Abstract
Acute myocardial infarction (AMI) is the leading cause of death worldwide, with early diagnosis still being difficult. Promising new cardiac biomarkers such as troponins and creatine kinase (CK) isoforms are being studied and integrated into clinical practice for early diagnosis of AMI. The cardiac-specific troponins I and T (cTnI and cTnT) have good sensitivity and specificity as indicators of myocardial necrosis and are superior to CK and its MB isoenzyme (CK-MB) in this regard. Besides being potential biologic markers, cardiac troponins also provide significant prognostic information. The introduction of novel high-sensitivity troponin assays has enabled more sensitive and timely diagnosis or exclusion of acute coronary syndromes. This review summarizes the available information on the potential of troponins and other cardiac markers in early diagnosis and prognosis of AMI, and provides perspectives on future diagnostic approaches to AMI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cardiovascular disease remains a leading cause of morbidity and mortality worldwide, with a higher death rate annually than that of any other disease [1, 2]. Millions of people suffer from one or more forms of cardiovascular disease, such as hypertension, coronary artery disease, or stroke. According to a World Health Organization report in 2011 [2], an estimated 17.3 million people died from cardiovascular disease in 2008, representing 30 % of all global deaths. Currently, a higher death rate is reported in high-income countries such as the USA, but the percentage is increasing rapidly in low-income countries as well. It is speculated that by 2030, around 23.6 million people will die from cardiovascular diseases, mainly from heart disease and stroke. The largest increase in the number of deaths will occur in the South-East Asia region [2].
Acute myocardial infarction (AMI) most commonly starts with a coronary artery blockage as a result of thrombosis at the site of rupture of a vulnerable atherosclerotic plaque. If the resulting ischemia exceeds a critical threshold and is left untreated for a sufficient period of time, it can cause irreversible myocardial cell damage (infarction) or death [3]. However, AMI need not always be associated with chest pain, and this makes diagnosis of AMI challenging. Early diagnosis of acute chest pain in patients presenting to the emergency room is critical to save lives.
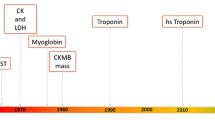
Diagnosis of AMI still predominantly depends on patient symptoms, electrocardiographic features, and assessment of classical cardiac markers [4, 5]. Many patients who do not have significant ST-segment elevation or ST-segment depression arrive at the hospital with nonspecific symptoms and have electrocardiographic features that turn out to be nondiagnostic in up to 50 % of cases [6]. Historically, total creatine kinase (CK), aspartate aminotransferase (AST), and total lactate dehydrogenase (LDH) levels have been measured as biomarkers of cardiac necrosis. However, these biomarkers have poor specificity for detection of cardiac injury because of their wide tissue distribution [7]. New markers of myocardial damage have challenged the place of traditional tests of cardiac injury [8, 9]. Cardiac troponins, in particular, have become the markers of choice for patients with acute coronary syndromes (ACS). Levels of cardiac troponins I and T (cTnI and cTnT) are more specific for diagnosis of AMI than previously used markers such as the MB isoenzyme of CK (CK-MB) [10]. In addition, frequent and serial measurements are not necessary, because an isolated measurement on the first postoperative day is enough to identify high-risk subgroups [5, 11]. Assessment of cTnI in patients undergoing myocardial revascularization surgery showed a characteristic immediate behavior, with a significant elevation in the serum cTnI level at intensive care unit admission and on the first postoperative day [12]. No correlation was observed in the duration of ischemia or the duration of extracorporeal circulation, suggesting that elevation of cTnI levels may be due to specific myocardial damage (native coronary obstruction, bypass occlusion, etc.) and not to extracorporeal circulation or the total time of ischemia. In view of this highly specific and sensitive behavior, the National Academy of Clinical Biochemistry (NACB) [13] proposed use of myoglobin and cTnI in conjunction as the ideal markers for the diagnosis of AMI.
The cTnI assay has been proven as a valuable diagnostic tool and has been shown to have prognostic importance in cardiac patients in conjunction with the results of clinical investigations, patient history, and symptoms [13, 14].
