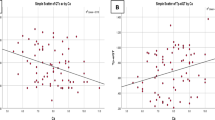
Abstract
Introduction
Serum uric acid (SUA) may contribute to the increased cardiovascular damage through direct injury to the endothelium and alteration of cardiovascular function.
Aim
To evaluate the association of SUA with the presence of the most recurrent electrographic alterations and with the length of the main ECG intervals in a large sample of general population.
Methods
For this study, on the database of the Brisighella Heart Study, we evaluated the available data of 790 men and 849 women, excluding subjects affected by gout or taking antihyperuricemic agents, those taking drug increasing the QT interval and those using beta-blockers or non-dihydropyridine calcium channel blockers at the moment of the ECG registration. Multiple ascending stepwise regression analyses were carried out to determine the independent predictors of the predefined ECG alterations.
Results
The prevalence of predefined ECG alterations was comparable between genders, with the exception of sinus bradicardia, left-anterior fascicular block, atrio-ventricular blocks and left ventricular hypertrophy (LVH), which appeared to be more frequent in men. The multivariate analysis revealed that SUA was associated to ischaemic alterations, LVH, sinus tachycardia and tachyarrhytmias. Age was associated to all evaluated ECG alterations beyond sinus tachycardia and LVH. Male sex was associated to sinus bradicardia, atrio-ventricular blocks, anterior-left fascicular block and LVH. Blood pressure was associated to different ECG alterations, but with clinically relevant OR with ischaemic alterations and LVH.
Conclusion
SUA level is related the prevalence of both organic and rhythm ECG alterations in a wide sample of general population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Uric acid (UA) is a diprotic acid which represents the final product of purine catabolism. In normal conditions its serum level is lower than 6 mg/dL in women and 7 mg/dL in men [1], due to a homeostatic regulation mainly ruled out by the kidney. Higher levels are defined as hyperuricemia, which could be the result both of overproduction or underexcretion, as many are the factors that could influence SUA. Some endogenous, such as age, sex, renal function and high cellular turnover conditions, and some others exogenous, such as purine intake and alcohol consumption.
The importance of determining SUA is due to the fact that, since it was initially considered to be an inert composite, it has been widely demonstrated that UA possesses opposite properties depending on its serum level: antioxidant at physiological concentrations and, on the contrary, pro-oxydant in hyperuricemia [2]. Thus, focusing on pathologies characterized by inflammation and oxidative stress, numerous proves have been collected so far to affirm that UA contributes to the development of some of the most important cardiovascular diseases, from hypertension to chronic heart failure and to the vast field of coronary artery diseases [3]. Its detrimental effects on the cardiovascular system include mediating immune response upon cell injury [4], increasing endotoxin-stimulated tumor necrosis factor-alpha production and hence proinflammatory immune activation [5]. Therefore, high levels of SUA may contribute to the increased cardiovascular damage through direct injury to the endothelium and alteration of cardiovascular function [6]. However there is a lack of studies about the relationship between UA and electrographic documented alterations. Thus, the aim of this study was to evaluate the association of SUA with the presence of the most recurrent electrographic alterations and with the length of the main ECG intervals in a large sample of general population.
2 Methods
The Brisighella Heart Study (BHS) is a prospective, population-based longitudinal epidemiological investigation involving 2,939 randomly selected subjects (1,491 men and 1,448 women), aged 14–84 years, free of cardiovascular disease at enrolment, resident in the northern Italy rural town of Brisighella. The study started in 1972 and it is still on-going. The town of Brisighella was originally selected as the site for the study, because of the homogeneity of life-style among its residents, with a very low rate of migration. Subjects were clinically evaluated at baseline and every 4 years thereafter by collecting an extensive amount of clinical and laboratory data [7, 8].
The BHS protocol and its sub-studies have been approved by the Ethical Board of the University of Bologna and all volunteers involved gave their signed consent to the participation to the study.
