Abstract
Introduction
Early uncontrolled studies reported large blood pressure reductions in subjects with resistant hypertension treated with renal denervation, however these results were not confirmed in several of the latest publications.
Aim
The aim of the current study was to evaluate the effectiveness of RDN in controlled studies comparing RDN to either a sham procedure or to medical therapy.
Method
Only controlled studies were included in the analysis. Both the unadjusted and control-adjusted BP changes were calculated.
Results
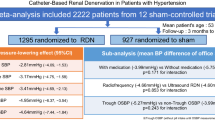
We identified 11 publications of which only 3 were double-blinded RCTs with a sham control, while 8 were open label studies where the control group was treated with medical therapy. Only 2 studies assessed adherence to medical therapy with robust methodologies. Office BP reduction (− 18/8 mmHg) significantly overestimated ABPM change (− 9/− 5 mmHg), with high heterogeneity between the included studies. When the treatment effect was adjusted for the BP change in the control group, BP changes became non significant (ABPM: − 1.8 for systolic BP [95% CI − 4.5 to 0.9] and − 0.6 for diastolic BP [95% CI − 2.3 to 1.2]). These results were confirmed when only the sham-controlled studies were analysed.
Conclusions
In spite of promising results in early reports, renal denervation fails to show superiority to a sham procedure or to medical therapy in recently published controlled studies. Lack of a sham control in most publications and heterogeneity in assessment of treatment adherence may account for part the variability reported in the studies.
Graphical abstract
Renal denervation fails to show superiority to a sham procedure or to medical therapy in recently published controlled studies.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Resistant hypertension (RH) is defined as an office blood pressure (BP) ≥ 140/90 mmHg despite the use of three or more antihypertensive drugs, including a diuretic. Studies demonstrated that the sympathetic nervous system plays a crucial role in the pathogenesis of RH [1, 2]. Renal denervation (RDN) uses radio frequency (RF) energy to disrupt both afferent and efferent renal sympathetic nerve fibers, resulting in a reduction of total sympathetic nerve activity [3]. In 2009, Krum and colleagues published a non-randomized proof-of-concept study (SYMPLICITY HTN-1), showing that percutaneous radio frequency (RF) catheter-based renal sympathetic nervous denervation (RDN) was effective (BP decrease: − 27/17 mmHg at 12 months) and safe in patients with RH [4]. One year later, the SYMPLICITY HTN-2 study, an open-label randomized clinical trial including 106 patients with RH, confirmed these results reporting an impressive BP reduction 6 months after RDN: in the RDN group (n = 49) office BP decreased by 32/12 mmHg (P < 0.0001), whereas BP remained unchanged in the control group (n = 51) [5]. In addition many new uncontrolled studies [6], showing the benefit of RDN in the treatment of RH, were published. However, in 2014, SYMPLICITY HTN-3, a US randomized controlled trial including 535 patients with RH randomized to RDN or a sham procedure failed to reach the efficacy endpoint: a reduction in official BP 6 months after RDN with the single-electrode SYMPLICITY RF catheter. In fact, in SYMPLICITY HTN-3 the mean decrease in office BP at 6 months was 14.1 mmHg in the RDN group vs 11.7 mmHg in the sham group. In particular the 24-h ambulatory blood pressure decrease was modest and of the same order of magnitude in both groups (− 6.8 and − 4.8 mmHg, respectively, with a non-significant difference between treatment and control arms) [7]. After SYMPLICITY HTN-3, other randomized controlled trials evaluating the efficacy of RDN in the treatment of RH reported conflicting results [8,9,10,11,12].
We therefore conducted a meta-analysis based on controlled clinical trials (RCT) to evaluate the efficacy of RDN as treatment modality in patients with RH.
2 Materials and methods
2.1 Literature research
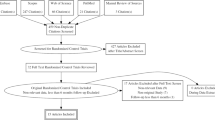
The systematic research was conducted through September 2016 through searching the PubMed, OVID and ISI-Web of Knowledge databases for publications on RDN as an invasive therapy for resistant hypertension. Different keyword combinations were used to identify research papers on RDN. In particular we chose as search terms for titles and abstracts: “resistant OR refractory OR severe OR uncontrolled” AND “hypertens*” AND “denervation OR RDN”. Finally, we examined the http://www.clinicaltrials.gov website for published and ongoing randomized trials of RDN in hypertensive patients.