2 Commonly Used Cardiac Biomarkers
A biomarker is a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention [15]. The ideal biomarker for detecting myocardial injury needs to be expressed in relatively high levels within cardiac tissue, with high clinical sensitivity and specificity that is detectable in the blood early after the onset of chest pain. Various cardiac markers used in early diagnosis of AMI are described in Table 1. Localization and diagnostic values of common cardiac markers are described below.
2.1 Creatine Kinase MB Isoenzyme
CK-MB resides in the cytosol and facilitates movement of high-energy phosphates into and out of mitochondria. It is distributed throughout many tissues and is found even in skeletal muscle. CK-MB levels rise about 6 h after the onset of myocardial infarction (MI), peak between 18 and 24 h, and return to normal within 2–4 days. A CK-MB to CK ratio of >0.025 is a good indicator of MI. The specificity and sensitivity of CK-MB for detection of MI are low because of its presence in skeletal muscle. It is relatively specific when skeletal muscle damage is not present, but CK-MB levels becomes elevated in conditions following acute or chronic muscle injury and in patients undergoing a surgical procedure [16].
2.2 Myoglobin
Myoglobin is a low molecular weight (17 kDa) heme protein. It is present in both cardiac and skeletal muscle, and therefore its specificity as a cardiac marker is low. However, it is released earliest from infarcted myocardium. The blood myoglobin level rises more rapidly than that of any other cardiac marker, making it very sensitive for early detection of AMI [17]. It rises significantly above its baseline within 2–3 h after the event, peaks at 9–12 h, and returns to baseline within 24 h. Therefore, its usefulness for late detection of AMI is low.
2.3 Heart Fatty-Acid Binding Protein
Heart fatty-acid binding protein (HFABP), which is present in large amounts in myocardium and also in skeletal muscle, is a sensitive biomarker for MI, as it rises within 3 h after myocardial injury and returns to normal within 24 h, but its specificity is suspect since it is also present in skeletal muscle. HFABP may be used in combination with myoglobin to increase specificity [18]. HFABP, together with several markers of myocardial injury, may also be of value in assessment of patients with congestive heart failure. In addition to its diagnostic potential for early diagnosis of AMI, it is an independent predictor of events within 6 months [19].
2.4 Glycogen Phosphorylase BB
Glycogen phosphorylase BB (GlyPH-BB) is a large-sized enzyme (molecular weight 188 kDa) involved in the breakdown of glycogen for energy and is released in myocardial cells that have oxygen deficiency. It has high sensitivity and specificity early after chest pain. This makes GlyPH-BB an important marker not only for MI but also for detecting ischemic tissue [20].
2.5 C-Reactive Protein
C-reactive protein (CRP) is an acute-phase reactant that has been used as a marker of inflammation. Various studies have suggested that CRP levels measured at admission may also be useful as an independent predictor of new coronary events, including MI and death, in patients with ischemic heart disease [21]. CRP is a useful prognostic indicator in patients with ACS, as elevated CRP levels are independent predictors of cardiac death, AMI, and congestive heart failure. In combination with cTnI and B-type natriuretic peptide (BNP), CRP may be a useful adjuvant, but its nonspecific nature limits its use as a diagnostic cardiac marker for ACS in the emergency room. Chronic inflammatory conditions such as rheumatoid arthritis or lupus also confuse interpretation of CRP levels.
2.6 Ischemia-Modified Albumin
Ischemia-modified albumin (IMA) is a relatively new marker of ischemia and is produced when circulating serum albumin comes into contact with ischemic heart tissues. IMA levels rise within minutes of transient ischemia, peak within 6 h, and can remain elevated for as long as 12 h. IMA is a useful marker for diagnosis of ACS, and the combination of cTnT and IMA can provide significant information about the presence of ACS and may be useful for triage of patients who present to the emergency room with chest pain. The high sensitivity of cTnT coupled with the high negative predictive value of IMA make them independent predictors of the risk of developing ACS. However, the increase in serum IMA levels that occurs in patients with symptoms of stroke suggest that it should be considered a marker of acute ischemic events and not specific for cardiac ischemia [22]. Its use is limited to ruling out ischemia rather than as a diagnostic test for the occurrence of ischemia.
