Abstract
Hypertension is one of the major risk factor able to promote development and progression of several cardiovascular diseases, including left ventricular hypertrophy and dysfunction, myocardial infarction, stroke, and congestive heart failure. Also, it is one of the major driven of high cardiovascular risk profile in patients with metabolic complications, including obesity, metabolic syndrome and diabetes, as well as in those with renal disease. Thus, effective control of hypertension is a key factor for any preventing strategy aimed at reducing the burden of hypertension-related cardiovascular diseases in the clinical practice. Among various regulatory and contra-regulatory systems involved in the pathogenesis of cardiovascular and renal diseases, renin-angiotensin system (RAS) plays a major role. However, despite the identification of renin and the availability of various assays for measuring its plasma activity, the specific pathophysiological role of RAS has not yet fully characterized. In the last years, however, several notions on the RAS have been improved by the results of large, randomized clinical trials, performed in different clinical settings and in different populations treated with RAS inhibiting drugs, including angiotensin converting enzyme (ACE) inhibitors and antagonists of the AT1 receptor for angiotensin II (ARBs). These findings suggest that the RAS should be considered to have a central role in the pathogenesis of different cardiovascular diseases, for both therapeutic and preventive purposes, without having to measure its level of activation in each patient. The present document will discuss the most critical issues of the pathogenesis of different cardiovascular diseases with a specific focus on RAS blocking agents, including ACE inhibitors and ARBs, in the light of the most recent evidence supporting the use of these drugs in the clinical management of hypertension and hypertension-related cardiovascular diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Arterial hypertension is a condition of great clinical relevance and scientific interest, given its large prevalence and the high social importance of its complications. Despite the large number of clinical studies performed on this area, there are still many unclear points regarding its pathogenesis, diagnosis and, mostly, therapeutic approach.
Among various regulatory and contra-regulatory systems involved in the pathogenesis of hypertension, renin-angiotensin system (RAS) plays a major role. Despite the identification of renin and the availability of various assays for measuring its plasma activity, the specific role of RAS in the pathogenesis of different cardiovascular diseases has not yet fully characterized. In particular, the lack of an effective evaluation of the tissue RAS probably represents the most relevant factor limiting the prevention of the onset of a clear relationship between increased RAS activity and the development or progression of different pathological conditions. However, the availability of drugs able to interfere with the RAS at various levels led to a better understanding of the pathophysiological role of this system, bringing it to the forefront in clinical practice.
Large, randomized clinical trials, performed in different clinical settings and in different populations treated with RAS inhibiting drugs, including angiotensin converting enzyme (ACE) inhibitors and antagonists of the AT1 receptor for angiotensin II (ARBs), provided relevant data. For example, pathological activation of tissue RAS in the heart is probably responsible for the initial stages of the hypertension-related heart disease, such as diastolic dysfunction, even in the absence of left ventricular (LV) hypertrophy, which is the first expression of hypertension-induced cardiac organ damage. Abnormal RAS activation can also explain at least, in part, the fact that regression of cardiac organ damage, namely LV hypertrophy, does not occur simply by lowering blood pressure (BP) levels. In fact, the only evidence of regression of these structural and functional abnormalities was observed in hypertensive patients without LV hypertrophy treated with ARBs, whereas similar BP reductions obtained with an ACE inhibitor did not improve LV diastolic function. In addition, it is well known that hypertensive heart disease is characterized less by myocyte hypertrophy than by increased tissue collagen deposition, which probably results from the abnormal RAS activation that stimulates collagen synthesis and inhibits collagen breakdown, thereby increasing the proportion of collagen in the connective tissue. It does, therefore, seem conceivable that hypertension activates tissue RAS, which first leads to the development of LV diastolic dysfunction and then triggers the progression to LV hypertrophy. Subsequently, systemic RAS activation mediates the progression from these conditions towards LV systolic dysfunction and congestive heart failure.
More recently, the pathophysiological notions on the RAS have been improved by the results of large, randomized clinical trials, which contributed to better define the role of renin in the clinical practice as a mechanism of transducing both structural and functional damage abnormalities in the presence of vascular atherosclerosis and hypertension. These findings suggest that the RAS should be considered to have a central role in the pathogenesis of different cardiovascular diseases, for both therapeutic and preventive purposes, without having to measure its level of activation in each patient.
2 Hypertension, Heart Failure, and Ischaemic Heart Disease: A Cardiovascular Continuum
Hypertension is a pathological condition characterized by a multifactorial pathogenesis. Its development is usually related to genetic predisposition, but also influenced by diet, stress and other environmental factors. Hypertension has been defined as a systolic BP greater than 140 mmHg and/or a diastolic BP greater than 90 mmHg [1]. The prevalence of this condition in the general population is about 30–45 % and increases with age. Hypertension is the most relevant risk factor for the development of cardiovascular diseases, including ischemic heart disease and congestive heart failure, and it is usually associated with other cardiovascular risk factors [1]. Therefore, the recommended strategy in order to obtain a satisfactory BP control, even in presence of additional cardiovascular risk factors, is represented by a larger use of combination therapies of different drug classes [1, 2]. One of the major concerns is to identification of a given therapeutic strategy that can be accepted by the patient in order to obtain good compliance to prescribed medications and effective BP control. This aspect of treatment is essential not only to obtain clinical response in hypertensive patients, but also to prevent the onset of overt cardiovascular diseases in patients who are asymptomatic. In fact, cardiovascular diseases, in particular those associated with hypertension and atherosclerotic disease, have reached pandemic proportions; they are responsible for 42 % of deaths in European women under the age of 75 years old and 38 % of deaths in males [3].