2.2 Selection criteria
Articles eligible for inclusion in our analysis were reports of controlled clinical trials evaluating the efficacy of RDN in patients with RH and comparing RDN with either no additional intervention (i.e. prosecution of the previous medical therapy), sham procedure or intensified medical therapy. Trials qualified for inclusion if the reports satisfied the following inclusion criteria: (1) a detailed study protocol with inclusion and exclusion criteria; (2) enrolment of patients affected with RH according to one of the currently accepted definitions (uncontrolled blood pressure values with 3 or more antihypertensive agents including or not a diuretic); (3) use of RDN, performed with a mono-electrode catheter, as blood pressure reduction in the treatment arm; (4) publication on peer-reviewed journal (conference abstracts and other unpublished data were excluded); (5) reporting of BP measuring technique(s) adopted; (6) reporting of the BP reduction obtained through intervention and reporting of a measure of variance of the BP reduction as standard deviation (SD) or confidence interval (CI), (7) presence of a control group (comparison of RDN with sham procedure or medical treatment). The studies were excluded in case of incomplete or incorrect reporting of data on the BP reduction or in case of complete/partial overlap of the study sample with the population of another study already included in the analysis.
2.3 Data extraction
In a first step, titles and abstracts of retrieved publications were reviewed. The articles were ordered by the last name of the first author and year of publication and entered into a literature database, using Mendeley Desktop, version 1.17.06 (https://www.mendeley.com). Next the articles were selected independently by two different investigators (MC and MP). At first articled were selected by title and abstracts. Then both investigators read all papers that passed the first stage of selection, assessed eligibility and computerized the relevant information. The reference lists of eligible manuscripts were also inspected to retrieve possibly missing information and to identify duplicate publication of the same data. Relevant data included general study characteristics (sample size for each arm, study design, type of catheter used for RDN and type of intervention for the control group), baseline characteristics of the study sample (age, sex, comorbidities, number of antihypertensive drugs, baseline office and ambulatory BP values), BP change at 6-month follow-up for each treatment arm and modality of assessment of drug adherence. When both per-protocol and intention-to-treat outcomes were reported, the intention-to-treat values were considered.
2.4 Statistical analysis
Our analysis aimed to assess the change in blood pressure in terms of both office and ambulatory values (when available) 6 months after the renal denervation procedure. Both unadjusted (i.e. BP change in the RDN cohort alone compared to baseline) and control-adjusted (BP reduction in the RDN group minus the BP reduction in the control group) changes were calculated.
Average unadjusted and adjusted BP changes were calculated by performing a study-level meta-analysis; results are expressed as mean change [95% confidence interval (CI)]. We conducted a sensitivity analysis by examining the publications in subgroups according to study design (blinded vs open label) and type of control arm (sham, intensified medical therapy and previous medical therapy).
The proportion of variability explained by true heterogeneity (i.e. between-studies variability) was estimated by calculating the I2 for each analysis. Random-effect (RE) models were used due to the significant heterogeneity of the studies included. Assessment for publication bias was performed by inspection of funnel plots followed by the trim-and-fill procedure. R software version 3.2.2 with the Metafor package version 1.9-2 was used for statistical analysis.
3 Results
3.1 Characteristics of the studies (Table 1)
We identified 1007 potential publications and included in the final analysis 11 articles published from 2010 to 2016, for a total of 732 patients treated with RDN and 504 controls; size of the RDN arm ranged from 11 to 364 patients. Only 3 studies [7, 11, 13] were double blinded randomized controlled trials with a sham control, while 8 publications were open label studies in which the control group was treated with medical therapy (unchanged previous medical therapy in 4 studies and intensified medical treatment in the remaining 4 publications). Only 2 of the 11 studies assessed adherence to medical therapy with robust methodologies (witnessed intake [9] and direct measurement of plasma drug concentrations [10]). 8 publications reported both office and ambulatory BP changes; 2 assessed only ambulatory blood pressure monitoring (ABPM) values [11, 13] and 1 reported only office BP [14]. All but one small study [14] (n = 13 RDN) used the Medtronic Symplicity catheter for the procedure.
