743
Ling LF, Marwick TH, Flores DR et al (2013) Identification of therapeutic benefit from revascularization in patients with left ventricular systolic dysfunction: inducible ischemia versus hibernating myocardium. Circ Cardiovasc Imaging 6:363–372
Slomka PJ, Nishina H, Berman DS et al (2004) Automatic quantification of myocardial perfusion stress-rest change: a new measure of ischemia. J Nucl Med 45:183–191
Slomka PJ, Nishina H, Berman DS et al (2005) Automated quantification of myocardial perfusion SPECT using simplified normal limits. J Nucl Cardiol 12:66–77
D’Egidio G, Nichol G, Williams KA et al (2009) Increasing benefit from revascularization is associated with increasing amounts of myocardial hibernation: a substudy of the PARR-2 trial. JACC Cardiovasc Imaging 2:1060–1068
Uebleis C, Hellweger S, Laubender RP et al (2013) The amount of dysfunctional but viable myocardium predicts long-term survival in patients with ischemic cardiomyopathy and left ventricular dysfunction. Int J Cardiovasc Imaging 29:1645–1653
Marshall RC, Huang SC, Nash WW, Phelps ME (1983) Assessment of the [18F]fluorodeoxyglucose kinetic model in calculations of myocardial glucose metabolism during ischemia. J Nucl Med 24:1060–1064
Nienaber CA, Gambhir SS, Mody FV et al (1993) Regional myocardial blood flow and glucose utilization in symptomatic patients with hypertrophic cardiomyopathy. Circulation 87:1580–1590
Schon HR, Schelbert HR, Najafi A et al (1982) C- 11 labeled palmitic acid for the noninvasive evaluation of regional myocardial fatty acid metabolism with positron-computed tomography. II. Kinetics of C-11 palmitic acid in acutely ischemic myocardium. Am Heart J 103:548–561
Brown MA, Myears DW, Bergmann SR (1988) Noninvasive assessment of canine myocardial oxidative metabolism with carbon-11 acetate and positron emission tomography. J Am Coll Cardiol 12:1054–1063
Walsh MN, Geltman EM, Brown MA et al (1989) Noninvasive estimation of regional myocardial oxygen consumption by positron emission tomography with carbon-11 acetate in patients with myocardial infarction. J Nucl Med 30:1798–1808
Gropler RJ, Geltman EM, Sampathkumaran K et al (1993) Comparison of carbon-11-acetate with fluorine-18-fluorodeoxyglucose for delineating viable myocardium by positron emission tomography. J Am Coll Cardiol 22:1587–1597
Schwaiger M, Schelbert HR, Keen R et al (1985) Retention and clearance of C-11 palmitic acid in ischemic and reperfused canine myocardium. J Am Coll Cardiol 6:311–320
Schwaiger M, Schelbert HR, Ellison D et al (1985) Sustained regional abnormalities in cardiac metabolism after transient ischemia in the chronic dog model. J Am Coll Cardiol 6:336–347
Kajander SA, Joutsiniemi E, Saraste M et al (2011) Clinical value of absolute quantification of myocardial perfusion with (15)O-water in coronary artery disease. Circ Cardiovasc Imaging 4:678–684
Danad I, Raijmakers PG, Appelman YE et al (2013) Hybrid imaging using quantitative H215O PET and CT-based coronary angiography for the detection of coronary artery disease. J Nucl Med 54:55–63
Nakazato R, Dey D, Alexanderson E et al (2012) Automatic alignment of myocardial perfusion PET and 64-slice coronary CT angiography on hybrid PET/CT. J Nucl Cardiol 19:482–491
Maddahi J, Schelbert H, Brunken R, Di Carli M (1994) Role of thallium-201 and PET imaging in evaluation of myocardial viability and management of patients with coronary artery disease and left ventricular dysfunction. J Nucl Med 35:707–715
Vancraeynest D, Pasquet A, Roelants V, Gerber BL, Vanoverschelde JL (2011) Imaging the vulnerable plaque. J Am Coll Cardiol 57:1961–1979
Rudd JH, Warburton EA, Fryer TD et al (2002) Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation 105:2708–2711
Tatsumi M, Cohade C, Nakamoto Y, Wahl RL (2003) Fluorodeoxyglucose uptake in the aortic wall at PET/CT: possible finding for active atherosclerosis. Radiology 229:831–837
Wykrzykowska J, Lehman S, Williams G et al (2009) Imaging of inflamed and vulnerable plaque in coronary arteries with 18F-FDG PET/CT in patients with suppression of myocardial uptake using a low-carbohydrate, high-fat preparation. J Nucl Med 50:563–568