2.7 B-Type Natriuretic Peptide
BNP is secreted primarily by the ventricular myocardium in response to wall stress, including volume expansion and pressure overload. Multiple studies have demonstrated that BNP may also be a useful prognostic indicator in ACS. Transient myocardial ischemia has been associated with an immediate rise in circulating BNP levels, and the magnitude of the rise was proportional to the severity of ischemia. These findings demonstrate an important link between the severity of an acute ischemic insult and circulating levels of BNP [23]. BNP measurement provides useful prognostic information on the mortality risk in patients with heart failure [24]. The BNP level measured in the first few days after an acute coronary event predicts the long-term risk of death or nonfatal cardiac events across the spectrum of ACS. BNP levels should be measured after an ACS in order to identify patients at high and low risk of adverse outcomes [25]. In one study, patients with elevated BNP levels (>80 pg/mL; n = 320) were found to be at higher risk of death within 7 days (2.5 % vs. 0.7 %; p = 0.006) and 6 months (8.4 % vs. 1.8 %; p < 0.0001), and patients with elevated BNP levels had a fivefold risk of developing new congestive heart failure within 30 days (5.9 % vs. 1.0 %; p < 0.0001). Elevated BNP levels (>80 pg/mL) at presentation add incremental information to cTnI and identify patients with non–ST-segment elevation ACS who are at higher risk of death and congestive heart failure [26]. BNP levels are potentially more useful when the patient’s baseline level is known, because the levels are proportional to the severity of heart failure [27].
Besides BNP, elevated levels of N-terminal (NT)-proBNP predict cardiovascular morbidity and mortality independently of other prognostic markers and identify at-risk individuals even in the absence of systolic or diastolic dysfunction on echocardiography [28].
3 Criteria for Cardiac Markers in the Blood
The NACB, in conjunction with leading researchers in the field of cardiac biomarkers, has proposed standards of laboratory practice for use of cardiac markers in ACS and heart failure [13]. Since no single biomarker fulfills all of the criteria of an ideal biomarker, the NACB proposes use of two biomarkers for diagnosis of AMI: an early marker and a definitive marker. Use of two markers facilitates diagnosis of patients who present early or late. Blood levels of the early marker must be consistently elevated within the first 6 h after the onset of symptoms. The NACB proposes myoglobin as the ideal early marker [13], as blood levels of myoglobin become elevated within 2 h in patients with AMI.
The definitive marker must be detectable in the blood within 6–9 h after the onset of symptoms, and must have high sensitivity and specificity for myocardial injury. Moreover, its blood levels must remain elevated for several days. The clinical utility of cTnI assays for assessment of myocardial injury has been demonstrated in several clinical studies, with improved cardiac specificity over other cardiac biomarkers [9, 29]. Although measurement of CK-MB levels has been historically considered the “gold standard” for diagnosis of AMI, levels of CK-MB can be elevated in noncardiac conditions as well, because of its presence in other tissues throughout the body [10]. This lack of absolute cardiac specificity of CK-MB has led to development of assays for proteins such as myoglobin and troponins, which have higher sensitivity and specificity for diagnosis of acute cardiac conditions. Many investigators have proposed that cTnI assays should replace CK-MB assays for assessment of patients with suspected cardiac conditions [9, 30]. However, CK-MB is still considered an important tool for assessment of re-infarction and for assessment of reperfusion after thrombolytic therapy [8].
4 Diagnostic Approaches to Detection of Acute Myocardial Infarction
The World Health Organization has put forward certain criteria for the diagnosis of AMI [31]. According to these criteria, the diagnosis should be based on the patient showing any two of the following three criteria: (1) a previous history of ischemic heart discomfort or angina; (2) changes on serially obtained electrocardiograms; and (3) significant changes (either increases or decreases) in blood levels of cardiac markers.
Because of the large variation in the pattern of these changes, diagnosis of AMI is still very challenging. Only about one third of patients who are admitted to the hospital with chest pain and ischemic discomfort finally turn out to be AMI sufferers, and 25 % of patients who are admitted with chest pain are later diagnosed with AMI [32]. Although electrocardiographic changes are fairly good indications of AMI, they do not always aid early diagnosis. Most patients with AMI do not necessarily show ST-segment elevation [8, 11], thus diagnosis of AMI is more challenging than is generally presumed. Therefore, more accurate and rapid methods need to be evaluated for early diagnosis.