Hypertension is a major contributor for development and progression of coronary artery disease. Stable coronary artery disease is characterized by episodes of hypoperfusion and reversible myocardial dysfunction, due to ischaemia at rest or during physical exercise, stress or even in the absence of a recognizable stimulus. Clinical manifestations of coronary artery disease are the result of obstruction of the epicardial arteries by atherosclerotic plaques, microvascular dysfunction and/or dysfunction due to a past myocardial infarct or ischaemic heart disease. The recommended treatments at the time of diagnosing coronary artery disease are beta-blockers, calcium-channel blockers and ACE inhibitors or ARBs [4, 5].
Coronary artery disease is responsible for over half the cases of heart failure [6]. This is a clinical syndrome caused by cardiac structural abnormalities with evident functional consequences. About 1–2 % of the adult population in industrialised countries suffers from heart failure and the prevalence exceeding 10 % among people over 70 years old [7]. LV ejection fraction is the main parameter used to interpret the severity of symptoms and describe the functional impairment in heart failure. RAS and sympathetic nervous system are the key neuro-humoral systems activated in the pathogenesis of heart failure. On the basis of the general pathophysiological profile, the recommended treatments at the time of diagnosing heart failure are diuretics (symptomatic drugs), but above all beta-blockers, ACE inhibitors or ARBs [8].
3 Treatment of Hypertension, Heart Failure and Ischaemic Heart Disease
According to the guidelines, the most important drugs used in treatment of hypertension are ACE inhibitors, ARBs, beta-blockers, calcium antagonists and diuretics [1]. Among these, the most widely used drugs in treatment of heart failure are diuretics (administered to control the symptoms caused by fluid retention), ACE inhibitors (indicated for all stages of heart failure), ARBs (mainly used in patients who develop adverse reactions to ACE inhibitors), beta-blockers (administered to reduce cardiac work and improve LV function) and digoxin. Finally, the drugs mainly used in treatment of ischaemic heart disease are ACE inhibitors (sometimes ARBs), beta-blockers, calcium antagonists, nitrates and antiplatelet agents. Thus, drugs that modulate RAS play an important role in all these pathologies because this system is central to the pathophysiology of these cardiovascular diseases.
3.1 Role of the RAS: Pathophysiological and Pharmacological Aspects
Renin was already indicated to be a fundamental regulator of BP at the end of the 19th century and is still now object of significant research in both pre-clinical and clinical fields. Renin is a glycoprotein which is synthesised, accumulated and secreted by myoepithelial cells of the juxtaglomerular apparatus of the nephron, originating initially from pre-prorenin from which a peptide is removed, to be transformed into prorenin [9].
Prorenin not only has its own enzymatic activity, by binding to its own, specific receptor, but that it also exerts biological effects independently of its enzymatic activity, resulting in cellular hypertrophy and fibrosis [10]. A further consideration on prorenin is related to the potential role of this enzyme as marker of microvascular complications in diabetes. Prorenin blood levels are increased in patients with type 1 and 2 diabetes mellitus with a greater tendency to develop microalbuminuria and could, therefore, predict the progression of renal microvascular damage [9].
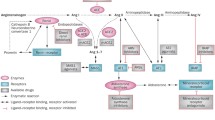
The enzymatic action of renin is to convert angiotensinogen to angiotensin I. This latter is the substrate for ACE (Fig. 1). The conversion of angiotensin I to angiotensin II, catalysed by ACE, is not the only known pathway for the biosynthesis of angiotensin II. It is currently hypothesised that there is an alternative pathway, involving an aminopeptidase, which transforms angiotensin I into des-aspartate-angiotensin I; this latter is converted by ACE into angiotensin II [11]. In addition, other non-ACE enzymes can take part in the transformation of angiotensin I into angiotensin II; the most important non-ACE enzymes are the chymases of the cardiovascular apparatus, the chemostatic angiotensin II generating enzyme (CAGE) enzyme system and various endopeptidases that can produce not only angiotensin II, but also other angiotensin fragments such as angiotensin III and IV [12]. As far as concerns the activity of angiotensin fragments, it has been seen that angiotensin IV, formed from angiotensin III through the action of aminopeptidase M, has strong effects on memory and cognition. The central and peripheral effects of angiotensin IV are mediated by specific receptors identified as membrane insulin-responsive aminopeptidases (IRAP), also known as AT4 receptors. When angiotensin IV binds to its receptors, it inhibits the catalytic activity of the IRAP and enables the accumulation of various neuropeptides related to reinforcing memory. Other effects of angiotensin IV binding to its own receptors include renal vasodilatation, natriuresis and extracellular matrix remodelling [13].
Schematic representation of the renin-angiotensin system. Modified from Reference [13]
The main product generated by ACE, and also by alternative pathways, is angiotensin II, which regulates cardiovascular homeostasis by modulating its own effects through binding to specific receptors. Nowadays, four angiotensin II receptors have been identified (Fig. 2). The AT1 receptor is involved in the main pathophysiological actions of angiotensin [14]. Activation of the AT2 receptor causes opposite effects on the vascular system compared those mediated by the AT1 receptor. The non-AT1/non-AT2 receptor was previously known as AT3 receptor. Its activation leads to the production of NO and is responsible for neuronal development. Finally, the AT4 plays a role in regulating blood flow, inhibiting the reabsorption of sodium, in memory processes and in vasodilatation [15].
Schematic representation of the angiotensin II AT1-subtype receptor. Modified from Reference [13]
Angiotensin II is not the only product that is generated from angiotensin I; this latter can also be metabolised to angiotensin 1–7 through the effect of the human ACE 2. This enzyme is a carboxypeptidase consisting of 805 aminoacids and has a short signal sequence. The preferential substrate of ACE 2 is angiotensin II, to which it binds with an affinity about 400-times higher than its affinity for angiotensin I, thus leading to the formation of angiotensin 1–7. ACE 2 can also catalyse the conversion of angiotensin I into angiotensin 1–9, which is subsequently converted to angiotensin 1–7 by ACE. Similarly, plasma endopeptidases can convert angiotensin I into angiotensin 1–7. The physiological significance of ACE 2 is still unclear. It may act as counter-regulatory mechanisms opposing the effects of ACE. Indeed, ACE 2 regulates the levels of angiotensin II and limits its effects by converting it to angiotensin 1–7.
