Abstract
Introduction
Glucagon-like peptide 1-receptor agonists (incretin mimetics) and dipeptidyl peptidase-4 inhibitors (incretin enhancers) have been recently introduced in the treatment of diabetes mellitus. In particular, incretin mimetics seems to have ancillary antioxidant/antinflammatory properties that might be involved in endothelial protection.
Aim
To investigate the effect of incretin mimetic therapy (liraglutide, exenatide) given to 11 patients with type 2 diabetes mellitus, on circulating endothelial progenitor cells (EPCs) (bone marrow-derived cells possibly participating in neovascularization and endothelial protection and repair) and capillary density.
Methods
Four diabetic patients were treated with exenatide (5 μg twice daily for 4 weeks and then 10 μg twice daily for 3 weeks) and 7 with liraglutide (0.6 mg per day for 1 week and then 1.2 mg per day for 3 weeks). Peripheral venous blood samples were obtained before treatment (basal) and after 4 week in patients treated with liraglutide, and after 4 and 7 weeks in patients treated with exenatide, since drug titration is usually longer. EPCs were evaluated by flow cytometry as CD34+/KDR+ cells. Capillary density was evaluated by videomicroscopy, before and after venous congestion, in the dorsum of the 4th finger.
Results
Patients treated with liraglutide (6 males 1 female, age 54 ± 12 years) showed a decrease in body mass index and blood pressure during treatment, while patients treated with exenatide (3 males 1 female, age 57 ± 6 years) did not show any relevant change. EPCs were significantly increased after treatment with exenatide, but not after treatment with liraglutide. Capillary density was slightly increased only after 4 weeks of treatment with exenatide, however the increase was no longer present at the final evaluation.
Conclusions
Treatment with exenatide, but not with liraglutide, was able to increase the number of circulating EPCs, possibly through an antioxidative/antiinflammatory effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Diabetes is associated with an increased risk of cardiovascular events and complications which are not completely justified with the simultaneous presence of hypertension or dyslipidemia. Endothelial dysfunction represents an important risk factor strongly associated with cardiovascular disease, in particular in diabetic patients [1]. Therefore, integrity of the endothelial monolayer plays a crucial role in the prevention of vascular inflammation and atherosclerosis. Several studies have demonstrated that the number of circulating endothelial progenitor cells (EPCs) reflects the endogenous ability to repair vascular endothelial damage. EPCs are recruited from the bone marrow to areas of endothelial injury, where they may differentiate and promote revascularization, restoring the integrity of the monolayer of the endothelium [2]. A reduced number or function of EPCs can therefore lead to endothelial dysfunction. The EPCs pool declines in the presence of cardiovascular risk factors [2, 3] and its dysfunction may play an important role in the development of cardiovascular disease in the setting of diabetes [4]. It has been demonstrated that a decrease in number of circulating EPCs and a decrease of EPCs function were correlated to atherosclerosis and vascular disease in diabetic patient [4,5,6]. Recent studies suggest that drugs commonly used in diabetic patients such as metformin, thiazolidinediones, glucagon-like peptide 1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 (DPP-4) inhibitors, insulin, statins and angiotensin converting enzyme-inhibitors may increase EPCs number and improve EPCs function [7]. In particular, GLP-1 receptor agonists (incretin mimetics) and DPP-4 inhibitors (incretin enhancers) have been recently introduced in the treatment of diabetes mellitus and both classes of drugs have been shown to improve endothelial function in patients with diabetes. It has been demonstrated that GLP-1 treatment enhanced the ability of EPCs to proliferate and differentiate [8] and that sitagliptin treatment is able to increase the number of circulating EPCs [9] and to increase neovascularization in a mouse model [10]. Treatment with linagliptin acutely increases putative vasculoregenerative and antiinflammatory cells in patients with type 2 diabetes (T2DM) [11]. In a recent case–control study, exenatide has been shown to improve vascular endothelial dysfunction [12]. Both drugs, GLP-1 and DPP-4, enhance the activation of endothelial nitric oxide synthase (eNOS) in endothelial cells. In addition incretin mimetics have anti-oxidative properties. Clinical studies reported that exenatide reduces high sensitivity C-reactive protein (hsCRP) and tumoral necrosis factor-alpha (TNF-alpha) in patients with diabetes [13]. GLP-1 administration to patients with T2DM, during a meal was able to reduce markers of oxidative stress [14]. Oeseburg and colleagues demonstrated that GLP-1 treatment decreases reactive oxygen species-induced endothelial cell senescence in a receptor-dependent manner involving the activation of protein kinase A and the induction of antioxidant genes [15]. Thus incretin mimetics and GLP-1 seem to have ancillary antioxidant/antinflammatory properties that may contribute to increased EPCs survival and function and also to neoangiogenesis.
