Abstract
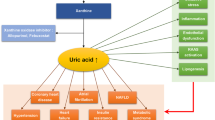
The relevance of cardiovascular role played by levels of serum uric acid is dramatically growing, especially as cardiovascular risk factor potentially able to exert either a direct deleterious impact or a synergic effect with other cardiovascular risk factors. At the present time, it still remains undefined the threshold level of serum uric acid able to contribute to the cardiovascular risk. Indeed, the available epidemiological case studies are not homogeneous, and some preliminary data suggest that the so-called “cardiovascular threshold limit” may substantially differ from that identified as a cut-off able to trigger the acute gout attack. In such scenario, there is the necessity to clarify and quantify this threshold value, to insert it in the stratification of risk algorithm scores and, in turn, to adopt proper prevention and correction strategies. The clarification of the relationship between circulating levels of uric acid and cardio-nephro-metabolic disorders in a broad sample representative of general population is critical to identify the threshold value of serum uric acid better discriminating the increased risk associated with uric acid. The Uric acid Right for heArt Health (URRAH) project has been designed to define, as primary objective, the level of uricemia above which the independent risk of cardiovascular disease may increase in a significantly manner in a general Italian population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The prevalence of hyperuricaemia and gout has been growing in Western countries for the last few decades [1, 2]. Gout is currently the most prevalent inflammatory arthritis in developed countries, especially in elderly men. A nationwide population based study by Trifirò et al [3] recently described an increase in the prevalence of gout in Italy from 0.7% in 2005 to 0.9% in 2009, with a male/female ratio of 4:1. A parallel trend towards an increasing prevalence of hyperuricaemia (serum uric acid > 6 mg/dL) was also observed (8.5% in 2005 vs 11.9% in 2009). These data appear quite lower than those observed in other countries; indeed, epidemiological data showed an estimated prevalence of hyperuricaemia of 21% in the United States (US) general population [4], while population-based studies reported a prevalence ranging from 13 to 25% in China [2, 5]. This worldwide rise in the prevalence of hyperuricemia and gout may be related to the epidemic diffusion of overweight and obesity [6], as well as the increased consumption of foods rich in purines [7], alcohol [8], and soft drinks sweetened with fructose [9, 10]. As a consequence, the mean serum uric acid levels in US is increased from 3.4 mg/dL in 1920s [11] to 6.25 mg/dL in 1970s [12]. This epidemic diffusion of hyperuricemia represents a major concern when considering that a growing body of evidence, deriving from both epidemiological and experimental studies, suggest a strong relationship between circulating levels of uric acid a number of cardio-nephro-metabolic disorders [13,14,15,16,17].
2 Uric Acid and Cardiovascular Risk
During the last few decades several epidemiological studies have reported a relationship between uric acid metabolism and traditional cardiovascular (CV) risk factors, including hypertension, metabolic syndrome and diabetes mellitus, suggesting a possible pathophysiological link between these conditions [13,14,15,16,17]. The association of gout with cardiovascular and renal diseases, observed as early as the late nineteenth century [17], is now well established. The prevalence of comorbidities increases with gout duration [18] and gout is associated with all components of the metabolic syndrome [19].
Over the last 15 years, the association of hyperuricaemia with CV diseases has been re-examined following the demonstration in animal models that hyperuricaemia could cause vascular disease. In humans, a large number of epidemiological studies have explored the link between hyperuricaemia and CV and renal outcomes [20, 21], and suggested an association of hyperuricaemia with increased frequency of cardiovascular death [14], coronary heart disease [22], heart failure, atrial fibrillation [23] and stroke [24]. Recently, a cross-sectional study suggested that coronary heart disease could be more severe in hyperuricaemic patients with asymptomatic monosodium urate crystal deposition than normouricaemic or hyperuricaemic patients free of crystal deposits [25]. Prospective cohorts have shown that hyperuricaemia leads to hypertension [26], which was recently found to be more refractory when associated with hyperuricaemia [27, 28], renal failure [29], type 2 diabetes mellitus [30, 31] and metabolic syndrome [32]. However, these impressive results did not prove causality because the observed associations could be explained by confounding factors, reverse causality or the intervention of a common causal factor. These limitations of previous epidemiological studies could be clarified by using an huge epidemiological database allowing multiple statistical adjustements allowing to clearly identify the relationship between serum uric acid levels and a cardio-nephrometabolic disorders.