Angiotensin 1–7 has pleiotropic effects, just like angiotensin II, and can influence the functions of many organs and systems. Its effects are mediated by a specific receptor, Mas-1. The proto-oncogene Mas encodes this orphan receptor, which has a protein G-mediated mechanism of transduction. By binding to this receptor, angiotensin 1–7 induces vasodilatation, natriuresis and diuresis. It is, therefore, considered that the ACE 2-Angiotensin 1–7-Mas-1 axis acts to counterbalance the vasoconstriction and hydrosalinic retention mediated by the classical ACE-angiotensin I-angiotensin II-aldosterone axis [16, 17].
Recent evidence demonstrated that angiotensin 1–7 could be also produced by the action of neprilysin. This is a ubiquitously distributed, zinc-dependent, type II membrane metalloprotease. Its expression is highest in the kidneys, but is also widely present in cardiovascular and other tissues. The most important biological function of neprilysin is hydrolysis of atrial natriuretic peptides; however, this enzyme has numerous other substrates, including substance P, kinins, opioid peptides, amyloid β protein, enkephalins, and gastrin as well as angiotensin I and II [18]. It has been demonstrated that the role of neprilysin within the RAS is to convert angiotensin I into angiotensin 1–7 and to hydrolyse angiotensin II [19].
From a clinical point of view, the most widely used classes of drugs act mainly at four levels: (1) inhibition of renin release (β-blockers, α2-adrenergic stimulators); (2) inhibition of ACE, which converts angiotensin I into angiotensin II (ACE inhibitors); (3) antagonism of the AT1 receptors of angiotensin II (ARBs); (4) inhibition of renin (direct inhibitors of renin).
3.2 ACE Inhibitors
3.2.1 Pharmacological Aspects
ACE inhibitors are currently the most widely used agents for the treatment of hypertension, heart failure and ischaemic heart disease. Based on their chemical structure, the ACE inhibitors can be divided into three groups (Table 1): (1) ACE inhibitors containing a sulphydryl group (alacepril, altiopril, captopril, spirapril and zofenopril); (2) ACE inhibitors containing a carboxylate group (benazepril, cilazapril, delapril, enalapril, lisinopril, perindopril, quinapril, ramipril, trandolapril and moexipril); (3) ACE inhibitors containing a phosphonate group (fosinopril).
The presence of a sulphydryl group confers potential benefits against ischaemic insults and atherosclerosis. On the other hand, it is thought that the sulphydryl group may contribute to specific toxicity profiles, such as dysgeusia, skin rashes and proteinuria [20].
This class of drugs acts on ACE by binding to the Zn2+ contained in the enzyme, in this way inhibiting the enzyme and blocking the conversion of angiotensin I into angiotensin II. The antihypertensive activity of the ACE inhibitors is the result of various effects. First of all, the inhibition of the effects of angiotensin II at both systemic and tissue levels, with vasodilatation in various districts, together with a reduction in plasma aldosterone, which translates into increased natriuresis and diuresis. Bradykinin is also involved in the antihypertensive action of the ACE inhibitors. Since ACE is identical to kininase II (responsible for the breakdown of bradykinin), the inhibition of this enzyme leads to an increase in the circulating levels of bradykinin, which induces peripheral vasodilatation. Furthermore, this peptide stimulates the secretion of prostaglandins (PGE2, PGI2), which contribute to the vasodilating action. Finally, the ACE inhibitors have an inhibitory effect on the release of antidiuretic hormone and reduce both central and peripheral sympathetic nervous system activity.
The vasodilating effect of ACE inhibitors plays a key role in the treatment of LV dysfunction, with or without symptoms of heart failure [21, 22]. In fact, these drugs induce both dilatation of the arteriolar vessels, with a consequent reduction in pre-load, and venous dilatation that diminishes systolic wall stress and causes an overall reduction in the LV end-diastolic volume. A further effect of ACE inhibitors in various experimental models is to prevent endothelial damage and the formation of atherosclerotic plaques [23]. Furthermore, ACE inhibitors have antimitogenic effects in the heart by inhibiting the action of angiotensin II on cardiac AT1 receptors. Thereby they can reduce LV hypertrophy [24].
Finally, ACE inhibitors are also able to slow the progression of chronic kidney disease, due to their beneficial effects on the kidney. The increase in pressure in the renal capillaries is responsible for the glomerular dysfunction in numerous renal disorders, including diabetic nephropathy. ACE inhibitors are able to lower glomerular capillary pressure by reducing systemic BP and by selective dilatation of the efferent arterioles. Furthermore, since angiotensin II is also implicated in the proliferation of mesangial cells and matrix production, ACE inhibitors have been shown to be effective in inhibiting the growth of these cells, thereby improving renal function and preventing the progression of microalbuminuria to overt proteinuria [25, 26].
3.2.2 Pharmacokinetics
Main pharmacokinetic properties of ACE inhibitors are reported in Table 2.
3.2.3 Clinical Aspects
Given their pharmacokinetic and pharmacodynamics characteristics, all ACE inhibitors are indicated for the treatment of hypertension (Table 3) [1]. With the exceptions of moexipril, spirapril, trandolapril and zofenopril, all ACE inhibitors are also indicated for treatment of heart failure [8]. Captopril, lisinopril, ramipril, trandolapril and zofenopril are also indicated for the treatment of acute myocardial infarction [4, 5]. Compared to other drugs of the same class, perindopril has also been registered for the treatment of stable coronary artery disease, because it demonstrated to reduce the risk of cardiac events in patients with a history of myocardial infarction and/or revascularization. There are other additional clinical indications for some selected drugs within this class. Ramipril is indicated for global cardiovascular risk reduction. Captopril, lisinopril and ramipril are indicated in diabetic nephropathy. Furthermore, ramipril is indicated for the treatment of non-diabetic glomerular nephropathy.