Capillary density is decreased in hypertension, diabetes and obesity [16, 17] and this might contribute to impaired tissue perfusion [17]. The potential effects of incretin mimetics and incretin enhancers in terms of vasculogenesis, possibly mediated by a modulation of acting on vascular endothelial growth factor (VEGF) [8], might therefore be assessed by evaluating capillary density [18].
Therefore the aim of the present study was to investigate the effect of incretin mimetic therapy (such as liraglutide and exenatide) given to patients with non-insulin dependent diabetes mellitus, on concentration of circulating EPCs and on capillary density.
2 Methods
Eleven patients with T2DM, with indication for GLP-1 treatment, were included in the study. Four diabetic patients were treated with exenatide 5 μg twice daily for 4 weeks and then 10 μg twice daily for 3 weeks. Seven patients were treated with liraglutide 0.6 mg per day for 1 week and then 1.2 mg per day for 3 weeks. Patients with previous cardiovascular o cerebral events, clinic or laboratory evidence of renal failure were excluded from the study. Previous treatment (at least 3 months before study entry) with antihypertensive agents, statins and antiplatelets agents was left unchanged until the end of the study. Peripheral venous blood samples were obtained from each patient, after overnight fasting, for laboratory tests before treatment (basal) and after 4 week in patients treated with liraglutide, and after 4 and 7 weeks in patients treated with exenatide, since drug titration is usually longer.
2.1 EPCs Isolation and Count
The number of EPCs was assessed using an in vitro assay as previously described [19, 20].
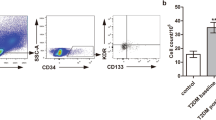
In particular, negative lineage (Lin-) mononuclear cells were obtained by enrichment from 20 mL peripheral blood mononuclear cells using 1 mL of a human progenitor cell enrichment cocktail (RosetteSep, StemCell technologies, Voden Medical Instruments S.p.A., Peschiera Borromeo, Milano, Italy). The enriched negative lineage mononuclear cells were collected by Ficoll density gradient centrifugation (Ficoll-Paque PLUS, GE-Healthcare, Fisher Scientific SAS, Illkirch Cedex, France), then washed and preincubated for 15 min with 50 μL of a FcR Blocking Reagent Human (Miltenyi Biotec, Calderara di Reno, Bologna, Italy) to inhibit non-specific binding or specific binding via Fc receptors. These cells were then subjected to double labelling with 5 μL of anti-CD34-fluorescein isothiocyanate (FITC) antibodies and 20 μL of anti-KDR (APC) antibodies. 7AAD (Beckman Coulter, Cassina De’ Pecchi, Milan, Italy) was used for real time viability staining to identify dead cells 20 min before flow cytometry. The labelled cells were analysed by four-colour flow cytometry with a FACS Calibur flow cytometer (fluorescence-activated cell sorter). Positive double staining for both CD34+ and KDR+ were considered as EPCs mature on peripheral blood.