3 Searching for a Desirable Normal Threshold for Serum Uric Acid
Serum uric acid values between 3.5 and 7.2 mg/dL in adult males and postmenopausal woman and between 2.6 and 6.0 mg/dL in premenopausal women have been identified as normal in many countries [15]. These ranges have been defined as the predicted interval including 95% of values of a reference group, in such a way that in 2.5% of cases a sample value will be smaller than the lower limit of this interval, and in 2.5% of cases it will be larger than the upper limit of this interval, whatever the distribution of these values. Indeed, in health-related fields, a reference range usually describes the variations of a measurement or value in healthy individuals. In particular, the standard definition of a reference range for a particular measurement. However, epidemiological and experimental data accumulated during the last few decades suggest that this methodological approach to identify reference range could present some weaknesses when applied to serum uric acid levels. Indeed, epidemiological data suggest a progressive worldwide increase of circulating levels of uric acid, which could lead to a “shift to right” (i.e. toward higher values) of normal range [12]. Thus, it seems reasonable to redefine the normal threshold values of uric acid according to its physiological role and its pathophysiological involvement in human diseases. Regarding the target population, healthy subjects are likely to be represented by individuals without clinical evidence of gout. However, a number of evidences suggest that articular damage may occur also without clinical evidence of acute arthritis [33,34,35,36,37]. In addition, the growing body of evidences presented above suggests that serum uric acid might also exert a detrimental influence on CV system, brain and kidney and negatively influence glucose metabolism [14, 15]. Interestingly, this influence seems to be evident also for circulating levels of uric acid below its saturation limit, indicating that it is likely independent of precipitation of urate monosodium crystals [14, 15]. Thus, the definition of normal range of serum uric acid in the general population is inevitably influenced by what we consider as “normal”, since the absence of gout flares does not necessarily imply the absence of uric acid related damage. On the other hand, there are also “optimal health ranges” identifying the optimal health impact on people. This might be the case for uric acid, since a threshold value < 6.0 mg/dL (< 360 μmol/L) seems to better identify true “healthy subjects” [15, 38]. Moreover, from an analytical perspective, considering that desirable analytical goals for imprecision and bias should be 4.3 and 4.8%, respectively [39], for a threshold of serum uric acid concentration of 6.0 mg/dL the imprecision should not exceed 0.26 mg/dL, giving a range of uncertainty between 5.74 and 6.26 mg/dL, i.e. substantially below the solubility limit of uric acid. Therefore, in the light of the new scientific knowledge on hyperuricemia, the recommendable upper threshold value for serum uric acid should reasonably be considered < 6.0 mg/dL [15, 38]. This methodological approach could represent also a strategy to sensitize clinicians towards clinical problems that now are widely recognized to be related to uric acid.
4 Unmet Knowledge Needs
The relevance of CV role played by levels of serum uric acid is dramatically growing, especially as a CV risk factor potentially able to exert either a direct deleterious impact or a synergic effect with other CV risk factors. Among others, at the present time it still remains undefined the threshold level of serum uric acid able to contribute to the CV risk. Indeed, the available epidemiological case studies are not homogeneous and some preliminary data suggest that the so-called “cardiovascular threshold limit” may substantially differ from that identified as a cut-off able to trigger the acute gout attack. In such scenario, there is the necessity to clarify and quantify this threshold value, to insert it in the stratification of risk algorithm scores and, in turn, to adopt proper prevention and correction strategies. The clarification of the relationship between circulating levels of uric acid and cardio-nephro-metabolic disorders in a broad sample representative of general population is critical to identify the threshold value of serum uric acid better discriminating the increased risk associated with uric acid.