3.2.4 OsMed Data on Drug Use
According to Osservatorio sull’impiego dei Medicinali (OsMed) data [27], in Italy during the first nine months of 2014, in line with the trend observed during the last few years, drugs used for the cardiovascular system were in the first place with regards to consumption, with 468 defined daily doses (DDD) every 1000 inhabitants. Among these, the most prescribed drugs were ACE inhibitors (120.5 DDD/1000 inhabitants). Ramipril is the most used active principle in class A of the Italian National Health System-subsidised drugs, with 59.9 DDD/1000 inhabitants, followed by enalapril with 12 DDD/1000 inhabitants. Ramipril also ranked first among the 30 active principles for which expenditure is highest, while combination therapy with perindopril and amlodipine was at the top of the 30 active principles for which Health System-subsidised expenditure differed most from 2013, with an increase of 39.8 %.
3.3 ARBs
3.3.1 Pharmacological Aspects
ARBs are used for the treatment of hypertension, heart failure and ischaemic heart disease [1, 4, 5, 8]. They predominantly act by blocking AT1 receptors for angiotensin II. The currently available compounds can be differentiated chemically into biphenylmethyl derivatives (azilsartan medoxomil, candesartan cilexetil, irbesartan, losartan, olmesartan medoxomil, telmisartan and valsartan) and thienylmethylacrylic derivatives (eprosartan). ARBs can also be differentiated from a molecular point of view on the basis of their affinity for AT1 receptors and the type of antagonism of these receptors. In decreasing order of affinity for the AT1 receptor, ARBs are: candesartan = olmesartan = azilsartan > irbesartan = eprosartan > telmisartan = valsartan = EXP 3174 (active metabolite of losartan) > losartan [28]. This pharmacodynamic difference has important clinical consequences. With regard to the antihypertensive activity of these drugs, comparative clinical studies have confirmed that the antihypertensive efficacy of losartan is less than that of the other ARBs [29, 30].
ARBs induce intense vasodilatation, reducing both pre-load and afterload, thus decreasing systolic wall stress and LV end-diastolic volume. Selective inhibition of the AT1 receptor has a series of clinical advantages. First of all there is also inhibition of the effects of angiotensin II, developed through non-ACE dependent pathways. Furthermore, free angiotensin II, which finds the AT1 receptors occupied, can exert its effects on the AT2 receptors, which mediate vasodilatation and improvement of vascular and cardiac function; ARBs preserve the activity of the AT4-angiotensin IV system and the synthesis of angiotensin 1-7, both mechanisms involved in vasodilatation. Since ACE is not inhibited, there isn’t an increase of bradykinin concentration; this determines a decrease of dry cough, typical side effect of ACE inhibitors and, consequently a possibly better adherence to therapy by patients. Finally, ARBs can exercise beneficial effects at the systemic level, independently of their binding to the AT1 receptor; they have, in fact, been shown to be able to activate the peroxisome proliferator-activated receptor (PPAR-gamma) and can also induce the release of adiponectin from adipocytes. These effects cause an increase in the sensitivity to insulin, reduce the levels of circulating lipids and promote anti-inflammatory activity [31].
3.3.2 Pharmacokinetics
Main pharmacokinetic properties of ARBs are reported in Table 4.
3.3.3 Clinical Aspects
All the drugs belonging to the class of ARBs have been approved for the treatment of hypertension (Table 5). Losartan and valsartan are also indicated for treatment of heart failure. Valsartan is also indicated for the treatment of adult patients with clinically stable symptomatic heart failure or asymptomatic LV dysfunction following a recent myocardial infarction (within the preceding 12 h–10 days). Furthermore, losartan, irbesartan and telmisartan are approved for the prevention of diabetic nephropathy in patients with type 2 diabetes mellitus. Finally, losartan is also approved for prevention of cerebrovascular events.
3.3.4 OsMed Data on Drug Use
According to OsMed data [27], from January to September 2014, ARBs are the second most frequently used drugs (98.1 DDD/1000 inhabitants). Valsartan, alone or in combination, is the leading ARB, with 14.3 DDD/1000 inhabitants, followed by telmisartan (9.3), irbesartan (9.2) and olmesartan (6.9).
4 ACE Inhibitors and ARBs
4.1 Clinical Indications
4.1.1 Hypertension
ACE inhibitors and ARBs have often been gather together for their ability to exert therapeutic effect on BP Although the medical literature is rich in indirect comparisons between these two classes of drugs, there are still only very few direct, randomised clinical trials.
4.1.2 Ischemic Heart Disease
Some meta-analyses [32, 33] suggested that the ARBs are less effective than ACE inhibitors in preventing acute myocardial infarction and all-cause mortality. This effect could be due to the lack of an additional mechanism of action on bradykinin and to the unfavourable impact of the increased amount of angiotensin II produced in response to blocking the AT1 receptor (even if this could act, in part, on AT2 receptors). However, the hypothesis raised by these meta-analyses [32, 33] were not confirmed by the results of several other inclusive and independent meta-analyses [34–37] and, mostly, by one large, randomized, head-to-head clinical trial, the ONgoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial (ONTARGET) [38]. This trial directly compared the clinical efficacy, safety and tolerability of an ACE inhibitor (ramipril 5–10 mg) with an ARB (telmisartan 40–80 mg) and this latter resulted equivalent to the former one with regard to incidence of major cardiovascular events, stroke and all-cause mortality [38].
