Abstract
Background
Asthma is a multifactorial respiratory disease determined by interactions of multiple disease susceptibility genes and environmental factors. Interleukin (IL)-18 is an important cytokine for initiating and perpetuating the catabolic and inflammatory response in allergic asthma. A number of single nucleotide polymorphisms that influence IL-18 production are found in the gene promoter region.
Objectives
The aim of this study was to investigate the association of IL-18 −607C/A promoter polymorphism with asthma and whether this polymorphism influenced the severity of asthma in affected children. The influence of this promoter gene polymorphism on total serum IgE level in studied subjects was also investigated.
Subjects and Methods
This study was carried out at the Allergy Clinic of Abu El Reesh Children’s Hospital at Cairo University, Egypt. This study included 40 asthmatic children, subdivided into four groups according to different degrees of asthma severity, and 20 apparently healthy subjects as the control group. All cases were subjected to history taking, clinical examination, and the following laboratory investigations: complete blood count, total serum IgE level assay by ELISA and genomic DNA extraction, and analysis for IL-18 −607C/A promoter gene polymorphism using the PCR-RFLP (restriction fragment length polymorphism) technique.
Results
In the present study the IL-18 −607AA genotype frequency was higher in cases (22.5 %) than in the control group (15 %); however, the difference was not statistically significant (p = 0.773). No statistically significant difference between the degree of asthma severity and IL-18 −607C/A polymorphism was found (p = 0.489). No significant association could be detected upon comparing the frequencies of C and A alleles among the two studied groups (p = 0.366). Also, no significant differences were demonstrated for the allele frequencies when the intermittent with mild [odds ratio (OR) = 2.72, 95 % CI 1.03–2.33, p = 0.067], intermittent with moderate, and severe (OR = 2.8, 95 % CI 1.01–8.5, p = 0.066) asthma groups were compared. The median value of the total serum IgE level in asthmatic cases with the mutant genotype (AA) was significantly higher [360 IU/L (96.6–1,340 IU/L)] than in the control group [119 IU/L (70.6–158.9 IU/L)] (p = 0.033).
Conclusion
No significant statistical difference was encountered regarding the distribution of IL-18 −607C/A genotypes and allele frequencies in asthma patients and healthy controls. Also, there were no significant associations between asthma severity and different genotypes or alleles. The median value of the total serum IgE level in asthmatic cases with the mutant genotype (AA) was significantly higher than in the control group. Thus, IL-18 −607AA genotype frequency might be related to higher total serum IgE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Asthma is a syndrome characterized by intermittent narrowing of the small airways of the lung, with subsequent airflow obstruction and symptoms of wheeze, cough, and breathlessness. An important characteristic of asthma is airways hyper-responsiveness, which is the exaggerated narrowing of the airways in response to provocative agents [1].
In Egypt, asthma is a common health problem that causes considerable morbidity and appreciable mortality and imposes much demand on the time and costs of medical and hospital care [2]. The prevalence of asthma in children (2–12 years) is nearly 8.2 %. In Giza, the prevalence of asthma among school children was found to be 5–15 and 2.46 % among high and low socioeconomic classes, respectively [3].
As a chronic inflammatory disease, much of the research related to asthma has been focused on pro-inflammatory mechanisms, and advances have been made in defining mechanisms that control inflammation and induce immune tolerance to specific antigens [4].
New insights in the pathogenesis of asthma suggest the role of lymphocytes; airway inflammation in asthma may represent a loss of normal balance between two “opposing” populations of T helper (Th) lymphocytes. Two types of Th lymphocytes have been characterized: Th1 and Th2. Th1 cells produce interleukin (IL)-2 and interferon (IFN)-γ, which are critical in cellular defense mechanisms and promote pro-inflammatory immune reaction in response to infection. Th2, in contrast, generates a family of cytokines (IL-4, IL-5, IL-6, IL-9, and IL-13) that can mediate allergic inflammation and promote antibody-dependent immune response. As a result, there is a great interest in using cytokines as markers of human immune function [5].