2.2 Evaluation of Capillary Density
Skin capillary density was assessed by capillaroscopy before and after venous congestion, as described elsewhere [21,22,23]. Briefly, after a period of rest in sitting position in a quiet and temperature controlled room (21–22 °C), capillaries from nailfold and the dorsum of the fourth finger of the non-dominant hand were visualized by using an epi-illuminated microscope containing a 100 W mercury vapour lamp light source, and pictures (final magnification of 200) were obtained by video-microscopy (Videocap 3.0 D1 200, DS Medica, Milano, Italy) in baseline conditions (basal capillary density) and after venous congestion (total capillary density), in order to visualize functionally excluded capillaries. Venous congestion was induced by inflating at to 60 mmHg for 2 min a miniature blood pressure cuff applied to the base of the fourth finger of the non-dominant hand [21,22,23]. Images (final magnification of 200) were also obtained before and after venous congestion at the distal third forearm on the sagittal line by using a traditional pressure cuff. Capillary density was defined as the number of capillaries per square millimeter of the microscopic field and was counted by hand. The first row of the nailfold capillaries was considered.
In addition, delta gain (absolute difference between total and basal capillary density) and % capillary recruitment (delta gain/total capillary density × 100) were calculated.
Capillary density was determined by two independent operators and findings were averaged.
2.3 Statistical Analysis
Results are expressed as the means ± standard deviation (SD). Comparison of continuous variables in the clinical study was performed by Student paired or unpaired t test, as appropriate. The statistical significance was set at the conventional level of 5%. All variables investigated were normally distributed.
3 Results
Demographic, haemodynamic, and humoral characteristics of the patients are summarized in Tables 1 and 2. Considering the two treated groups together (Table 1) after 3 weeks of treatment with full dose, no difference in anthropometric characteristics and in hemodynamic profile was detected. Conversely, between basal and final visit, a significant reduction in total-cholesterol and in fasting blood glucose was detected. In patients treated with exenatide no difference in anthropometric and hemodynamic characteristics was observed in each visit, except for a not statistically significant reduction in SBP during an intermediate visit (p ~ 0.10). No difference in humoral parameters was found.
In patients treated with liraglutide a significant reduction in weight, body mass index and abdominal circumference was detected between basal visit and final visit respectively (Table 2). An improvement of glycemic control was evident after 3 weeks of treatment with liraglutide with a reduction of glycated hemoglobin and fasting blood glucose (Table 2). Furthermore a reduction of total cholesterol was observed in patients treated with liraglutide after 3 weeks of treatment (Table 2).
3.1 EPC Number
Double positive EPCs (CD34+/KDR+) is expressed as number of EPCs × 106 Lyn-cells. Considering the two treated groups together, no significant difference in EPC number was observed between the basal and final visit (Table 3). However, when patients were subdivided according to the drug treatment, the EPC number significantly increased in patients treated with exenatide (Fig. 1) at the intermediate visit (Table 3). This increase was maintained also after 3 weeks. No significant difference in EPC count was detected in patients treated with liraglutide in each visit (Table 3, Fig. 2).
3.2 Evaluation of Capillary Density
Considering the two treated groups together, no significant differences were observed in total capillary density at the end of the study in both the dorsum of the fourth finger and the forearm (Table 4). Considering the two groups separately, in the patients treated with exenatide a slight increase in total capillary density in the forearm was detected at the intermediate visit, but not at the end of the study (Fig. 3). No statistically significant difference in the dorsum of the fourth finger was found in the group treated with exenatide. No significant difference in the total capillary density was detected in the liraglutide group in the forearm (Fig. 4) or in the dorsum of the fourth finger.