3.2 Effectiveness of Renal Denervation
Unadjusted 6-month office systolic BP reduction across 9 studies ranged from − 32 mmHg [95% CI − 38.3 to − 25.7] [5] to − 8 mmHg [95% CI − 17.8 to 1.8] [9]. In the pooled analysis, the unadjusted change in office BP was − 17.9 [95% CI − 22.5 to − 13.2] for systolic BP and − 7.9 [− 9.6 to − 6.3] for diastolic BP, with significant heterogeneity among the studies (I2: 88 and 74% respectively) (Fig. 1). Mean unadjusted ABPM change in 10 studies corresponded to approximately one half of the office BP change: − 8.6 [95% CI − 10.7 to − 6.5] for systolic and − 4.9 [− 6.4 to − 3.4] for diastolic BP (I2: 64 and 76% respectively) (Fig. 2).
When the treatment effect was adjusted for the BP change in the control group, BP changes were non significant for both Office (− 3.5 [95% CI − 13.0 to + 6.1]/− 2.8 [− 6.0 to + 0.4]) and ABPM (− 1.8 [95% CI − 4.5 to 0.9]/− 0.6 [− 2.3 to 1.2]).
3.3 Subgroup Analysis
When the 3 sham-controlled studies were analyzed separately, the results of the main analysis were confirmed (adjusted ABPM change: − 2.3 [95% CI − 4.6 to 0.1]/− 0.7 [− 2.0 to 0.7]) with low heterogeneity (I2 = 0 for both SBP and DBP change). Adjusted Ambulatory BP change was not significant for studies comparing RDN and intensified medical therapy (4.0 [95% CI − 5.8 to 13.8]/1.2 [− 2.9 − 5.4]. Ambulatory BP reduction was significant when the 3 studies comparing RDN to previous medical therapy were analyzed (adjusted ABPM change: − 5.3 [95% CI − 9.7 to − 0.9]/− 3.4 [− 6.2 to − 0.7]; one study [14] was not included because ABPM values were not reported (Fig. 3).
In this meta-analysis we examined the efficacy of RDN as treatment modality in patients with true RH by considering only controlled RCTs. Compared to previous meta-analyses on RDN, the current includes the newest controlled studies [13, 15]. Moreover, it compares the effect of RDN on BP decrease according to the type of controlled group and finally it includes the trial of Pokushalov et al. [14], where only office BP values were provided. This study is affected by many limitations and its inclusion in meta-analysis of RDN studies has been already questioned [24].
4 Discussion
The main pooled analysis showed that the effect of RDN both on office and ambulatory BP, after adjustment for the effects of the control groups, is modest and non significant for both measurement techniques. Furthermore, similarly to what has been observed initially with the results of the Symplicity HTN-3, BP decrease was rather modest when using ABPM, probably due to the white-coat effect, which is highly prevalent in patients with RH [17]. Another explanation for the large discrepancy we observed between office and ambulatory BP changes is the so-called “regression to the mean” effect, which is a consequence of using a specified office BP threshold for enrolment in these trials [18].
When all studies were analyzed together, a significant amount of heterogeneity was observed, with BP changes varying over a wide range of values. This should be expected, as heterogeneity reflects in part the differences in study design, specifically the type of control group. Heterogeneity was significantly reduced in the subgroup analysis, with the exception of the “Intensified medical therapy” subgroup, again reflecting differences in study protocols.
More specifically, the 3 sham-controlled studies [8, 11, 13] showed no significant advantage for RDN over sham with a non-significant amount of heterogeneity. A sham procedure is considered the most appropriate type of control in studies evaluating interventional procedures as RDN, as it is the only method allowing accounting for the placebo effect resulting from undergoing the procedure. Furthermore by using a sham-control group, other confounding factors, such as the regression to the mean and the improvement of the adherence to medical treatment after the procedure, can be eliminated.