Rogers IS, Nasir K, Figueroa AL et al (2010) Feasibility of FDG imaging of the coronary arteries: comparison between acute coronary syndrome and stable angina. JACC Cardiovasc Imaging 3:388–397
Cheng VY, Slomka PJ, Le Meunier L et al (2012) Coronary arterial 18F-FDG uptake by fusion of PET and coronary CT angiography at sites of percutaneous stenting for acute myocardial infarction and stable coronary artery disease. J Nucl Med 53:575–583
Dweck MR, Chow MW, Joshi NV et al (2012) Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology. J Am Coll Cardiol 59:1539–1548
Abdelbaky A, Corsini E, Figueroa AL et al (2013) Focal arterial inflammation precedes subsequent calcification in the same location: a longitudinal FDG-PET/CT study. Circ Cardiovasc Imaging 6:747–754
Rogers IS, Tawakol A (2011) Imaging of coronary inflammation with FDG-PET: feasibility and clinical hurdles. Curr Cardiol Rep 13:138–144
Joshi NV, Vesey AT, Williams MC et al (2014) 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet 383:705–713
Panin VY, Kehren F, Michel C, Casey M (2006) Fully 3-D PET reconstruction with system matrix derived from point source measurements. IEEE Trans Med Imaging 25:907–921
Bybee KA, Lee J, Markiewicz R et al (2010) Diagnostic and clinical benefit of combined coronary calcium and perfusion assessment in patients undergoing PET/CT myocardial perfusion stress imaging. J Nucl Cardiol 17:188–196
Arsanjani R, Xu Y, Dey D et al (2013) Improved accuracy of myocardial perfusion SPECT for detection of coronary artery disease by machine learning in a large population. J Nucl Cardiol 20:553–562
Zaidi H, Nkoulou R, Bond S et al (2013) Computed tomography calcium score scan for attenuation correction of N-13 ammonia cardiac positron emission tomography: effect of respiratory phase and registration method. Int J Cardiovasc Imaging 29:1351–1360
Mylonas I, Kazmi M, Fuller L et al (2012) Measuring coronary artery calcification using positron emission tomography-computed tomography attenuation correction images. Eur Heart J Cardiovasc Imaging 13:786–792
Kajander S, Ukkonen H, Sipilä H, Teräs M, Knuuti J (2009) Low radiation dose imaging of myocardial perfusion and coronary angiography with a hybrid PET/CT scanner. Clin Physiol Funct Imaging 29:81–88
Kajander S, Joutsiniemi E, Saraste M et al (2010) Cardiac positron emission tomography/computed tomography imaging accurately detects anatomically and functionally significant coronary artery disease. Circulation 122:603–613
Achenbach S, Goroll T, Seltmann M et al (2011) Detection of coronary artery stenoses by low-dose, prospectively ECG-triggered, high-pitch spiral coronary CT angiography. JACC Cardiovasc Imaging 4:328–337
White JA, Rajchl M, Butler J, Thompson RT, Prato FS, Wisenberg G (2013) Active cardiac sarcoidosis: first clinical experience of simultaneous positron emission tomography–magnetic resonance imaging for the diagnosis of cardiac disease. Circulation 127:e639–e641
Martinez-Möller A, Zikic D, Botnar RM et al (2007) Dual cardiac–respiratory gated PET: implementation and results from a feasibility study. Eur J Nucl Med Mol Imaging 34:1447–1454
Büther F, Dawood M, Stegger L et al (2009) List mode-driven cardiac and respiratory gating in PET. J Nucl Med 50:674–681
Slomka PJ, Nishina H, Berman DS et al (2004) “Motion-frozen” display and quantification of myocardial perfusion. J Nucl Med 45:1128–1134
Conflict of interest
Cedars-Sinai Medical Center receives royalties for the quantitative assessment of function, perfusion, and viability, a portion of which is distributed to the authors of this manuscript (DB, GG, PS).
Human and Animal Studies
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Color figures online at http://springerlink.bibliotecabuap.elogim.com/article/10.1007/s40336-014-0070-2.
Rights and permissions
About this article
Cite this article
Slomka, P., Berman, D.S., Alexanderson, E. et al. The role of PET quantification in cardiovascular imaging. Clin Transl Imaging 2, 343–358 (2014). https://doi.org/10.1007/s40336-014-0070-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-014-0070-2