4 Discussion
In the present study, the effects of the incretin mimetics exenatide and liraglutide on EPCs and capillary density were evaluated in patients with T2DM. A short treatment with exenatide was able to increase circulating EPCs number and total capillary density. These effects seems to be independent of glycemic control, lipid profile or blood pressure values, since no change in these parameters were observed. Endothelial dysfunction is a well-accepted marker of early vascular damage both in the coronary and peripheral vasculature [24]; in T2DM, endothelial impairment occurs also in patients with impaired glucose tolerance or at the early stage of the disease, and play an important role in atherosclerosis progression [25]. Furthermore, diabetic patients usually are characterized by the presence of multiple cardiovascular risk factors such as hyperlipidemia, smoking, obesity, and insulin resistance which contribute to endothelial dysfunction. Therefore, the study of endothelial function in patients with T2DM may have important clinical implications and may contribute to understand the mechanisms underlying the pathogenesis of diabetic end organ damage and atherosclerosis progression. Growing evidence suggests that bone marrow–derived EPCs represent a reliable marker of endothelial function and play an important role in vascular homeostasis in adults. Bone marrow derived EPCs maintain endothelial function by contributing to reendothelialization and neovascularization [2, 3] and impaired mobilization or depletion of these cells may lead to endothelial dysfunction and cardiovascular disease progression.
It has been demonstrated that diabetic patients have a decreased number of circulating EPCs and impaired EPCs functions, as differentiation, migration and adhesion [7]. Egan et al. observed a reduction of progenitor cells in patients with T2DM [26]; Fadini et al. also showed an EPCs decrease in peripheral artery disease [27]. In both studies the disease severity was negatively correlated with the EPCs number [26, 27], especially CD34+KDR+ phenotype which seems to be the most appropriate phenotype to identify the EPCs because those cells are more closely linked to cardiovascular damage in diabetes [28]. The EPC abnormalities may correlate and contribute to increase cardiovascular risk seen in patient with type 2 diabetes also after adjusting for traditional risk factors. In a study of Werner et al, EPCs number predicted severe endothelial dysfunction independently from classic cardiovascular risk factors [29].
The mechanism by which diabetes affects endothelial progenitor number and function is still debated. An increased apoptosis combined with a reduction in proliferation of EPCs was described in diabetic patients [30, 31]; furthermore a decreased nitric oxide (NO) bioavailability, induced by hyperglycemia, may to reduce EPCs migration [32].
These observation led to search for treatments able to improve endothelial monolayer integrity through EPCs replacement, and to explain the mechanisms responsible for endothelial progenitor dysfunction. Several drugs commonly used in diabetes, included metformin and insulin, were recently found to increase EPCs number and ameliorate EPCs function [7]. In particular GLP-1 receptor agonists and DPP-4 inhibitors contribute to restore endothelial monolayer by improving EPCs number and migration. These recent antidiabetic drugs are endowed with antinflammatory and antioxidant properties, acting trough several molecular pathways [33]. It has been demonstrated that the GLP-1 agonist exendin-4 is able to stimulate proliferation of human coronary artery endothelial cells through eNOS, protein kinase A (PKA) and phosphoinositol-3 kinase—protein kinase B (PI3K/Akt)-dependent pathways [34]. GLP-1 was found to induce EPCs proliferation acting on VEGF [8]. Interestingly the stromal derived factor (SDF)-1α, which is one of the most important soluble regulator of EPCs, is a physiological substrate of DPP-4 [35], therefore DPP-4 inhibition may increase SDF-1α bioavailability and activity, with consequent stimulation of EPCs. In a randomized study of 46 patients with T2DM 4-day treatment with linagliptin was able to significantly increase CD34+CD133+ progenitor cells, CD34+KDR+ EPCs, and CX3C chemokine receptor 1 (CX3CR1) bright monocytes, with a concomitant up-regulation of SDF-1α without any effect on metabolic parameters [11]. Finally, it has been observed that in both EPCs and endothelial cell cultures exposed to high glucose concentrations (25 mmol/L), liraglutide was able to increase sirtuin 6 (SIRT6) and to decrease nuclear factor-kB expression [36]. However, sodium-glucose transporter inhibitors such as dapagliflozin ere unable to increase circulating EPCs [37].