IL-18 is a unique cytokine that enhances innate immunity and both Th1- and Th2-driven immune responses. Originally described as an IFN-γ-inducing factor that is produced by Kupffer cells, activated macrophages, keratinocytes, intestinal epithelial cells, osteoblasts, and adrenal cortex cells, IL-18 was found to act in synergy with IL-12 to promote the development of Th2 immune response by induction of IL-13. So, according to its role in regulation of the Th1/Th2 balance, IL-18 is considered to be a candidate asthma susceptibility gene [6].
The gene for human IL-18 is located on chromosome 11q22.2–22.3, a region that has been previously linked to atopy-related traits [7]. Several polymorphisms have been identified in the IL-18 gene; among them, −607C/A and −137C/G in the promoter region have been studied in detail with respect to etiopathogenesis of allergic diseases [8, 9].
The −607C/A variant disrupts a cyclic adenosine 5′-monophosphate (cAMP) responsive element-binding protein-binding site. It influences the quantitative expression of the IL-18. Thus, this polymorphism seems to account for differential IL-18 expression and changes in the production of this cytokine [10].
Previous studies have suggested that genetic polymorphisms of IL-18 and its receptor were associated with elevated serum IgE levels, atopy, and/or asthma in various populations [11–13].
In an extension of this research, we sought to investigate the association between the IL-18 −607C/A promoter polymorphism and asthma in group of Egyptian children with bronchial asthma and whether this polymorphism influenced the severity of asthma in affected children. Also, the relationship between the IL-18 gene polymorphism and the serum total IgE level was determined.
2 Subjects and Methods
This study was carried out at the Allergy Clinic of Abu El Reesh Children’s Hospital at Cairo University, Egypt. Informed oral consents were taken from all participants and the study was approved by the local ethical committee. Sixty subjects participated in this study: 40 asthmatic patients (19 males and 21 females) divided into intermittent asthma [15 (37 %)] and persistent asthma [25 (63 %)], which were further subdivided according to severity into mild [13 (32 %)], moderate [10 (25 %)], and severe [2 (5 %)]. Severity of asthma was diagnosed mainly according to symptoms and the degree of pulmonary function tests. Twenty healthy subjects (11 males and nine females) with no personal or family history of asthma or other atopic manifestations were included as control group. The age of both groups ranged from 2 to 12 years. The diagnosis of asthma was according to Global Initiative for Asthma (GINA) guidelines [14]. Patients with other health problems such as gastroesophageal reflux disease, cardiac disease, chronic chest diseases other than asthma, any systemic diseases, or who were treated with systemic corticosteroids within 2 weeks before sampling were excluded from the study (exclusion criteria).
All patients were subjected to a detailed clinical evaluation including history, clinical assessment of asthma severity, and physical examination (both general and chest examination).
All subjects were subjected to complete blood count (CBC), total serum IgE level, and genomic DNA extraction and analysis for IL-18 −607C/A promoter polymorphism.
2.1 Specimen Collection
From each patient, 5 mL venous blood was withdrawn; 1 mL was collected in an EDTA tube for CBC assay, 2 mL in plain tubes, sera were separated for assay of the total serum IgE level, and 2 mL in sterile EDTA vacutainers for DNA extraction and analysis for IL-18 −607C/A promoter polymorphism using PCR-RFLP (restriction fragment length polymorphism) analysis. Samples were stored at −20 °C until the time of assay.
2.2 Methodology
The total serum IgE level was determined using a Human IgE ELISA kit (quantitative solid-phase sandwich enzyme immunoassay technique) provided by IDLabs™ Biotechnology Inc. (http://www.idlabs.com).
2.2.1 DNA Isolation and Interleukin (IL)-18 −607C/A Promoter Gene Polymorphism
Genomic DNA was isolated from peripheral blood leukocytes using QIAamp DNA blood Mini kit (Qiagen GmbH) (Hoffmann-La Roche AG, Hilden, Germany).
Enzymatic amplification was performed by PCR using Dream Taq™ Green PCR Master Mix (supplied by Fermentas UAB, Vilnius, Lithuania) and Hybaid thermal cycler (Promega Corporation, Madison, WI, USA) (Table 1).