Many non-AMI patients who are appropriately admitted to the hospital with chest pain have unstable angina or congestive heart failure; however, a large number of patients are inappropriately admitted with a noncardiac cause of chest pain [33]. Not only do “missed AMI” patients have a higher mortality rate than patients admitted to a coronary care unit, but also missed AMI is the leading cause of malpractice lawsuits and settlements among emergency room patients. Thus, proper triage of emergency room patients with chest pain will not only reduce morbidity and mortality but will also decrease health care expenditure. In addition to determining which patients are most likely experiencing an AMI, emergency room physicians need to quickly and accurately identify those AMI patients who should receive thrombolytic therapy. The greatest benefit occurs when thrombolytic therapy is initiated within the first 3 h after the onset of symptoms [11].
The role of cardiac biomarkers in evaluation of emergency room patients presenting with chest pain continues to increase in importance. Biomarkers can serve to confirm the diagnosis in symptomatic patients with diagnostic electrocardiographic changes [8]. These biomarkers are found in cardiac tissue and are released into the bloodstream following the onset of myocardial necrosis during an AMI. With the exception of cTnI, these biomarkers are also found in other tissues throughout the body. Therefore, a myriad of noncardiac conditions can result in elevated levels of these biomarkers. This lack of cardiac specificity is one of the major weaknesses of most biomarkers [34].
5 Cardiac Troponin Complex
Troponin is a complex molecule present in cardiac tissues. It confers calcium sensitivity on muscle actinomycin adenosine triphosphatase activity. It consists of three subunits with distinct functions: (1) troponin C (cTnC), (2) cTnI, and (3) cTnT. Troponins are also present in skeletal muscle, and because the amino acid sequence of cTnC is similar to those of skeletal troponins, cTnC cannot be used as a cardiac-specific marker. However the isoforms of cTnI and cTnT differ significantly from skeletal muscle troponins.
The myocardium contains bundles of striated muscle fibers (thick and thin filaments) that are composed of cardiac-specific contractile proteins (actin and myosin); regulatory proteins (troponins and tropomyosin); proteins (myoglobin) required for the conversion of chemical energy into work (muscle contraction); and the enzymes CK and LDH. The thick filament is mainly composed of myosin, while the thin filaments consist primarily of actin, tropomyosin, and troponins. Troponin complex consists of three single-chain polypeptide subunits: the Ca2+-binding subunit troponin C, the tropomyosin-binding subunit troponin T, and the inhibitory subunit troponin I. Troponin I binds to actin in thin myofilaments to hold the actin-tropomyosin complex in place, and consequently myosin cannot bind the actin in relaxed muscle. When calcium binds to troponin C, it causes certain conformational changes, which lead to dislocation of troponin I, with tropomyosin finally leaving the binding site for myosin on actin, thus leading to contraction of the muscle. The letter “I” in “troponin I” stands for its inhibitory effect on adenosine triphosphatase activity. Together with tropomyosin and under the influence of calcium, troponins function as molecular switches, thereby regulating contraction of striated muscle (fast-skeletal, slow-skeletal, and cardiac muscle), while smooth-muscle contraction is regulated by calmodulin [35, 36]. As a result of cardiac tissue injury, troponins and other key proteins of myocardium are released into the peripheral circulation and thus serve as clinical diagnostic markers.