4.1 Limitations of the Study
This study has some limitations. First, the number patients enrolled is relatively small and the observation period is short. Second, we did not consider potential confounding factors as hypertension, dyslipidemia, obesity, smoking history which could influence both circulating EPC number and capillary density. Third, the precise mechanism by which exenatide may influence EPCs has not been investigated in the present study, and remains elusive.
Finally, there is no general agreement about the best methodological approach to EPC count, since no gold standard is acknowledged. However, the methods used in the present study seems to provide a reasonable accuracy, specificity and sensitivity [19, 20, 28, 50].
In conclusion, the improvement of endothelial function and the circulating EPCs may represent a potential therapeutic target in patient with type 2 diabetes. The treatment with GLP-1 agonists, such as exenatide, may be considered an useful therapeutic option. Further studies are needed to confirm this hypothesis.
5 Conclusions
The present study showed that exenatide but not liraglutide, is able to significantly increase the number of circulating EPCs and the capillary density. Conversely, liraglutide was able to reduce weight, body mass index, abdominal circumference and total cholesterol and to improve glycemic control without any significant effect on endothelial cells and microcirculation. Hyperglycemia shift differentiation of EPCs into an inflammatory phenotype [38]. Thus, exenatide could restore EPCs pool through its antiinflammatory and antioxidant capability. Murthy et al demonstrated that exenatide is able to attenuate intimal hyperplasia following balloon catheter induced vascular injury in an animal model of type 2 diabetes [39]. Some study described antiinflammatory effect of exenatide demonstrating the ability of exenatide to reduce weight and inflammatory factors and to increase the expression of adiponectin [40, 41]; exenatide is able to improve endothelial dysfunction by inhibiting the expression of monocyte chemotactic protein-1 (MCP-1) and vascular cell adhesion molecule-1 (VCAM-1) and promoting the phosphorylation of eNOS [42].
The evaluation of capillary density showed a slight increase only after 4 weeks of treatment with exenatide; however the increase was no longer present at the final evaluation. It’s well demonstrated that essential hypertension is associated with capillary rarefaction [22]. Different results were observed in patient with diabetes. A significant correlation between retinopathy stages and functional alterations during dynamic capillaroscopy was observed [43]. In a cross-sectional observational study including patients with type 1 diabetes, it was shown that skin microvascular functional alterations were present in both extremities, namely an absence of capillary reserve [44]. On the other hand it was demonstrated that skin capillary density was not altered in type 2 diabetes, or in subjects with impaired glucose tolerance compared with age, sex and BMI matched controls [45]. In several studies it has been shown that in hypertensive patients with good control of blood pressure an increase in capillary density was observed suggesting that some antihypertensive drugs, such as angiotensin-converting enzyme inhibitors and dihydropyridine calcium channel blockers, improve microvessels structure and network density [46,47,48]. In the present study no significant modification on blood pressure values was observed. Therefore, the increase of capillary density observed might be secondary to a reduction of arteriovenous shunts caused by NO-mediated vasodilation during exenatide treatment. On the other hand, Smits et al showed an augmented capillary perfusion in healthy overweight men acutely treated with exenatide, independent of NO, opening other fields of investigation [49].
References
Fonseca V, Desouza C, Asnani S, Jialal I. Nontraditional risk factors for cardiovascular disease in diabetes. Endocr Rev. 2004;25(1):153–75.
Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85(3):221–8.
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–7.
Fadini GP, Sartore S, Schiavon M, Albiero M, Baesso I, Cabrelle A, Agostini C, Avogaro A. Diabetes impairs progenitor cell mobilisation after hindlimb ischaemia-reperfusion injury in rats. Diabetologia. 2006;49(12):3075–84.
Moon JH, Chae MK, Kim KJ, Kim HM, Cha BS, Lee HC, Kim YJ, Lee BW. Decreased endothelial progenitor cells and increased serum glycated albumin are independently correlated with plaqueforming carotid artery atherosclerosis in type 2 diabetes patients without documented ischemic disease. Circ J. 2012;76(9):2273–9.