5 Aim of the Research Project
The Working Group on uric acid and CV risk of the Italian Society of Hypertension has designed the Uric acid Right for heArt Health (URRAH) project. The primary objective of this project is to define the level of uricemia above which the independent risk of CV disease may increase in a significantly manner in a general Italian population. Secondary objectives will include the relationship between serum uric acid and a defined cluster of demographic, clinic and laboratory variables, able to affect significantly the CV risk profile.
6 Methods and Analysis
6.1 Study Design
A multinational and multicentre retrospective, observational cohort study which will involve the collection of data on outpatients and subjects from general population with a follow-up period of at least 20 years up to 31 July 2017.
6.2 Study Population
Participants will be included into the general database according to availability of one or more serum uric acid levels determination at the index date (i.e. July 1st or early) and complete informations about several study variables (see below)
6.3 Setting
The study will be performed by including in a general database the data deriving from several cohorts in our country from Italian Centers of Hypertension, distributed in almost all the Italian regions and recognised by the Italian Society of Hypertension. The URRAH Project participating centres and investigators are listed in the Acknowledgements.
6.4 Data Collection
For participants recruited into the study, data will be collected retrospectively. These data items will come from different database. For all participants, a standardised set of information will be recorded. This will include demographics, metabolic parameter, smoking habit, blood pressure, renal function, target organ damage (intima-media thickness, left ventricular hypertrophy, urinary albumin excretion, cardiovascular, renal and brain disease and concomitant treatments.
In details, renal function will be evaluated through glomerular filtration rate, estimated using a standardized serum creatinine assay and the Chronic Kidney Disease Epidemiology Collaboration (CKDEPI) equation [40]. Cardiac geometric parameters will be assessed by echocardiography. Left ventricular hypertrophy will be defined as a Left Ventricular Mass Index > 115 g/m2 in men and 95 g/m2 in women, indexed for the body surface area, as indicated by the 2013 European Society of Hypertension/European Society of Cardiology Guidelines [41]. Urinary albumin excretion will be obtained from urinary albumin-to-creatinine ratio in the spot urine samples, and expressed in mg/g.
At the end of the follow-up, the following heard endpoints will be evaluated: fatal and non-fatal acute myocardial infarction, heart failure, fatal and non-fatal stroke, and the coronary revascularization. The renal outcome will be established as a double increment of serum creatinine.
6.5 Statistical Analysis
A preliminary power analysis based on differences from stratified values of uric acid for α = 0.05 and power (1 − β) = 0.80 will be performed time to time in the different protocols of the URRAH project. Considering a medium effect size based on prior research of about 1 mg/dl, the number of subjects in the database represents a sample largely sufficient to avoid β error also after stratification into deciles.
A full descriptive analysis will be performed of all considered variables. The Kolmogorov–Smirnov normality test will be performed for the continuous variables. The continuous variables will be compared among the different renal function classes by ANOVA followed by the Tukey post hoc test. Non-normally distributed parameters will be then log‐transformed before continuing with further analyses. The analysis will be repeated by the predefined participant categories (younger and older men, premenopausal and postmenopausal women). All tests will be carried out using SPSS 21.0 for Windows (IBM Corp). A significance level of 0.05 will be considered for every test.
6.6 Ethical Issues
The study will only use data routinely collected. No extra tests or interventions will be undertaken on patients, and there will be no impact on patient care or outcome. Prior to initiation of a study site, approval will be sought from Ethical Committee of the coordinating centre (Division of Internal Medicine, University of Bologna).The processing of the patients’ personal data collected in this study will comply with the European Directive on the Privacy of Data. All data to be collected, stored and processed will be anonymised. All study-related documents will be retained on site in a secure location. No personal information will be stored on local computers during conduct of the study or after completion.
7 Expected Result
The study will increase knowledge of the relationship between serum uric acid levels and cardio-nephro-metabolic disorder and should allow to define the “cardiovascular threshold limit” for circulating levels of uric acid.