4.1.3 Heart Failure
ACE inhibitors and ARBs are recommended by international guidelines for patients with heart failure, in view of their favourable effects on cardiovascular mortality observed in the Cooperative New Scandinavian Enalapril Survival Study (CONSENSUS) [39, 40], Studies Of Left Ventricular Dysfunction (SOLVD)-Treatment [41], Survival and Ventricular Enlargement (SAVE) [42], Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) [43], Valsartan Heart Failure Trial (VaL-HeFT) [44], and Evaluation of Losartan in the Elderly Study (ELITE) I-II trials [45, 46]. In addition, the VALsartan in Acute myocardial iNfarction Trial (VALIANT) [47] and SOLVD-Prevention [48] trials evaluated the efficacy of both classes of drugs in patients with asymptomatic LV dysfunction associated with post-ischemic heart disease. On the basis of these findings, in patients with asymptomatic or symptomatic ventricular dysfunction (heart failure), ACE inhibitors remained the first choice drug. In this setting, however, ARBs have demonstrated a similar efficacy compared to ACE inhibitors, thereby excluding any further evaluations to establish greater or lesser efficacy.
4.1.4 Stroke
RAS inhibitors have a variable effect on stroke prevention. An all-inclusive meta-analysis involving more than 180 studies and 500,000 patients (31) showed that the ARBs were more effective than the other classes in reducing ischaemic stroke, probably because of the results of the Losartan Intervention For Endpoint reduction in hypertension study (LIFE) study [49], while the ACE inhibitors remain the drugs of first choice for the treatment of ischaemic heart disease and myocardial infarction.
4.1.5 Kidney Disease
BP control is fundamental in patients with kidney disease in order to slow the progression towards renal failure. The use of RAS inhibitors reduces BP levels (including intraglomerular pressure) and proteinuria and the progression towards chronic kidney disease. Both classes of drugs, including ACE inhibitors and ARBs, have been recently demonstrated to be effective in preventing the onset of microalbuminuria [50, 51], thus confirming the primary protective role of RAS blockade in patients with potential or overt kidney disease.
4.1.6 Atrial Fibrillation
The RAS seems to play a key role in the electrical and structural remodelling that underlies atrial fibrillation and probably in limiting the oxidative stress (and consequent activation of the renin-angiotensin system), which is currently implicated in the development of arrhythmias. Treatment with ACE inhibitors or ARBs has been found to be equally effective in reducing atrial fibrillation in patients with heart failure (ACE inhibitors OR: 0.64; 95 % CI 19–50 %; p = 0.0003; ARBs OR: 0.64; 95 % CI 22–48 %; p < 0.0001) [52].
4.1.7 Diabetes Mellitus
The metabolic abnormalities associated with diabetes mellitus, even in the initial stages, activate the RAS and increase levels of angiotensin II and aldosterone. Thus, RAS inhibitors may contribute in decreasing the production of pro-inflammatory mediators and oxidative stress and increase the sensitivity of tissues to insulin. ACE inhibitors promote an increased sensitivity to insulin in skeletal muscles, reduce the concentration of angiotensin II and increase that of bradykinin. Furthermore, they improve the microcirculation in hyperglycaemic patients, lowering the risk of microvascular complications that are often associated with diabetes. Some ARBs (telmisartan and, to a lesser degree, irbesartan), have demonstrated to act as PPAR-gamma agonists, improving sensitivity to insulin and reduce oxidative stress, beyond their BP lowering activities.
4.2 Adherence to Therapy
The factors responsible for the lack of BP control in clinical practice include a high rate of treatment discontinuation, due to poor adherence to antihypertensive therapy. This poor adherence is conditioned by numerous factors, such as the type of antihypertensive drugs, the concomitant use of other therapies, the patient’s clinical condition and sometimes also the conditions and place in which the patient lives [53]. Even a switch from one compound to another, both for ACE inhibitors and for ARBs, can negatively affect adherence to therapy. According to data from studies by Mancia et al. [54, 55], adherence to therapy is better with ARBs than with ACE inhibitors (Hazard Ratio [HR], 1.33; 95 % Confidence Intervals [CI] 1.13–1.57), although still sub-optimal. Furthermore, within the classes of ACE inhibitors and ARBs there are differences in treatment interruption [56]. Among the ACE inhibitors, captopril and moexipril are the drugs with the highest rates of suspension, while ramipril, zofenopril and fosinopril are those with the lowest rates. Among the ARBs, the rate of treatment interruption is highest for losartan and lowest for olmesartan.
4.3 Adverse Reactions and Safety
ACE inhibitors are generally well-tolerated drugs. The most frequent adverse effects are cough, hyperkalaemia and first-dose hypotension. Cough is characteristically non-productive and has been attributed to accumulation of bradykinin, substance P and prostaglandins in the lungs. Hyperkalaemia is a direct consequence of the reduction of aldosterone and is more marked the worse the renal impairment. Other adverse effects include morbilloform or maculopapular skin rashes, with or without pruritus, dysgeusia, neutropenia (generally reversible and tends to disappear within 3 weeks of treatment suspension) and hepatic toxicity [57, 58]. A very small percentage (0.1–0.2 %) of subjects can develop angioedema, which is manifested by rapid swelling of the nose, lips, tongue and glottis, with or without laryngeal oedema. The angioedema resolves within a few hours of interrupting ACE inhibitor treatment; however, the patient’s airways must be protected and, if necessary, the patient must be given adrenaline, antihistamines and glucocorticoids [59].
Given their selectivity of action, ARBs were initially introduced onto the market as drugs with a potentially lower risk of causing the typical adverse reactions to ACE inhibitors. Actually these drugs can cause symptomatic hypotension, particularly in patients already taking high doses of diuretics, and may exacerbate the effect of other BP-lowering drugs when taken concomitantly. Other documented reactions are headache, dizziness, back pain and gastrointestinal disorders. Furthermore, these drugs may be responsible for increased levels of serum potassium in patients with kidney disease or taking potassium-sparing diuretics.