In the 4 studies comparing RDN to intensified medical therapy [9, 10, 15, 19] over a period of 6 months, no differences were found in terms of both ABPM and office BP reduction, between the two groups of treatment. The intensified medical therapy consisted in adding 1 or more drugs, such as sequential sympathetic nervous system blockade (bisoprolol, prazosin, rilmenidine) [19] and of aldosterone receptor antagonists [10, 15, 19], to a three-drug therapy or in adjusting medical therapy according to noninvasive haemodynamic parameters [9]. Spironolactone was added by protocol to the standard regimen in the control group of one study [15], and it was the most frequently drug added in the control groups of 2 other studies [10, 19]. In Fadl Elmula et al. [9] spironolactone was already prescribed at baseline in 60% of patients in the control arm vs 33% in the RDN arm and it was not added after randomization to any subject.
While heterogeneity was high among studies comparing RDN with intensified medical therapy, there is some evidence that spironolactone was at least comparable to RDN in terms of BP reduction. This finding supports the use of spironolactone in patients with RH who are candidates for RDN. This recommendation is also supported by the possibility that spironolactone may improve the efficacy of RDN [21] since its mechanisms of action include effects similar to those of RDN [22]. The role of spironolactone in true RH has already been evaluated in the addition of spironolactone in patients with resistant arterial hypertension trial [23], in which spironolactone as an add-on treatment showed significantly greater decreases in both office SBP (14.6 mmHg) and 24-h SBP (10.6 mmHg), than in control group, in which baseline antihypertensive treatment was maintained. All these data underline the crucial role of spironolactone in the treatment of true RH and probably may indicate that RDN in RH may not be indicated unless a previous add-on treatment with spironolactone.
When the 4 studies comparing RDN to previous medical therapy are considered [5, 12, 14, 20], three of which were open-label [5, 12, 20], both office [12, 14, 20] and ambulatory BP reductions [5, 12, 20] after RDN were significant. This finding could be explained by several factors such as a possible improvement in adherence to treatment in the RDN group, placebo effect related to the invasive procedure, and differences in antihypertensive therapy.
About that, more recently new sham-controlled trials are ongoing and some are terminated with the newest catheters both in patients with resistant hypertension and in those with milder forms, including patients that do not take medications, such as the Spyral HTN-OFF MED trial (not included in our analysis due to the use of the new multi-electrode catheters unlike those used in the included studies). This choice could avoid the confounding effects deriving from using different classes of antihypertensive drug and patient’s variable adherence to treatment [24, 25]. In particular in the Spyral HTN-OFF MED trial, comparing RDN with sham intervention in patients with mild-to-moderate hypertension who were off medication, significant differences in 24-h ABPM in favour of RDN have been reported. These preliminary results are of the same magnitude of the blood pressure reduction found in our subgroup analysis when the 3 studies comparing RDN to previous medical therapy were analyzed [26]. It’s important to underline that the SPYRAL HTN-OFF MED trial did not meet the inclusion criteria for this metanalysis and its results derive from using the catheters’ new generation of (Spyral Medtronic) that guarantee a more effective denervation in comparison to that used in all the previous RCTs.
Another element that should be considered in RCTs evaluating the effectiveness of any therapy is represented by non-adherence to prescribed antihypertensive drugs. This may represent an important confounder especially if adherence improves in patients undergoing an invasive procedure. Among the 11 selected studies, only 2 assessed adherence to medical therapy with robust methods such as measurements of plasma drug levels [10] and witnessed intake [9], for a total of 61 patients treated with RDN and 64 controls (about 10.1% of the entire population examined). In 6 other studies adherence was assessed using indirect methods, such as questionnaires [15, 19] and compliance diary [5, 8, 11, 13]. Considering only the two studies assessing adherence with direct methodologies [9, 10], the adjusted BP changes were non-significant and these results were confirmed in the main pooled analysis. Non-adherence is extremely common: almost 50% of hypertensive patients do not take antihypertensive medications as prescribed and prevalence of non-adherence increases in patients with RH [27]; this condition may be difficult to detect unless direct and/or invasive techniques are used, as demonstrated in several recent studies, while clinical assessment of non-adherence in routine practice remains challenging [28].