References
Walace KL, Riedel AA, Joseph-Ridge N, Wortnmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004;31:1582–7.
Roddy E, Doherty M. Epidemiology of gout. Arthritis Res Ther. 2010;12:223.
Trifirò G, Morabito P, Cavagna L, Ferrajolo C, Pecchioli S, Simonetti M, Bianchini E, Medea G, Cricelli C, Caputi AP, Mazzaglia G. Epidemiology of gout and hyperuricaemia in Italy during the years 2005–2009: a nationwide population-based study. Ann Rheum Dis. 2013;72(5):694–700.
Zhu Y, Padya BJ, Vjoi HK. Prevalence of gout and hyperuricemia in the US general population: The National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63:3136–41.
Miao Z, Li C, Chen Y, Zhao S, Wang Y, Wang Z, Chen X, Xu F, Wang F, Sun R, Hu J, Song W, Yan S, Wang CY. Dietary and lifestyle changes associated with highprevalence of hyperuricemia and gout in the Shandong coastal cities of Eastern China. J Rheumatol. 2008;35(9):1859–64.
Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the Health Professionals Follow-up Study. Arch Intern Med. 2005;165:742–8.
Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350:1093–103.
Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Alcohol intake and risk of incident gout in men: a prospective study. Lancet. 2004;363:1277–81.
Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ. 2008;336:309–12.
Choi JW, Ford ES, Gao X, Choi HK. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2008;59:109–16.
Fishberg AM. The interpretation of blood uric acid in hypertension. Arch Intern Med (Chic). 1924;34:503–7.
Glynn RJ, Campion EW, Silbert JE. Trends in serum uric acid levels 1961–1980. Arthritis Rheum. 1983;26:87–93.
Borghi C. The role of uric acid in the development of cardiovascular disease. Curr Med Res Opin. 2015;31(Suppl 2):1–2.
Borghi C, Rosei EA, Bardin T, Dawson J, Dominiczak A, Kielstein JT, Manolis AJ, Perez-Ruiz F, Mancia G. Serum uric acid and the risk of cardiovascular and renal disease. J Hypertens. 2015;33(9):1729–41.
Desideri G, Castaldo G, Lombardi A, Mussap M, Testa A, Pontremoli R, Punzi L, Borghi C. Is it time to revise the normal range of serum uric acid levels? Eur Rev Med Pharmacol Sci. 2014;18(9):1295–306.
Grassi D, Desideri G, Di Giacomantonio AV, Di Giosia P, Ferri C. Hyperuricemia and cardiovascular risk. High Blood Press Cardiovasc Prev. 2014;21(4):235–42.
Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–21.
Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Comorbidities in patients with gout prior to and following diagnosis: case-control study. Ann Rheum Dis. 2016;75(1):210–7.
Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007;57(1):109–15.
Richette P, Perez-Ruiz F, Doherty M, Jansen TL, Nuki G, Pascual E, Punzi L, So AK, Bardin T. Improving cardiovascular and renal outcomes in gout: what should we target? Nat Rev Rheumatol. 2014;10(11):654–61.
Park JJ, Roudier MP, Soman D, Mokadam NA, Simkin PA. Prevalence of birefringent crystals in cardiac and prostatic tissues, an observational study. BMJ Open. 2014;4(7):e005308.
Nozue T, Yamamoto S, Tohyama S, Fukui K, Umezawa S, Onishi Y, Kunishima T, Hibi K, Terashima M, Michishita I. Correlations between serum uric acid and coronary atherosclerosis before and during statin therapy. Coron Artery Dis. 2014;25(4):343–8.
Tamariz L, Hernandez F, Bush A, Palacio A, Hare JM. Association between serum uric acid and atrial fibrillation: a systematic review and meta-analysis. Heart Rhythm. 2014;11(7):1102–8.
Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and risk of stroke: a systematic review and meta-analysis. Arthritis Rheum. 2009;61(7):885–92.