Amplification of IL-18 −607C/A (rs1946518) promoter gene region as proposed by Folwaczny et al. [15] was performed using two sets of primers provided by Operon Biotechnologies (GmbH/Biocampus, Germany): forward primer 5′-GCCCTCTTACCTGAATTTTGGTAGCCCTC-3′ and reverse primer 5′-AGATTTACTTTTCAGTGGAACAGGAGTCC-3′. The PCR reaction mixture (25 μL) contained 12.5 μL 2 × PCR Master Mix [10 × PCR buffer, 4 mmol/L MgCl2, 0.5 units Taq DNA polymerase/μL, and 400 μmol/L of each deoxyribonucleotide triphosphate (dNTP) (dATP, dCTP, dGTP, dTTP)], 1 μL of each primer (10 pmol), 3 μL of genomic DNA, and 7.5 μL sterilized nuclease-free water. The reaction was carried out with the following cycles: 94 °C for 5 min; 35 cycles of 30 s denaturation at 94 °C, 30 s annealing at 55 °C and 1 min extension at 72 °C; and a 10-min final extension at 72 °C after completion of the cycles (Table 2; Fig. 1).
The presence of amplified 171 bp on 1.5 % agarose gel containing ethidium bromide was detected by performing E/P on the gel electrophoresis apparatus and visualized by UV transillumination (Promega, USA). Then, 7 μL of the amplified products were digested with 5 units of FastDigest Tru1I restriction enzyme (recognition sequence: 5′ T↓T A A 3′ , 3′ A A T↑T 5′ ) at 65 °C for 10 min (supplied by Fermentas, Vilnius, Lithuania) (Table 3).
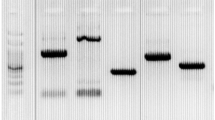
The digested products were then detected on 3.5 % agarose gel electrophoresis containing ethidium bromide: CC wild pattern appeared as a single band of 171 bp, CA heterozygous pattern appeared as three bands of 171, 101, and 70 bp, and AA homozygous pattern appeared as two bands of 101 and 70 bp (Table 4; Fig. 2).
2.3 Statistical Analysis
The data were coded and entered using the statistical package SPSS® version 15 (SPSS Inc., Chicago, IL, USA). The data were summarized using descriptive statistics: mean, standard deviation, minimal and maximum values for quantitative variables, and number and percentage for qualitative values. Genotype frequencies fulfilled Hardy-Weinberg equilibrium. Statistical differences between groups were tested using the Chi-square test for qualitative variables, independent sample t test and ANOVA with post hoc test for quantitative normally distributed variables, and the non-parametric Mann–Whitney test and Kruskal–Wallis test were used for quantitative variables which aren’t normally distributed. Correlations were performed to test for linear relations between variables. p values <0.05 were considered significant (Table 5; Fig. 3).
3 Results
There was no statistically significant difference in the frequency of the A allele between asthmatics with mild persistent severity and those with intermittent severity (p = 0.067); however, the mutant A allele was found to be a risk factor for asthma severity between the two groups [OR = 2.72, 95 % CI 2.72 (1.1–8.08)] (Fig. 4).
There was no statistically significant difference in the frequency of the A allele between asthmatics with intermittent severity and those with moderate and severe persistent type asthma (p = 0.066); however, the mutant A allele was found to be a significant risk factor for asthma severity between the two groups (OR = 2.8, 95 % CI 1.01–8.5) (Fig. 5).
It was found that the median value of the total serum IgE level in asthmatic cases with mutant genotype AA was significantly higher than in the control group (p = 0.033) (Table 5).
4 Discussion
With regards to sex distribution, both the asthmatic group and control group were comparable. In asthmatics, males constituted 47 % in comparison with 52 % females, while in the control group, males represented 55 % in comparison with 45 %females. Asthma prevalence is three times higher in boys than in girls before puberty [5], yet this was not observed in this present study, possibly due to our small sample size.
Upon comparing asthmatic patients according to severity, it was observed that the most common type was intermittent (37 %), followed by mild persistent, moderate and severe persistent (33, 25, and 5 %, respectively).
In the current study, upper respiratory tract infection represented the most common precipitating factor for asthma in asthmatic patients (37 %), followed by passive smoking as the second most common precipitating factor (22.5 %); this was consistent with the findings of Loengard [16], who studied asthmatic patients admitted to a hospital in England over the course of 1 year. He found that most of the adult patients (37 %) had evidence of a viral infection and that the majority of children with episodes of wheezing have viral infections. Our study was also consistent with Sawicki [17] who suggested that exposure to tobacco smoke during pregnancy and throughout childhood increases the risk of developing asthma.