Finally, both ACE inhibitors and ARBs are absolutely contraindicated in pregnancy. In the first months they can be teratogenic, in later months they can cause problems to the foetus and oligohydramnios [60].
4.4 Are There Differences Between the Various ACE Inhibitors and ARBs?
Comparisons between the various ACE inhibitors indicate that they are remarkably similar in their capacity to lower BP. In a comparison of 14 active principles, the estimated decrease in BP was between −6/−4 and −9/−5 mmHg (40). Similarly, a comparison of ARBs showed estimated decreases of BP levels in the range from −6/−3 to −10/−7 mmHg.
As far as concerns ARBs, three aspects should be considered: (1) different ARBs not only have different efficacy, but also different durations of action; (2) efficacy is not always the same between the different compounds if administered during the day or the night; (3) efficacy in the last 4 h, (the period furthest from the last dose) seems to be dependent on the type of ARB rather than on its dose. This could be because the influence of dose on antihypertensive efficacy is less relevant than the specific pharmacological characteristics of the individual active principles. Furthermore, the differences seem to be more evident for diastolic BP.
Tables 6 and 7 summarise the main efficacy and safety characteristics of ACE inhibitors and ARBs.
5 Appropriateness of the Use of RAS Inhibitors
Nowadays, it is no longer justifiable to consider only antihypertensive treatment because less than 20 % of patients have isolated hypertension, while more than 75 % of the hypertensive population have at least one associated risk factor (on average 2 or 3). Furthermore, one of the still unresolved problems is that of subjective tolerance, which seems, overall, to be good for RAS inhibitors although better for the ARBs than for the ACE inhibitors. Nowadays the RAS inhibitors are used mainly for all states of hypertension associated with any form of heart disease, whether ischaemic or LV dysfunction of variable severity. Appropriateness of use also involves the identification of specific compounds, since simple “equivalence” based on a prevailing mechanism of action cannot be automatically assumed to translate into the same levels of efficacy. The appropriateness of use of RAS inhibitors also depends on some management aspects, including the patient’s preference (nowadays determinant for every drug), the need to ensure temporal stability of successful treatments, periodic estimates of the clinical result and subjective tolerability and the level of loyalty of the patient with regards to a specific product that has been demonstrated, in that case, to be effective. Quantitative and qualitative changes in treatment should be taken into consideration in patients with unsatisfactory or partial results and after making sure that the principles of therapeutic adequacy (type of patient, doses and methods of administration) have been respected.
6 Conclusions
The following conclusions can be drawn from the data discussed in this paper. First of all, all RAS inhibitors are similarly effective in lowering BP levels. However, they have differences with regard to pharmacological characteristics (pharmacokinetics and pharmacodynamics) and efficacy in the treatment of different cardiovascular co-morbidities often associated with disorders such as systemic hypertension (for example, acute myocardial infarction, chronic heart failure, diabetes mellitus, stroke and kidney disease with or without proteinuria). For these reasons, when choosing the most appropriate RAS inhibitor, it is essential to consider both the clinical characteristics of the patient and, in particular, the presence of co-morbid conditions, and the pharmacological characteristics of the different compounds with the aim of choosing the drug able to ensure the greatest short and long-term efficacy in terms of cardiovascular prevention.
Once the expected therapeutic objectives have been reached, the on-going effective treatment should be continued to consolidate the results in the long-term. A key aspect of this strategy is to promote adherence to treatment which is a concrete aim that can be met easily with RAS inhibitors because of their very good tolerability, provided that the choice focuses on: (a) adequacy of the drug with respect to the patient, (b) intrinsic tolerability of the different compounds, and (c) continuity of effective therapeutic choices.
Although the RAS has been very well studied, recent research revealed that there are still many aspects to clarify and, in particular, more mediators to take into consideration, also in terms of developing new compounds that can act on this system. At the moment the most important drugs that modulate the renin angiotensin system, ACE-inhibitors and ARBs, are effective in systemic hypertension, heart failure and ischaemic heart disease, as demonstrated by experimental findings and many clinical data. These drugs have excellent tolerability as shown by the fact that they are the most frequently prescribed drugs for the indicated disorders. These drugs have pharmacokinetic and pharmacodynamics differences, and so each doctor, general practitioner and/or specialist, plays a central role when choosing the most appropriate drugs, taking into account any co-morbidities that may be present.
References
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159–219.
Volpe M, Rosei EA, Ambrosioni E, Cottone S, Cuspidi C, Borghi C, et al. 2012 consensus document of the Italian Society of Hypertension (SIIA): strategies to improve blood pressure control in Italy: from global cardiovascular risk stratification to combination therapy. High Blood Press Cardiovasc Prev. 2013;20(1):45–52.
Angeli F, Angeli E, Cavallini C, Ambrosio G, Mazzotta G, Reboldi G, et al. Electrocardiographic abnormalities of left ventricular repolarization: prognostic implications in hypertensive post-menopausal women. Maturitas. 2010;67(2):159–65.
Task Force on the management of STseamiotESoC, Steg PG, James SK, Atar D, Badano LP, Blomstrom-Lundqvist C, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33(20):2569–619.
Task Force M, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949–3003.
Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm. 2011;8(8):1308–39.
Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93(9):1137–46.
McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787–847.
Hamada K, Taniguchi Y, Shimamura Y, Inoue K, Ogata K, Ishihara M, et al. Serum level of soluble (pro)renin receptor is modulated in chronic kidney disease. Clin Exp Nephrol. 2013;17(6):848–56.
Batenburg WW, Danser AH. (Pro)renin and its receptors: pathophysiological implications. Clin Sci (Lond). 2012;123(3):121–33.
Sim MK. Des-aspartate-angiotensin I, a novel angiotensin AT(1) receptor drug. Eur J Pharmacol. 2015;5(760):36–41.