Andres M, Quintanilla MA, Sivera F, Sanchez-Paya J, Pascual E, Vela P, Ruiz-Nodar JM. Silent monosodium urate crystal deposits are associated with severe coronary calcification in asymptomatic hyperuricemia: an exploratory study. Arthritis Rheumatol. 2016;68(6):1531–9.
Grayson PC, Kim SY, LaValley M, Choi HK. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2011;63(1):102–10.
Viazzi F, Rebora P, Giussani M, Orlando A, Stella A, Antolini L, Valsecchi MG, Pontremoli R, Genovesi S. Increased serum uric acid levels blunt the antihypertensive efficacy of lifestyle modifications in children at cardiovascular risk. Hypertension. 2016;67(5):934–40.
Cicero AF, Rosticci M, Fogacci F, Grandi E, D’Addato S, Borghi C. High serum uric acid is associated to poorly controlled blood pressure and higher arterial stiffness in hypertensive subjects. Eur J Intern Med. 2017;37:38–42.
Li L, Yang C, Zhao Y, Zeng X, Liu F, Fu P. Is hyperuricemia an independent risk factor for new-onset chronic kidney disease? A systematic review and metaanalysis based on observational cohort studies. BMC Nephrol. 2014;15:122.
Lv Q, Meng XF, He FF, Chen S, Su H, Xiong J, Gao P, Tian XJ, Liu JS, Zhu ZH, et al. High serum uric acid and increased risk of type 2 diabetes: a systemic review and meta-analysis of prospective cohort studies. PLoS One. 2013;8(2):e56864.
Mazza A, Zamboni S, Rizzato E, Pessina AC, Tikhonoff V, Schiavon L, Casiglia E. Serum uric acid shows a J-shaped trend with coronary mortality in non-insulin-dependent diabetic elderly people. The CArdiovascular STudy in the ELderly (CASTEL). Acta Diabetol. 2007;44:99–105.
Yu TY, Jee JH, Bae JC, Jin SM, Baek JH, Lee MK, Kim JH. Serum uric acid: a strong and independent predictor of metabolic syndrome after adjusting for body composition. Metabolism. 2016;65(4):432–40.
Bomalaski JS, Lluberas G, Schumacher HR Jr. Monosodium urate crystals in the knee joints of patients with asymptomatic nontophaceous gout. Arthritis Rheum. 1986;29:1480–4.
Rouault T, Caldwell DS, Holmes EW. Aspiration of the asymptomatic metatarsophalangeal joint in gout patients and hyperuricemic controls. Arthritis Rheum. 1982;25:209–12.
Carter JD, Kedar RP, Anderson SR, Osorio AH, Albritton NL, Gnanashanmugam S, Valeriano J, Vasey FB, Ricca LR. An analysis of MRI and ultrasound imaging in patients with gout who have normal plain radiographs. Rheumatology. 2009;48:1442–6.
Pineda C, Amezcua-Guerra LM, Solano C, Rodriguez-Hneriquez P, Hernandez-Diaz C, Vargas A, Hofmann F, Gutierrez M. Joint and tendon subclinical involvement suggestive of gouty arthritis in asymptomatic hyperuricemia: an ultrasound controlled study. Arthritis Res Ther. 2011;13:R4.
Wright SA, Filippucci E, McVeigh C, Grey A, McCarron M, Grassi W, Wright GD, Taggart AJ. Highresolution ultrasonography of the first metatarsal phalangeal joint in gout: a controlled study. Ann Rheum Dis. 2007;66:859–64.
Bardin T. Hyperuricemia starts at 360 micromoles (6 mg/dL). Joint Bone Spine. 2015;82(3):141–3.
Ricos C, Alvarez V, Cava F, Garcia-Lario JV, Hernandez A, Jmenez CV, Minchella J, Perich C, Simon M. Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest. 1999;59:491–500.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31(7):1281–357.