Sibal L, Aldibbiat A, Agarwal SC, Mitchell G, Oates C, Razvi S, Weaver JU, Shaw JA, Home PD. Circulating endothelial progenitor cells, endothelial function, carotid intima-media thickness and circulating markers of endothelial dysfunction in people with type 1 diabetes without macrovascular disease or microalbuminuria. Diabetologia. 2009;52(8):1464–73.
Desouza CV. Does drug therapy reverse endothelial progenitor cell dysfunction in diabetes? J Diabetes Complicat. 2013;27(5):519–25.
Xiao-Yun X, Zhao-Hui M, Ke C, Hong-Hui H, Yan-Hong X. Glucagon-like peptide-1 improves proliferation and differentiation of endothelial progenitor cells via upregulating VEGF generation. Med Sci Monit. 2011;17(2):35–41.
Gonçalves A, Leal E, Paiva A, Teixeira Lemos E, Teixeira F, Ribeiro CF, Reis F, Ambrósio AF, Fernandes R. Protective effects of the dipeptidyl peptidase IV inhibitor sitagliptin in the blood-retinal barrier in a type 2 diabetes animal model. Diabetes Obes Metab. 2012;14(5):454–63.
Huang CY, Shih CM, Tsao NW, Lin YW, Huang PH, Wu SC, Lee AW, Kao YT, Chang NC, Nakagami H, et al. Dipeptidyl peptidase-4 inhibitor improves neovascularization by increasing circulating endothelial progenitor cells. Br J Pharmacol. 2012;167(7):1506–19.
Fadini GP, Bonora BM, Cappellari R, Menegazzo L, Vedovato M, Iori E, Marescotti MC, Albiero M, Avogaro A. Acute effects of linagliptin on progenitor cells, monocyte phenotypes, and soluble mediators in type 2 diabetes. J Clin Endocrinol Metab. 2016;101(2):748–56.
Hu Y, Liu J, Wang G, Xu Y. The effects of exenatide and metformin on endothelial function in newly diagnosed type 2 diabetes mellitus patients: a case–control study. Diabetes Ther. 2018;9:1295–305.
Derosa G, Maffioli P, Salvadeo SA, Ferrari I, Ragonesi PD, QuerciF Franzett IG, Gadaleta G, Ciccarelli L, Piccinni MN, et al. Exenatide versus glibenclamide in patients with diabetes. Diabetes Technol Ther. 2010;12(3):233–40.
Ceriello A, Esposito K, Testa R, Bonfigli AR, Marra M, Giugliano D. The possible protective role of glucagon-like peptide 1 on endothelium during the meal and evidence for an “endothelial resistance” to glucagon-like peptide 1 in diabetes. Diabetes Care. 2011;34(3):697–702.
Oeseburg H, de Boer RA, Buikema H, van der Harst P, van Gilst WH, Silljé HH. Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arterioscler Thromb Vasc Biol. 2010;30(7):1407–14.
Agabiti-Rosei E, Rizzoni D. Microvascular structure as a prognostically relevant endpoint. J Hypertens. 2017;35(5):914–21.
Levy BI, Schiffrin EL, Mourad JJ, Agostini D, Vicaut E, Safar ME, Struijker-Boudier HA. Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation. 2008;118(9):968–76.
Rizzoni D, De Ciuceis C, Porteri E, Agabiti-Rosei C, Agabiti-Rosei E. Use of antihypertensive drugs in neoplastic patients. High Blood Press Cardiovasc Prev. 2017;24(2):127–32.
Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rütten H, Fichtlscherer S, Martin H, Zeiher AM. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Investig. 2001;108(3):391–7.
De Ciuceis C, Pilu A, Cappelli C, Porteri E, Zani F, Santoro A, Gandossi E, Boari GF, Rizzardi N, Castellano M, et al. Decreased number of circulating endothelial progenitor cells in patients with Graves’ hyperthyroidism. J Endocrinol Investig. 2011;34(5):335–9.