The limitations of a meta-analysis reflect those of the included studies. We decided to include studies with different designs and type of control arm to increase the number of patients and to maximize the likelihood of detecting a treatment effect. However, we consider the results from the subgroup analyses according to control type to be the most meaningful, because of the lower heterogeneity. Specifically, the pooled analysis of the 3 sham-controlled trials provides the clearest evidence for lack of efficacy of RDN.
An additional limitation concerns the comparatively large size of the Simplicity-HTN3 trial, which accounts for more than half of the total number of RDN patients; however the results from the two smaller sham-controlled RCTs appear to confirm the findings of the HTN3.
Lastly, almost all procedures (664 out of 677) were performed using the first generation mono-electrode Medtronic Simplicity ablation catheter; therefore our results cannot be generalized to more recent devices (like the Spyral multielectrode radiofrequency catheter), whose effectiveness and practicity seems to be better but is still under evaluation [26].
5 Conclusion
Our meta-analysis confirms the results of the most recent studies providing evidence of the poor effectiveness of renal denervation in terms of BP reduction in comparison both to sham procedure and to intensified medical therapy. A significant BP reduction was observed only in open-label studies comparing RDN to previous medical therapy, however such studies have important limitations as they cannot account for procedure-related placebo effect and changes in treatment adherence in patients undergoing an invasive procedure; such limitations are shared with the studies comparing RDN with intensified medical therapy, for which a high degree of heterogeneity was observed.
On the other hand, there is evidence that procedure-related factors (such as number or sites of ablations with consequent variable degrees of damage to the nerve fibers) might also have contributed to the poor blood pressure response. It must however be noted that the guidance technologies applicable to RDN continue to evolve and may offer a more effective denervation in the present and future.
In light of the results of our analysis, high-quality research is needed before widespread adoption of this expensive technology. Future studies should include sham control, use of ABPM for enrolment and follow-up, objective assessment of drug adherence, a fully optimized medical therapy with the inclusion of aldosterone-receptor antagonists and use of newer-generation catheters for ablation.
Other fields of investigation should address the use of RDN in highly selected subgroups of hypertensives such as patients with metabolic syndrome and sympathetic over-activity [29, 30] and those developing significant side effects with antihypertensive drugs.
References
Mancia G, Fagard R, Narkiewicz K, Task Force for the Management of Arterial Hypertension of the European Society of Hypertension and the European Society of Cardiology, et al. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press. 2014;23:3–16.
Bakris G, Nathan S. Renal denervation and left ventricular mass regression: a benefit beyond blood pressure reduction? J Am Coll Cardiol. 2014;63(18):1924–5.
Laurent S, Schlaich M, Esler M. New drugs, procedures, and devices for hypertension. Lancet. 2012;380(9841):591–600.
Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet Lond Engl. 2009;373:1275–81.
Symplicity HTN-2 Investigators, Esler MD, Krum H, Sobotka PA, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet Lond Engl. 2010;376:1903–9.
Fadl Elmula FE, Hoffmann P, Fossum E, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension after witnessed intake of medication before qualifying ambulatory blood pressure. Hypertension. 2013;62(3):526–32.
Bhatt DL, Kandzari DE, O’Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–401.
Bakris GL, Townsend RR, Liu M, Cohen SA, D’Agostino R, Flack JM, et al. Impact of renal denervation on 24-hour ambulatory blood pressure results from SYMPLICITY HT. J Am Coll Cardiol. 2014;64(11):1071–8.
Fadl Elmula FE, Hoffmann P, Larstorp AC, et al. Adjusted drug treatment is superior to renal sympathetic denervation in patients with true treatment-resistant hypertension. Hypertension. 2014;63(5):991–9.
Rosa J, Widimský P, Toušek P, et al. Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true-resistant hypertension: six-month results from the Prague-15 study. Hypertension. 2015;65(2):407–13.
Desch S, Okon T, Heinemann D, et al. Randomized sham-controlled trial of renal sympathetic denervation in mild resistant hypertension. Hypertension. 2015;65(6):1202–8.
Kario K, Ogawa H, Okumura K, SYMPLICITY HTN-Japan Investigators, et al. SYMPLICITY HTN-Japan—first randomized controlled trial of catheter-based renal denervation in Asian patients. Circ J. 2015;79(6):1222–9.