In this study, only 45 % of cases had family history of asthma or other atopic manifestations; this is in agreement with Bjerg et al. [18] who found that although a family history of asthma is an important risk factor, it is neither necessary nor sufficient for predisposing an individual to developing asthma and that it is likely that a family history of asthma also includes environmental and social factors.
On the contrary, by reviewing population-based studies that evaluated family history of asthma and atopic disease in children with asthma, Bruke et al. [19] observed that a family history of asthma in one or more first-degree relatives was consistently identified as a risk factor for asthma.
The serum IgE level is often increased in allergic diseases, but a normal serum IgE level does not mean that atopy does not exist. On the other hand, a very high serum IgE level can be accepted as one of the important pieces of data showing the existence of allergy in the absence of parasitic infestations [20]. This was also found in the present study, in which a high serum IgE level was detected in asthmatic patients with a median level of (140 IU/L) compared with a lower median level (103 IU/L) in control cases. However, no statistical significance was detected between both groups (p = 0.695).
In the current study, results showed a higher absolute eosinophilic count in cases (median 100 cells/mm3) than in control cases (median 49 cells/mm3), but they were not of significant statistical value (p = 0.339). However, Hussein et al. [21] revealed a significant increase in eosinophilic count in atopic asthmatics compared with healthy controls.
Although the IL-18 −607 mutant homozygous AA genotype frequency was higher in cases (22.5 %) than in the control group (15 %), no statistically significant difference could be detected (p = 0.773). This result was in accordance with Pawlik et al. [22], who observed that the relative frequencies of CC, CA, and AA genotypes among asthmatic patients were 39, 41.1, and 19.9 %, respectively, and were 35.1, 47.5, and 7.4 %, respectively, among control group, and that the frequency of this polymorphism did not differ significantly between asthmatic patients and control individuals in a study carried out in a Polish population (p = 0.065). Heinzmann et al. [23] also denied that there was any association between the IL-18 −607C/A promoter polymorphism and bronchial asthma in children in a German population.
However, a study by Lachheb et al. [6] revealed that the distribution of genotypes was significantly different in asthma patients and controls (p = 0.008). Another study by Ma et al. [24] suggested through a meta-analysis that IL-18 −607C/A polymorphism in the promoter region was associated with asthma risk. They found that individuals carrying the AC/CC genotype of −607 C/A promoter polymorphism were associated with an increased asthma risk.
The discordance between some of the results of the current study and those revealed by other studies might reflect the genetic heterogeneity among different ethnic populations that influences allele and genotype frequencies.
In the current study, no statistically significant difference between the degree of asthma severity and IL-18 −607C/A polymorphism was found (p = 0.489). This result was in accordance with Holla et al. [25] who studied IL-18 −607C/A gene polymorphism in adult asthmatic Czech patients and did not find a relationship between genotypes and different degrees of asthma severity. Also, the study by Lachheb et al. [6] was unable to find a relationship between IL-18 −607C/A genotypes and groups of asthma severity (p = 0.58).
Allelic variants of cytokine genes associated with promoter gene region polymorphisms do not influence the protein amino-acid sequence but can result in changes in cytokine production. In consequence, they may alter the immune responses mediated by the cytokine in question and could be associated with various immunological diseases [26].
In the current study, no significant association could be detected upon comparing the frequencies of the two alleles (C, A) among the two studied groups (p = 0.366). Also, no significant differences were demonstrated for the allele frequencies when the intermittent with mild (OR = 2.72, 95 % CI 1.03–2.33, p = 0.067) and intermittent with moderate and severe [OR = 2.8, 95 % CI 1.01–8.5, p = 0.066) asthma groups were compared.
The previous result disagree with Lachheb et al. [6], who observed that carriers of the A allele were significantly more frequent in the asthma group than in the control group (43 vs. 33 %) (p = 0.02). They suggested that asthma patients carrying the −607A allele exhibited an increased risk of developing asthma than controls (OR = 1.55, 95 % CI 1.03–2.33), but in accordance with our results they observed that no significant differences were demonstrated for the allele frequencies of the IL-18 −607C/A polymorphism when the intermittent with mild (p = 0.58) and moderate with severe (p = 0.54) asthma groups were compared.