Antonios TF, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Rarefaction of skin capillaries in borderline essential hypertension suggests an early structural abnormality. Hypertension. 1999;34(4 Pt 1):655–8.
Antonios TF, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Structural skin capillary rarefaction in essential hypertension. Hypertension. 1999;33(4):998–1001.
De Ciuceis C, Rossini C, Porteri E, La Boria E, Corbellini C, Mittempergher F, Di Betta E, Sarcar A, Agabiti-Rosei C, Casella C, et al. Circulating endothelial progenitor cells, microvascular density and fibrosis in obesity before and after bariatric surgery. Blood Press. 2013;22(3):165–72.
Grover-Paèz F, Zavalza-Gomez AB. Endothelial dysfunction and cardiovascular risk factor. Diabetes Res Clin Pract. 2009;84(1):1–10.
Su Y, Liu XM, Sun YM, Wang YY, Luan Y, Wu Y. Endothelial dysfunction in impaired fasting glycemia, impaired glucose tolerance, and type 2 diabetes mellitus. Am J Cardiol. 2008;102:497–8.
Egan CG, Lavery R, Caporali F, Fondelli C, Laghi-Pasini F, Dotta F, Sorrentino V. Generalised reduction of putative endothelial progenitors and CXCR4-positive peripheral blood cells in type 2 diabetes. Diabetologia. 2008;51(7):1296–305.
Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A, Agostini C, Avogaro A. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45(9):1449–57.
Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, Grego F, Vigili de Kteutzenberg S, Tiengo A, Agostini C, Avogaro A. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 2006;26(9):2140–6.
Werner N, Wassmann S, Ahlers P, Schiegl T, Kosiol S, Link A, Walenta K, Nickenig G. Endothelial progenitor cells correlate with endothelial function in patients with coronary artery disease. Basic Res Cardiol. 2007;102(6):565–71.
Chen Q, Dong L, Wang L, Kang L, Xu B. Advanced glycation end products impair function of late endothelial progenitor cells through effects on protein kinase Akt and cyclooxygenase-2. Biochem Biophys Res Commun. 2009;381(2):192–7.
Rosso A, Balsamo A, Gambino R, Dentelli P, Falcioni R, Cassader M, Pegoraro L, Pagano G, Brizzi MF. p53 Mediates the accelerated onset of senescence of endothelial progenitor cells in diabetes. J Biol Chem. 2006;281(7):4339–47.
Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Reduced number of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89(1):E1–7.
Fadini GP, Avogaro A. Cardiovascular effects of DPP-4 inhibition: beyond GLP-1. Vasc Pharmacol. 2011;55(1–3):10–6.
Erdogdu O, Nathanson D, Sjöholm A, Nyström T, Zhang Q. Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Mol Cell Endocrinol. 2010;325(1–2):26–35.
De La Luz Sierra M, Yang F, Narazaki M, Salvucci O, Davis D, Yarchoan R, Zhang HH, Fales H, Tosato G. Differential processing of stromal-derived factor-1alpha and stromal-derived factor-1beta explains functional diversity. Blood. 2004;103(7):2452–9.
Balestrieri ML, Rizzo MR, Barbieri M, Paolisso P, D’Onofrio N, Giovane A, Siniscalchi M, Minicucci F, Sardu C, D’Andrea D, et al. Sirtuin 6 expression and inflammatory activity in diabetic atherosclerotic plaques: effects of incretin treatment. Diabetes. 2015;64(4):1395–406.
Bonora BM, Cappellari R, Albiero M, Avogaro A, Fadini GP. Effects of SGLT-2 inhibitors on circulating stem and progenitor cells in patients with type 2 diabetes J Clin Endocrinol Metab. 2018 (Epub ahead of print).