Mathiassen ON, Vase H, Bach NJ, et al. Renal denervation in treatment-resistant essential hypertension. A randomized, SHAM-controlled, double-blinded 24-h blood pressure-based trial. J Hypertens. 2016;34:1639–47.
Pokushalov E, Romanov A, Corbucci G, et al. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol. 2012;60(13):1163–70.
Oliveras A, Armario P, Clarà A, et al. Spironolactone versus sympathetic renal denervation to treat true resistant hypertension: results from the DENERVHTA study—a randomized controlled trial. J Hypertens. 2016;34:1863–71.
Fadl Elmula FE, Jin Y, Yang WY, et al. Meta-analysis of randomized controlled trials of renal denervation in treatment-resistant hypertension. Blood Press. 2015;24:263–74.
Persu A, Azizi M, Jin Y, et al. Hyperresponders vs. nonresponder patients after renal denervation: do they differ? J Hypertens. 2014;32(12):2422–7.
Howard JP, Cole GD, Sievert H, et al. Unintentional overestimation of an expected antihypertensive effect in drug and device trials: mechanisms and solutions. Int J Cardiol. 2014;172(1):29–35.
Azizi M, Sapoval M, Gosse P, et al. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trials. Lancet. 2015;385(9981):1957–65.
Bakris GL, Townsend RR, Flack JM, et al. 12-month blood pressure results of catheter-based renal artery denervation for resistant hypertension—the SYMPLICITY HTN-3 trial. J Am Coll Cardiol. 2015;65(13):1314–21.
Pancholy SB, Shantha GP, Patel TM, Sobotka PA, Kandzari DE. Meta-analysis of the effect of renal denervation on blood pressure and pulse pressure in patients with resistant systemic hypertension. Am J Cardiol. 2014;114(6):856–61.
Gaddam KK, Nishizaka MK, Pratt-Ubunama MN, et al. Association between resistant hypertension, aldosterone, and persistent intravascular volume expansion. Arch Intern Med. 2008;168(11):1159–64.
Vàclavik J, Sediàk R, Plachy M, et al. Addition of spironolactone in patients with resistant arterial hypertension (ASPIRANT): a randomized, double-blind, placebo-controlled trial. Hypertension. 2011;57(6):1069–75.
Mahfoud F, Bohm M, Azizi M, et al. Proceedings from the European clinical consensus conference for renal denervation: considerations on future clinical trial design. Eur Heart J. 2015;36(33):2219–27.
Mahfoud F, Schmieder RE, Azizi M, et al. Proceedings from the 2nd European clinical consensus conference for device-based therapies for hypertension: state of the art and considerations for the future. Eur Heart J. 2017;38(44):3272–81.
Townsend RR, Mahfoud F, Kandzari DE, et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomized, sham-controlled, proof-of-concept trial. Lancet. 2017. https://doi.org/10.1016/S0140-6736(17)32281-X.
Rabbia F, Fulcheri C, Di Monaco S, et al. Adherence to antihypertensive therapy and therapeutic dosage of antihypertensive drugs. High Blood Press Cardiovasc Prev. 2016;23(4):341–5.
Burnier M, Santschi V, Favrat B, Brunner HR. Monitoring compliance in resistant hypertension: an important step in patient management. J Hypertens Suppl. 2003;21(2):S37–42.
Wenwen C, Chang Y, He L, et al. Effect of renal sympathetic denervation on hepatic glucose metabolism and blood pressure in a rat model of insulin resistance. J Hypertens. 2016;34:2465–74.
Tsioufis C, Dimitriadis K, Kasiakogias A, et al. Effects of multielectrode renal denervation on elevated sympathetic nerve activity and insulin resistance in metabolic syndrome. J Hypertens. 2017;35(5):1100–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare no competing interests.
Ethical approval
The following meta-analysis included researches involving human participants.
Informed consent
All participants in each study provided written informed consent.
Rights and permissions
About this article
Cite this article
Pappaccogli, M., Covella, M., Berra, E. et al. Effectiveness of Renal Denervation in Resistant Hypertension: A Meta-Analysis of 11 Controlled Studies. High Blood Press Cardiovasc Prev 25, 167–176 (2018). https://doi.org/10.1007/s40292-018-0260-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40292-018-0260-5