Lorenz JN. Chymase: the other ACE? Am J Physiol Ren Physiol. 2010;298(1):F35–6.
Romero CA, Orias M, Weir MR. Novel RAAS agonists and antagonists: clinical applications and controversies. Nat Rev Endocrinol. 2015;11(4):242–52.
Dzau VJ. Tissue renin-angiotensin system in myocardial hypertrophy and failure. Arch Intern Med. 1993;153(8):937–42.
Capuano F, Rossi F. Farmaci del sistema cardiovascolare del sangue e del rene. In: Medica EM, editor. Farmacologia: principi di base e applicazioni terapeutiche; 2011.
Ferrao FM, Lara LS, Lowe J. Renin-angiotensin system in the kidney: what is new? World J Nephrol. 2014;3(3):64–76.
Etelvino GM, Peluso AA, Santos RA. New components of the renin-angiotensin system: alamandine and the MAS-related G protein-coupled receptor D. Curr Hypertens Rep. 2014;16(6):433.
von Lueder TG, Atar D, Krum H. Current role of neprilysin inhibitors in hypertension and heart failure. Pharmacol Ther. 2014;144(1):41–9.
Gu J, Noe A, Chandra P, Al-Fayoumi S, Ligueros-Saylan M, Sarangapani R, et al. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi). J Clin Pharmacol. 2010;50(4):401–14.
Shliakhto EV, Konradi AO, Moiseeva OM. Molecular, genetic, and cellular aspects of the heart and vessel remodeling in hypertension (review). Ter Arkh. 2004;76(6):51–8.
Sankaranarayanan K, Chakraborty R, Boerwinkle EA. Ionizing radiation and genetic risks. VI. Chronic multifactorial diseases: a review of epidemiological and genetical aspects of coronary heart disease, essential hypertension and diabetes mellitus. Mutat Res. 1999;436(1):21–57.
Williams RR, Dadone MM, Hunt SC, Jorde LB, Hopkins PN, Smith JB, et al. The genetic epidemiology of hypertension: a review of past studies and current results for 948 persons in 48 Utah pedigrees. Prog Clin Biol Res. 1984;147:419–42.
Schlager G. Spontaneous hypertension in laboratory animals. A review of the genetic implications. J Hered. 1972;63(1):35–8.
Andreadis EA, Angelopoulos ET, Kolyvas GN, Agaliotis GD, Mousoulis CG, Mousoulis GP. The effect of aliskiren versus ramipril-based treatment on the Ambulatory Arterial Stiffness Index in hypertensive patients. Int Angiol. 2014;33(1):78–83.
Ohsawa M, Tamura K, Kanaoka T, Wakui H, Maeda A, Dejima T, et al. Addition of aliskiren to Angiotensin receptor blocker improves ambulatory blood pressure profile and cardiorenal function better than addition of benazepril in chronic kidney disease. Int J Mol Sci. 2013;14(8):15361–75.
Kanaoka T, Tamura K, Ohsawa M, Wakui H, Maeda A, Dejima T, et al. Effects of aliskiren-based therapy on ambulatory blood pressure profile, central hemodynamics, and arterial stiffness in nondiabetic mild to moderate hypertensive patients. J Clin Hypertens (Greenwich). 2012;14(8):522–9.
Agenzia Italiana per il Famraco (AIFA). Rapporto dell’Osservatorio sull’impiego dei Medicinali (OsMed) 2014. Uso dei Farmaci in Italia. 2015 [cited 2015 August, 20]. http://www.agenziafarmaco.gov.it/it/content/luso-dei-farmaci-italia-rapporto-osmed-2014.
Weir MR, Yadao AM, Purkayastha D, Charney AN. Effects of high- and low-sodium diets on ambulatory blood pressure in patients with hypertension receiving aliskiren. J Cardiovasc Pharmacol Ther. 2010;15(4):356–63.
Chen Y, Meng L, Shao H, Yu F. Aliskiren vs. other antihypertensive drugs in the treatment of hypertension: a meta-analysis. Hypertens Res. 2013;36(3):252–61.
Harel Z, Gilbert C, Wald R, Bell C, Perl J, Juurlink D, et al. The effect of combination treatment with aliskiren and blockers of the renin-angiotensin system on hyperkalaemia and acute kidney injury: systematic review and meta-analysis. BMJ. 2012;344:e42.
Nickenig G, Ostergren J, Struijker-Boudier H. Clinical evidence for the cardiovascular benefits of angiotensin receptor blockers. J Renin Angiotensin Aldosterone Syst. 2006;7(Suppl 1):S1–7.
Zhenfeng Z, Huilan S, Junya J, Dong L, Shan L. A systematic review and meta-analysis of aliskiren and angiotension receptor blockers in the management of essential hypertension. J Renin Angiotensin Aldosterone Syst. 2011;12(2):102–12.
Strauss MH, Hall AS. Angiotensin receptor blockers may increase risk of myocardial infarction: unraveling the ARB-MI paradox. Circulation. 2006;114(8):838–54.
Bangalore S, Kumar S, Wetterslev J, Messerli FH. Angiotensin receptor blockers and risk of myocardial infarction: meta-analyses and trial sequential analyses of 147,020 patients from randomised trials. BMJ. 2011;342:d2234.
Epstein BJ, Gums JG. Angiotensin receptor blockers versus ACE inhibitors: prevention of death and myocardial infarction in high-risk populations. Ann Pharmacother. 2005;39(3):470–80.
Tsuyuki RT, McDonald MA. Angiotensin receptor blockers do not increase risk of myocardial infarction. Circulation. 2006;114(8):855–60.
Volpe M, Tocci G, Trimarco B, Mancia G. Angiotensin II receptor blockers and risk of myocardial infarction: a meta-analysis of randomized clinical trials updated until May 1, 2008. High Blood Press Cardiovasc Prev. 2008;15(3):171–215.
Investigators O, Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547–59.