Loomans CJ, van Haperen R, Duijs JM, Verseyden C, de Crom R, Leenen PJ, Drexhage HA, de Boer HC, de Koning EJ, Rabelink TJ, et al. Differentiation of bone marrow-derived endothelial progenitor cells is shifted into a proinflammatory phenotype by hyperglycemia. Mol Med. 2009;15(5–6):152–9.
Murthy SN, Hilaire RC, Casey DB, Badejo AM, McGee J, McNamara DB, Kadowitz PJ, Fonseca VA. The synthetic GLP-I receptor agonist, exenatide, reduces intimal hyperplasia in insulin resistant rats. Diabetes Vasc Dis Res. 2010;7(2):138–44.
Koska J, Sands M, Burciu C, D’Souza KM, Raravikar K, Liu J, Truran S, Franco DA, Schwartz EA, Schwenke DC, et al. Exenatide protects against glucose- and lipid-induced endothelial dysfunction: evidence for direct vasodilation effect of GLP-1 receptor agonists in humans. Diabetes. 2015;64(7):2624–35.
Kim Chung le T, Hosaka T, Yoshida M, Harada N, Sakaue H, Sakai T, Nakaya Y. Exendin-4, a GLP-1 receptor agonist, directly induces adiponectin expression through protein kinase A pathway and prevents inflammatory adipokine expression. Biochem Biophys Res Commun. 2009;390(3):613–8.
Wei R, Ma S, Wang C, Ke J, Yang J, Li W, Liu Y, Hou W, Feng X, Wang G, Hong T. Exenatide exerts direct protective effects on endothelial cells through the AMPK/Akt/eNOS pathway in a GLP-1 receptor-dependent manner. Am J Physiol Endocrinol Metab. 2016;310(11):E947–57.
Chang CH, Tsai RK, Wu WC, Kuo SL, Yu HS. Use of dynamic capillaroscopy for studying cutaneous microcirculation in patients with diabetes mellitus. Microvasc Res. 1997;53(2):121–7.
Tibirica E, Rodrigues E, Cobas RA, Gomes MB. Endothelial function in patients with type I diabetes evaluated by skin capillary recruitment. Microvasc Res. 2007;73:107–12.
Shore AC. Capillaroscopy and measurement of capillary pressure. Br J Clin Pharmacol. 2000;50(6):501–13.
Debbabi H, Uzan L, Mourad JJ, Safar M, Levy BI, Tibiriçà E. Increased skin capillary density in treated essential hypertensive patients. Am J Hypertens. 2006;19(5):477–83.
Debbabi H, Bonnin P, Levy BI. Effects of blood pressure control with perindopril/indapamide on the microcirculation in hypertensive patients. Am J Hypertens. 2010;23(10):1136–43.
De Ciuceis C, Salvetti M, Paini A, Rossini C, Muiesan ML, Duse S, Caletti S, Coschignano MA, Semeraro F, Trapletti V, et al. Comparison of lercanidipine plus hydrochlorothiazide vs. lercanidipine plus enalapril on micro and macrocirculation in patients with mild essential hypertension. Intern Emerg Med. 2017;12(7):963–74.
Smits MM, Muskiet MHA, Tonneijck L, Kramer MH, Diamante M, van Raalte DH, Sernè EH. GLP-1 receptor agonist exenatide increases capillary perfusion independent of nitric oxide in healthy overweight men. Artheriscelr Thromb Vasc Biol. 2015;35(6):1538–43.
Lithell H. Hypertension and hyperlipidemia. Am J Hypertens. 1993;6:303S–8S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
The procedures followed were in accordance with the institutional guidelines.
Informed consent
Informed consent was obtained from all individual participants.
Rights and permissions
About this article
Cite this article
De Ciuceis, C., Agabiti-Rosei, C., Rossini, C. et al. Microvascular Density and Circulating Endothelial Progenitor Cells Before and After Treatment with Incretin Mimetics in Diabetic Patients. High Blood Press Cardiovasc Prev 25, 369–378 (2018). https://doi.org/10.1007/s40292-018-0279-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40292-018-0279-7