According to Cardon and Bell [27], some identified cytokine polymorphisms have been associated with allergies, asthma, or elevated IgE levels; therefore, the impact of this promoter polymorphism on the total serum IgE level was investigated in all study subjects. It was found that the median value of the total serum IgE level in asthmatic cases with mutant genotype AA was significantly higher [360 IU/L (96.6–1,340 IU/L)] than in the control group 119 IU/L (70.6–158.9 IU/L) (p = 0.033), while there was no statistical difference observed with the wild (CC) and heterozygous (CA) genotypes.
This was in part consistent with Nieters et al. [28] who suggested a role of IL-18 polymorphism (and other cytokines) in elevated IgE levels (p = 0.013). Their study was carried out in a German population with hay fever and atopy. However, these results disagree with Lachheb et al. [6] who did not find a similar association in asthmatic children; neither did Holla et al. [25] in their study of an adult asthmatic Czech population.
The population in the present study was geographically different from other comparative studies with different ethnic backgrounds, leading to a wide range of environmental triggers to which different populations were exposed, and this may explain why our results differ from other results carried out in different populations. Besides, the limited number of patients in this study may explain these contradictory results.
5 Conclusion
The distribution of IL-18 −607C/A genotypes and allele frequencies was not significantly different in asthma patients and controls. Also, there was no significant association between asthma severity and different genotypes and alleles. The median value of total serum IgE levels in asthmatic cases with mutant genotype AA was significantly higher than in the control group.
References
Settin A, Zedan M, Farag M, Ezz El Regal M, Osman G. Gene polymorphisms in IL-6 G/C and IL-1Ra VNT in asthmatic children. Indian J Pediatr. 2008;75:1019–23.
El-Hefny A, Nassar S, El-Heneidy F, Said M, El-Beleidy A, El-Marsafy E, Moustafa N, El-Falaky M, Haddad Z. Epidemiology of childhood asthma in Cairo. Med J Cairo Univer. 1994;62:505–18.
Yamamah G, Abdel Dayemm S, Mohamoud N. Bronchial asthma and hypertension among school children of Giza governorate, screening and epidemiology study. Med J Cairo Univ. 1999;67:229–37.
Gern JE, Seroogy CM. Role of T regulatory cells in asthma. J Allergy Clin Immunol. 2005;46:996–9.
Sharma G. Asthma overview (2010). http://emedicine.com. Accessed May 2010, revised Jun 2010.
Lachheb J, Chelb H, Ammar J, Hamzaoui K, Hamzaoui A. Promoter polymorphism of the IL-18 gene is associated with atopic asthma in Tunisian children. Int J Immunogenet. 2007;35:63–8.
Blumenthal MN, Langefeld CD, Beaty TH, Bleecker ER, Ober C, Lester L. A genome-wide search for allergic response (atopy) genes in three ethnic groups: Collaborative Study on the Genetics of Asthma. Hum Genet. 2003;114:157–64.
Kruse S, Kuehr J, Moseler M, Kopp MV, Kurz T, Deichmann KA. Polymorphisms in the IL-18 gene are associated with specific sensitization to common allergens and allergic rhinitis. J Allergy Clin Immunol. 2003;111:117–22.
Sebelova S, Izakovicova-Holla L, Stejskalova A, Schüller M, Znojil V, Vasku A. Interleukin-18 and its three gene polymorphisms relating to allergic rhinitis. J Hum Genet. 2007;52:152–8.
Giedraitis V, He B, Huang WX, Hillert J. Cloning and mutation analysis of the human IL-18 promoter: a possible role of polymorphisms in expression regulation. J Neuroimmunol. 2001;112:146–52.
Watanabe M, Kaneko H, Shikano H, Aoki M, Sakaguchi H, Matsui E. Predominant expression of 950delCAG of IL-18R alpha chain cDNA is associated with reduced IFN-gamma production and high serum IgE levels in atopic Japanese children. J Allergy Clin Immunol. 2002;109:669–73.