A detailed description of the protocol of the BHS and the full protocol have been largely described elsewhere [9]. Briefly, all-cause mortality and morbidity, as well as the incidence of the main cardiovascular risk factors, were recorded throughout the duration of the entire study. During each survey we perform an update of familial and personal history (with a specific attention to life-style habits and pharmacological treatments), a physical examination (with a specific attention to anthropometric measurements, blood pressure measuring, heart and breath rate evaluation), and we collect a fasting blood sample [10, 11]. Hematochemical analyses have been evaluated according to standardized methods [12] by trained personnel and include fasting plasma glucose (FPG), total cholesterol (TC), low-density lipoprotein-cholesterol (LDL-C), triglycerides (TG), high-density lipoprotein-cholesterol (HDL-C), non-HDL cholesterol, aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, creatinine, SUA, and creatine-phospho-kinase (CPK). Glomerular filtration rate was derived using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [13].
Standard 12-lead resting ECG recordings were performed using a Marquette MAC 12 electrocardiograph (Marquette Medical Systems, Inc., Milwaukee, WI) with signals sampled at 250 Hz per channel. A representative P-QRS-T cycle was then derived by selective averaging using the Dalhousie ECG Analysis Program [14]. The automated diagnosis of the ECG software was confirmed in blind manner by a trained cardiologist. Sinus bradycardia was defined by a sinusal rhythm with a heart rate lower than 60 beats per minute (BPM); same but non sinusal frequencies were grouped and considered as bradyarrhytmias. Sinusal frequencies higher than 100 BPM were defined as sinus tachycardia; same but non sinusal frequencies were grouped and considered as tachyarrhytmias. Left ventricular hypertrophy was calculated according to the Sokolow–Lyon formula, as recommended by current guidelines [15], and the diagnosis was further confirmed by the R-wave voltage in lead aVL, that seems to present a greater diagnostic ability in detecting left ventricular hypertrophy at a population level [16].
Signs of previous myocardial infarction were detected by the presence of Q waves, according to current recommendations from European guidelines [17]. We excluded unspecific and/or uncommon alterations from our analysis.
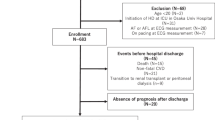
For this study, considering the 2012 population survey, we evaluated the available data of 790 men and 849 women, excluding subjects affected by gout or taking antihyperuricemic agents, those taking drug increasing the QT interval at the moment of the ECG registration [18] and those using beta-blockers or non-dihydropyridine calcium channel blockers.
Continuous parameters have been described separately by sex, being the most part of them differently distributed between genders, in particular ECG wave intervals, and their values have been compared by t test for independent samples. Univariate regression analysis with Bonferroni’s correction and the Pearson correlation coefficients were used to assess the association between sampled anthropometric, biochemical, clinical parameters and ECG intervals.
Multiple ascending stepwise regression analyses were carried out to determine the independent predictors of the predefined ECG alterations. All variables at p ≤ 0.2 in univariate analysis were candidates to the model.
A p value less than 0.05 was regarded as statistically significant. Statistical analyses were performed using the SPSS 21.0 statistical software package (IBM corporation, Armonk, NY, USA).
3 Results
The main clinical and laboratory characteristics of the studied population, grouped by sex, are resumed in Table 1. All the considered continuous parameters were significantly higher in men, except for HDL-C, Apo-AI and estimated Glomerular Filtration Rate that were significantly higher in women, while age and LDL-C were similar between the genders.
The ECG diagnoses, distributed by sex, are resumed in Table 2. For each considered electrocardiographic alteration the percentage of diagnosed ECG was comparable between the two sexes, with the exception of sinus bradicardia, left-anterior fascicular block, atrio-ventricular blocks and left ventricular hypertrophy which appeared to be more frequent in men.
The multivariate analysis (Table 3) revealed that SUA was associated to previous myocardial infarction, left ventricular hypertrophy, sinus tachycardia and tachyarrhytmias. Age was associated to all evaluated ECG alterations beyond sinus tachycardia and left ventricular hypertrophy. Male sex was associated to sinus bradicardia, atrio-ventricular blocks, anterior-left fascicular block and left ventricular hypertrophy. Blood pressure was associated to different ECG alterations, but with clinically relevant OR with ischaemic alterations and left ventricular hypertrophy (Table 4).