Acknowledgements
URRAH Project participating centres and investigators: Dipartimento di Scienze Mediche e Chirurgiche Alma Mater Studiorum, Università di Bologna. Claudio Borghi (coordinator), Arrigo F.G. Cicero. Dipartimento di Scienze Cliniche e Sperimentali, Università di Brescia. Maria Lorenza Muiesan (coordinator), Enrico Agabiti Rosei, Massimo Salvetti. Dipartimento di Geriatria e Terapia Intensiva Geriatrica, Università di Firenze, Azienda Ospedaliero Universitaria Careggi, Firenze. Andrea Ungar (coordinator), Giulia Rivasi. Dipartimento di Medicina Interna e Specialità Mediche, Università degli Studi di Genova e Policlinico Universitario San Martino-IST Genova. Roberto Pontremoli (coordinator), Francesca Viazzi. Dipartimento di Medicina Clinica, Sanità Pubblica, Scienze della Vita e dell’Ambiente, Università degli Studi dell’Aquila. Giovambattista Desideri. Dipartimento di Scienze della Salute, Università di Milano-Bicocca, Ospedale S Gerardo dei Tintori, Monza (MB). Guido Grassi (coordinator), Michele Bombelli, Rita Facchetti. Dipartimento di Medicina Clinica, Sanità Pubblica, Scienze della Vita e dell’Ambiente, Università degli Studi dell’Aquila. Claudio Ferri (coordinator), Bruno Bernardino. Dipartimento di Medicina Clinica e Chirurgia, Università degli Studi di Napoli “Federico II”. Ferruccio Galletti (coordinator), Lanfranco D’Elia. Dipartimento di Medicina, Università di Padova. Paolo Palatini. Dipartimento di Medicina, Università di Padova. Edoardo Casiglia (coordinator), Valerie Tikhonoff. Dipartimento Biomedico di Medicina Interna e Specialistica, Università degli Studi di Palermo. Carlo M. Barbagallo. Dipartimento di Medicina, Ospedale di Assisi (PG). Paolo Verdecchia. Dipartimento di Medicina Clinica e Sperimentale, Università di Pisa. Agostino Virdis (coordinator), Stefano Masi. Azienda Ospedaliera “Bianchi-Melacrino-Morelli” & CNR-IFC, Reggio Calabria. Francesca Mallamaci. Dipartimento di Scuola medica Salernirtana, Università di Salerno. Massimo Cirillo. Dipartimento di Medicina, Ospedale Ca’ Foncello, Azienda ULSS 2 Marca Trevigiana, Treviso. Marcello Rattazzi (coordinator), Paolo Pauletto. Dipartimento di Emergenza e Trapianto d’Organo, Azienda Ospedaliero-Universitaria Consorziale Policlinico, Università “Aldo Moro”, Bari. Pietro Cirillo (coordinator), Loreto Gesualdo. Ospedale Santa Maria della Misericordia, Rovigo. Alberto Mazza. Dipartimento Cardio-toraco-vascolare A. De Gasperis, ASST Grande Ospedale Metropolitano Niguarda, Milano. Cristina Giannattasio (coordinator), Alessandro Maloberti. Dipartimento di Medicina Clinica e Molecolare, Università di Roma Sapienza, Ospedale S Andrea, Roma. Massimo Volpe (coordinator), Giuliano Tocci. Dipartimento di Medicina Interna, Chirurgia e Odontoiatria, Scuola Medica Salernitana, Università di Salerno. Guido Iaccarino. Dipartimento di Neuroscienze ed Organi di Senso, Università degli Studi di Bari. Pietro Nazzaro.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Investigators of Working Group on Uric Acid and Cardiovascular Risk of the Italian Society of Hypertension are listed in the Acknowledgments section.
Rights and permissions
About this article
Cite this article
Desideri, G., Virdis, A., Casiglia, E. et al. Exploration into Uric and Cardiovascular Disease: Uric Acid Right for heArt Health (URRAH) Project, A Study Protocol for a Retrospective Observational Study. High Blood Press Cardiovasc Prev 25, 197–202 (2018). https://doi.org/10.1007/s40292-018-0250-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40292-018-0250-7