Liang XH, Cheung W, Heng CK, Wang DY. Reduced transcriptional activity in individuals with IL-18 gene variants detected from functional but not association study. Biochem Biophys Res Commun. 2005;33:736–41.
Lee KS, Kim SR, Park SJ, Min KM, Lee KY, Jin SM, Yoo WH, Lee YC. Antioxidant down-regulates interleukin-18 expression in asthma. Mol Pharmacol. 2006;70:1184–93.
Global Initiative for Asthma (GINA) program. Asthma classification, in diagnosis and classification; 2010. pp. 21–3. Available from http://www.ginasthma.org
Folwaczny M, Glas J, Torok HP, Tonenchi L, Paschos E, Bauer B, Limbersky O, Folwaczny C. Polymorphisms of the interleukin-18 gene in periodontitis patients. J Clin Periodontol. 2005;32:530–4.
Loengard A. Viral-induced asthma; 2008. http://asthma.about.com. Accessed Dec 2011.
Sawicki G. Asthma symptoms and diagnosis in children; 2010. http://www.uptodate.com. Accessed Jan 2011.
Bjerg A, Hedman L, Perzanowski MS, Platts-Mills T, Lundbäck B, Rönmark E. Family history of asthma and atopy: in-depth analyses of the impact on asthma and wheeze in 7- to 8-year-old children. Pediatrics. 2007;120:741–8.
Bruke W, Freinmeyer M, Reed K, Hampson L, Carlsten C. Family history as a predictor of asthma risk. Am J Prev Med. 2003;24:160–9.
Aral M, Arican O, Gul M, Sasmaz S, Kocturk SA, Kastal U, Ekerbicer HC. The relationship between serum levels of total IgE, IL-18, IL-12, INF-γ and disease severity in children with atopic dermatitis. Mediat Inflamm. 2006;2006:1–4.
Hussein YM, Ahmad AS, Ibrahim MM, El Tarhouny SA, Shalaby SM, El Shal AS, El Said M. INF-γ gene polymorphism as a biochemical marker in Egyptian atopic patients. J Investig Allergol Clin Immunol. 2009;19:292–8.
Pawlik A, Kaminski M, Kuśnierczyk P, Kurzawski M, Dziedziejko V, Adamska M, Safranow K, Gawronska-Szklarz B. Interleukin 18 promoter polymorphism in patients with atopic asthma. Tissue Antigens. 2007;70:314–8.
Heinzmann A, Gerhold K, Ganter K, Kurz T, Schuchmann L, Keitzer R. Association study of polymorphisms within interleukin-18 in juvenile idiopathic arthritis and bronchial asthma. Allergy. 2004;59:845–51.
Ma Y, Zhang B, Tang RK, Liu Y, Peng GG. Interleukin-18 promoter polymorphism and asthma risk: a meta-analysis. Mol Biol Rep. 2012;39:1371–6.
Holla LI, Hrdlickova B, Schuller M, Buckova D, Kindlova D, Izakovic V, Vasku A. Haplotype analysis of the interleukin-18 gene in Czech patients with allergic disorders. Hum Immunol. 2010;71:592–7.
Khripko OP, Sennikova NS, Lopatnikova JA, Khripko JI, Filipenko ML, Khrapov EA, Gelfgat EL, Yakushenko EV, Kozlov VA, Sennikov SV. Association of single nucleotide polymorphisms in the IL-18 gene with production of IL-18 protein by mononuclear cells from healthy donors. Mediat Inflamm. 2008;18:1–6.
Cardon LR, Bell JI. Association study designs for complex diseases. Nat Rev. 2001;2:91–9.
Nieters A, Linseisen J, Becker N. Association of polymorphisms in Th1, Th2 cytokine genes with hay fever and atopy in a sub-sample of EPIC-Heidelberg. Clin Exp Allergy. 2004;34:346–53.
Acknowledgement and Disclosures
The author has no conflicts of interest that are directly relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shaaban, H.H., Mohy, A.M., Abdel-Razek, AR.A. et al. Interleukin-18 −607C/A Gene Polymorphism in Egyptian Asthmatic Children. Mol Diagn Ther 18, 427–434 (2014). https://doi.org/10.1007/s40291-014-0097-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-014-0097-0