4 Discussion
Beyond the most well known cardiovascular risk factors UA has also been supposed to contribute to the development of cardiovascular diseases. Thus, despite the need of further prospective and randomized trials to better establish the burden of this assumption, mainly due to the fact that hyperuricemia is commonly associated with metabolic syndrome and renal function impairment which are themselves cardiovascular risk factors, medical literature has widely described the involvement of SUA in several clinical entities as incident hypertension [19], stroke [20], acute coronary syndrome [19, 21, 22] and chronic heart failure [23, 24]. What we decided to focus on instead was the relationship between SUA and pure electrocardiographic alterations, without any referral to clinics, in a group of general population, as to our knowledge no other study had analyzed the relationship before, with the only exception of few works on atrial fibrillation (AF) we will discuss further.
According to our results, speaking about electrocardiographic alterations indicative of organic cardiac modifications, UA was found to be associated firstly with signs of previous myocardial infarction and it is worthy to underline its Odds Ratio, comparable with that of LDL-C and higher than that of SBP, revealing the strength of the parameter. Another correlation was UA with signs of left ventricular hypertrophy. The explanation of this findings is based on the microvascular damage induced by UA on the coronary wall which in time may lead to the loss of endothelium-dependent vasodilatation. UA reduces the compliance of vessel walls due to a lower NO bioavailability both by blocking l-arginine uptake [25] and by facilitating its degradation [26] as observed in murine and cellular models. Moreover UA can trigger microvascular inflammation stimulating the production of chemokines and inflammatory markers [27]. Additionally, UA stimulates renin-angiotensin system (RAS) [28] and vascular smooth cell proliferation [29], thus substantiating the relationship with electrocardiographic signs of left ventricular hypertrophy.
Considering instead tracings of rhythm alterations, we found UA was associated with tachyarrhytmias, which consisted principally of atrial fibrillation, and with sinus tachycardia. The results confirmed the recent TROMSO study [30] which showed the direct influence of baseline SUA on the risk of developing atrial fibrillation in a wide sample of general population and another study in which the authors supposed the larger left atrium dimension observed in hyperuricemic patients to represent the possible cause [31]. Anyway, we think that the inflammatory and oxidative properties of UA may play a part even this time, as inflammatory markers have been associated to the development of arrhythmias [32]. As for sinus tachycardia, it could be framed considering the higher heart rate as compensatory of the increased myocardial oxygen request due to the over-mentioned UA induced ventricular remodelling, while sinus bradicardia was not found to be associated with UA level. No correlations were found with bundle branch and atrio-ventricular blocks. As for bundle branch blocks the result could be partially in contrast with one previous study [33] which demonstrated a relatively high prevalence of the ECG alteration in patients on haemodialysis compared to general population, assuming that UA accumulation could play a part in altering the electric cardiac conduction. Anyway, the study population was not comparable with ours, as it was composed of patients with final-stage renal function impairment, leading to higher values of SUA, without considering any other potential confounding factors.
This study has some relevant limitations, the main of which is the transversal design of the study that did not allow concluding if SUA was higher in subjects developing ECG alterations before the change itself. The second one is that we did not exclude from the selection patients already taking lipid-lowering drugs or antihypertensive drugs (beyond beta-blockers or non-dihydropyridine calcium channel blockers) as the choice was made to preserve the sample representativity of the whole Brisighella Heart Study cohort, excluding however the main iatrogenic causes of rhythm alterations. Finally, our results could not directly applicable to different populations, since the Brisighella Heart Study cohort as specific characteristics, among which relevant could be highly prevalent sinus bradycardia, observed in all study surveys [7, 8, 10, 12].