Swedberg K, Held P, Kjekshus J, Rasmussen K, Ryden L, Wedel H. Effects of the early administration of enalapril on mortality in patients with acute myocardial infarction. Results of the Cooperative New Scandinavian Enalapril Survival Study II (CONSENSUS II). N Engl J Med. 1992;327(10):678–84.
The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316(23):1429–35.
The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293–302.
Vantrimpont P, Rouleau JL, Wun CC, Ciampi A, Klein M, Sussex B, et al. Additive beneficial effects of beta-blockers to angiotensin-converting enzyme inhibitors in the Survival and Ventricular Enlargement (SAVE) Study. SAVE Investigators. J Am Coll Cardiol. 1997;29(2):229–36.
Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362(9386):759–66.
Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345(23):1667–75.
Pitt B, Poole-Wilson PA, Segal R, Martinez FA, Dickstein K, Camm AJ, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial–the Losartan Heart Failure Survival Study ELITE II. Lancet. 2000;355(9215):1582–7.
Pitt B, Segal R, Martinez FA, Meurers G, Cowley AJ, Thomas I, et al. Randomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study, ELITE). Lancet. 1997;349(9054):747–52.
Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Kober L, Maggioni AP, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349(20):1893–906.
The SOLVD Investigattors. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327(10):685–91.
Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995–1003.
Castro Hevia J, Antzelevitch C, Tornes Barzaga F, Dorantes Sanchez M, Dorticos Balea F, Zayas Molina R, et al. Tpeak-Tend and Tpeak-Tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. J Am Coll Cardiol. 2006;47(9):1828–34.
Haller H, Ito S, Izzo JL Jr, Januszewicz A, Katayama S, Menne J, et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364(10):907–17.
Schneider MP, Hua TA, Bohm M, Wachtell K, Kjeldsen SE, Schmieder RE. Prevention of atrial fibrillation by renin-angiotensin system inhibition a meta-analysis. J Am Coll Cardiol. 2010;55(21):2299–307.
Wolk R, Mazurek T, Lusawa T, Wasek W, Rezler J. Left ventricular hypertrophy increases transepicardial dispersion of repolarisation in hypertensive patients: a differential effect on QTpeak and QTend dispersion. Eur J Clin Invest. 2001;31(7):563–9.
Corrao G, Parodi A, Zambon A, Heiman F, Filippi A, Cricelli C, et al. Reduced discontinuation of antihypertensive treatment by two-drug combination as first step. Evidence from daily life practice. J Hypertens. 2010;28(7):1584–90.
Corrao G, Zambon A, Parodi A, Poluzzi E, Baldi I, Merlino L, et al. Discontinuation of and changes in drug therapy for hypertension among newly-treated patients: a population-based study in Italy. J Hypertens. 2008;26(4):819–24.
Mancia G, Parodi A, Merlino L, Corrao G. Heterogeneity in antihypertensive treatment discontinuation between drugs belonging to the same class. J Hypertens. 2011;29(5):1012–8.
Wolk R, Stec S, Kulakowski P. Extrasystolic beats affect transmural electrical dispersion during programmed electrical stimulation. Eur J Clin Invest. 2001;31(4):293–301.
Zhao X, Xie Z, Chu Y, Yang L, Xu W, Yang X, et al. Association between Tp-e/QT ratio and prognosis in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Clin Cardiol. 2012;35(9):559–64.
Sauer A, Wilcox JE, Andrei AC, Passman R, Goldberger JJ, Shah SJ. Diastolic electromechanical coupling: association of the ECG T-peak to T-end interval with echocardiographic markers of diastolic dysfunction. Circ Arrhythm Electrophysiol. 2012;5(3):537–43.
Passino C, Magagna A, Conforti F, Buralli S, Kozakova M, Palombo C, et al. Ventricular repolarization is prolonged in nondipper hypertensive patients: role of left ventricular hypertrophy and autonomic dysfunction. J Hypertens. 2003;21(2):445–51.
Acknowledgments
The Authors wish to thank Mrs Daniela Cimmaruta and Mrs Annalisa Capuano for their help and assistance in preparing the English version of this consensus document.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
C. Borghi: On behalf of the SIIA Task Force.
F. Rossi: On behalf of the SIF Task Force.
The list of the members of the SIIA and SIF Task Force are given in “Appendix”.
Appendix
Appendix
Members of the SIIA Task Force: Enrico Agabiti Rosei (Brescia), Nicola De Luca (Napoli), GiovanBattista Desideri (L’Aquila), Giuseppe Mancia (Milan), Paolo Pauletto (Padova), Gianfranco Parati (Milan), Roberto Pontremoli (Genova), Giuseppe Schillaci (Chieti), Michele Stornello (Siracusa). Giuliano Tocci (Rome), Franco Veglio (Torino), Agostino Virdis (Pisa), Massimo Volpe (Rome).
Members of the SIF Task Force: Pier Luigi Canonico (Novara), Giuseppe Cirino (Napoli), Salvatore Cuzzocrea (Messina), Romano Danesi (Pisa), Monica Di Luca (Milano), Giorgio Cantelli Forti (Bologna), Armando Genazzani (Torino), Giovan Battista Leproux (Roma), Luca Steardo (Roma).
Scientific Advisor: Prof. Bruno Trimarco, President of the Italian Society of Cardiovascular Prevention (SIPREC), Hypertension Research Center, and Department of Advanced Biomedical Sciences, Federico II University, Naples, Italy.
Rights and permissions
About this article
Cite this article
Borghi, C., SIIA Task Force., Rossi, F. et al. Role of the Renin-Angiotensin-Aldosterone System and Its Pharmacological Inhibitors in Cardiovascular Diseases: Complex and Critical Issues. High Blood Press Cardiovasc Prev 22, 429–444 (2015). https://doi.org/10.1007/s40292-015-0120-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40292-015-0120-5