To conclude, even if confounding factors could hamper the interpretation of UA involvement in the development of cardiovascular diseases and even if its burden to be considered as a new cardiovascular risk factor still has to be established with further trials, we demonstrated that UA serum level is related to the development of both organic and both rhythm ECG alterations in a wide sample of general population.
References
Jin M, Yang F, Yang I, Yin Y, Luo JJ, Wang H, Yang XF. Uric acid, hyperuricemia and vascular diseases. Front Biosci. 2012;17:656–69.
Borghi C, Verardi FM, Pareo I, Bentivenga C, Cicero AF. Hyperuricemia and cardiovascular disease risk. Expert Rev Cardiovasc Ther. 2014;12:1219–25.
Kivity S, Kopel E, Maor E, Abu-Bachar F, Segev S, Sidi Y, Olchovsky D. Association of serum uric acid and cardiovascular disease in healthy adults. Am J Cardiol. 2013;111:1146–51.
Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–21.
Netea MG, Kullberg BJ, Blok WL, Netea RT, van der Meer JW. The role of hyperuricemia in the increased cytokine production after lipopolysaccharide challenge in neutropenic mice. Blood. 1997;89:577–82.
Katsiki N, Karagiannis A, Athyros VG, Mikhailidis DP. Hyperuricaemia: more than just a cause of gout? J Cardiovasc Med. 2013;14(6):397–402.
Cicero AF, D’Addato S, Reggi A. Marchesini Reggiani G, Borghi C. Hepatic steatosis index and lipid accumulation product as middle-term predictors of incident metabolic syndrome in a large population sample: data from the Brisighella Heart Study. Intern Emerg Med. 2013;8:265–7.
Cicero AF, D’Addato S, Santi F, Ferroni A, Borghi C, Brisighella Heart Study. Leisure-time physical activity and cardiovascular disease mortality: the Brisighella Heart Study. J Cardiovasc Med. 2012;13:559–64.
Cicero AF, Dormi A, D’Addato S, Borghi C, Brisighella Heart Study Staff. From risk factor assessment to cardiovascular disease risk and mortality modification: the first 40 years of the Brisighella Heart Study. Clin Lipidol. 2011;6:269–76.
Cicero AF, Reggi A, Tartagni E, Grandi E, D’Addato S, Borghi C, Brisighella Heart Study. Dietary determinants of oxidized-low-density lipoprotein antibodies in a sample of pharmacologically untreated non-smoker subjects: data from the Brisighella heart study. Adv Clin Exp Med. 2013;22:69–76.
Cicero AF, Rosticci M, D’Addato S, Baronio C, Grossi G, Grandi E, Borghi C, Brisighella Heart Study Group. Population health needs assessment and healthcare services use in a three years follow-up on administrative and clinical data: results from the Brisighella Heart Study. High Blood Press Cardiovasc Dis. 2013;21:45–51.
Cicero AF, D’Addato S, Veronesi M, Rosticci M, Santi F, Dormi A, Borghi C, Brisighella Heart Study Group. Relationship between blood pressure, cholesterolemia and serum apolipoprotein B in a large population sample: the Brisighella Heart Study. J Hypertens. 2012;30:492–6.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
Rautaharju PM, MacInnis PJ, Warren JW, Wolf HK, Rykers PM, Calhoun HP. Methodology of ECG interpretation in the Dalhousie program; NOVACODE ECG classification procedures for clinical trials and population health surveys. Methods Inf Med. 1990;29:362–74.
Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Members Task Force. ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;2013(31):1281–357.
Rodrigues SL, Angelo LC, Baldo MP, et al. Detection of left ventricular hypertrophy by the R-wave voltage in lead aVL: population-based study. Clin Res Cardiol. 2013;102:653–9.
Thygesen K, Alpert JS, Jaffe AS, Dantas EM, Barcelos AM, Pereira AC, Krieger JE, Mill JG, Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551–67.
http://www.azcert.org/medical-pros/drug-lists/bycategory.cfm. Last accessed 1 Sept 2014.
Grayson PC, Kim SY, Lavalley M, et al. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2010;63:102–10.
Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke. 2006;37:1503–7.
Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and coronary artery disease: a systematic review and meta-analysis. Arthritis Care Res. 2010;62:170–80.
Wannamethee SG, Shaper AG, Whincup PH. Serum urate and the risk of major coronary heart disease events. Heart. 1997;78:147–53.
Duan X, Ling F. Is uric acid itself a player or a bystander in the pathophysiology of chronic heart failure? Med Hypotheses. 2008;70(3):578–81.
Kaufman M, Guglin M. Uric acid in heart failure: a biomarker or therapeutic target? Heart Fail Rev. 2013;18(2):177–86.
Schwartz IF, Grupper A, Chernichovski T, Grupper A, Hillel O, Engel A, Schwartz D. Hyperuricemia attenuates aortic nitric oxide generation, through inhibition of arginine transport, in rats. J Vasc Res. 2011;48:252–60.
Zharikov S, Krotova K, Hu H, et al. Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am J Physiol Cell Physiol. 2008;295:C1183–90.
Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, Wamsley A, Sheikh-Hamad D, Lan HY, Feng L, Johnson RJ. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41:1287–93.
Yu MA, Sánchez-Lozada LG, Johnson RJ, Kang DH. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens. 2010;28(6):1234–42.
Corry DB, Eslami P, Yamamoto K, Nyby MD, Makino H, Tuck ML. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J Hypertens. 2008;26(2):269–75.
Nyrnes A, Toft I, Njølstad I, Mathiesen EB, Wilsgaard T, Hansen JB, Løchen ML. Uric acid is associated with future atrial fibrillation: an 11-year follow-up of 6308 men and women—the Tromso Study. Europace. 2014;16:320–6.
Chao TF, Hung CL, Chen SJ, Wang KL, Chen TJ, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Chen SA. The association between hyperuricemia, left atrial size and new-onset atrial fibrillation. Int J Cardiol. 2013;168(4):4027–32.
Höglund N, Andersson J, Almroth H, Tornvall P, Englund A, Rosenqvist M, Jensen SM, Boman K. The predictive value of C-reactive protein on recurrence of atrial fibrillation after cardioversion with or without treatment with atorvastatin. Int J Cardiol. 2013;167(5):2088–91.
Mandić A, Tomić M, Petrov B, Romić Z. Atrial fibrillation, atrioventricular blocks and bundle branch blocks in hemodialysis patients. Coll Antropol. 2012;36(4):1395–9.
Acknowledgments
No author has a specific conflict of interest in the publication of this paper.
We particularly acknowledge Marina Giovannini and Elisabetta Rizzoli for their support to the Brisighella Heart Study laboratory activity and Marcella Cagnati for her work on data managing. We also sincerely thank the Faenza public health district and all the General Practitioners of Brisighella for their continuous support to the study.
Funding sources
This work has been conducted with funding of the University of Bologna and with an unrestricted grant from the “Fondazione del Monte” (Bank foundation).
Author information
Authors and Affiliations
Corresponding author
Additional information
The Brisighella Heart Study: the members are listed in Appendix.
Appendix: The Brisighella Study Group
Appendix: The Brisighella Study Group
Arrigo F.G. Cicero, Martina Rosticci, Cristina Baronio, Martino Morbini, Angelo Parini, Giulia Grossi, Elisa Grandi, Sergio D’Addato, Elena Ancarani, Silvia Palmesano, Marina Giovannini, Elisabetta Rizzoli, Marcella Cagnati, Giovanni Gardini, Riccardo Urso, Giuseppe Derosa, Stefano Bacchelli, Claudio Borghi.
Rights and permissions
About this article
Cite this article
Cicero, A.F.G., Rosticci, M., Reggi, A. et al. Relationship Between Serum Uric Acid and Electrocardiographic Alterations in a Large Sample of General Population: Data From the Brisighella Heart Study. High Blood Press Cardiovasc Prev 22, 129–134 (2015). https://doi.org/10.1007/s40292-014-0077-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40292-014-0077-9