Monoclonal antibodies specific to cTnI and cTnT have been identified and used for development of rapid tests for early detection of MI [37–39]. Several studies have confirmed that cTnI has greater sensitivity and specificity than LDH isoenzymes and CK-MB in detecting MI [9, 40]. The release kinetics of cTnI and cTnT are more or less similar, but some reports have suggested that cTnI may have better predictive value in early detection of AMI [41]. Although cTnT and cTnI values are useful tools for diagnosis of AMI, their interpretation must take into account the number of hours from the onset of chest pain. The sensitivity of both cTnT and cTnI increases from 10–45 % within 1 h of the onset of pain (depending on the cutoff) to >90 % at 8 or more hours. The specificity of cTnT declines gradually from 87 % to 80 % between 1 and 12 h after the onset of chest pain, and the specificity of cTnI is approximately 95 %. After the onset of chest pain, the percentages of measured cTnT values above the cutoff value (0.1 ng/mL) are 54.5 % at 1 h, 57.1 % at 2 h, 68.2 % at 3 h, 69.6 % at 4 h, 88.5 % at 4 h, 93.3 % at 6 h, and 94.9 % at 7–36 h. Cardiac troponin tests are useful to rule out MI when the value is negative at 8 or more hours after the onset of chest pain [42, 43]. However, the studies that evaluated the sensitivity and specificity of troponins looked at patients with chest pain (i.e. with a high pre-test probability), thus the sensitivity and specificity may not be generalizable to other populations.
6 Diagnostic Significance of Cardiac Troponins in Acute Myocardial Infarction
Each year, millions of patients with chest pain are admitted to the emergency room as a result of cardiovascular disease. Accurate triage of patients with chest pain remains a challenge. An analysis of 10,689 patients from the ACI-TIPI (Acute Cardiac Ischemia Time-Insensitive Predictive Instrument) trial suggested that of patients presenting to the emergency room with chest pain, ~2 % were missed AMI cases (i.e. they were discharged after initial evaluation) and 2.3 % were missed unstable angina cases [44]. The cardiac specificity of cTnI has resolved assessment difficulties to some extent, as in the past, interpretation of the results of older cardiac biomarker tests could be severely confounded by conditions unrelated to AMI [30].
Once the absolute cardiac specificity of cTnI was identified, the Joint European Society of Cardiology/American College of Cardiology Committee issued a consensus document re-evaluating the established definitions of MI [45]. The Committee stated that “[m]yocardial infarction is diagnosed when blood levels of sensitive and specific biomarkers, such as cardiac troponins and the MB fraction of CK (CK-MB), are increased in the clinical setting of acute ischemia….The most recently described and preferred biomarker for myocardial damage is cardiac troponin (I or T), which has nearly absolute myocardial tissue specificity, as well as high sensitivity, thereby reflecting even microscopic zones of myocardial necrosis”. More specifically, blood should be obtained for testing on hospital admission, at 6–9 h, and again at 12–24 h if the earlier samples are negative and the clinical index of suspicion is high. For patients in need of an early diagnosis, a rapidly appearing cardiac biomarker (e.g. CK-MB or myoglobin) plus a biomarker that rises later (e.g. a cardiac troponin) are recommended for confirmation of the diagnosis. The cardiac specificity of troponins enables clinicians to diagnose or exclude AMI in uncertain cases within 8–12 h after the onset of chest discomfort. Once the diagnosis is confirmed, clinicians may follow the 2011 updated American College of Cardiology/American Heart Association guidelines for the management of patients with AMI [11].
7 Antibodies Against Cardiac Troponin I
Because cTnI has its distinct immunologic epitopes, there is potential for production of cTnI-specific antibodies that can be used for diagnosis of AMI. During incubation in the necrotic muscle after AMI, cTnI is cleaved by endogenous proteases. The most stable fragment resulting from this process lies between amino acids 30 and 110 of the parent protein. Its stability is possibly due to its protection by cTnC. For good sensitivity and reproducibility in assay design, most antibodies act against this stable part of cTnI.
Because of the cardiac specificity of troponin, Cummins et al. [46] realized that an immunoassay for cTnI could be a valuable diagnostic aid in detection of myocardial cell necrosis. The development of monoclonal antibodies for an immunoassay of cTnI made possible its introduction as a cardiac biomarker [47]. Of the currently available testing strategies, troponin immunoassays provide the best risk-stratification tool for patients with ACS. By integrating information from the patient history, physical examination, electrocardiogram, and initial cardiac biomarker tests, clinicians can assign patients to one of four categories: a noncardiac diagnosis, chronic stable angina, possible ACS, and definite ACS.
8 Advances in Rapid Assay Development for Cardiac Markers
Point-of-care testing for CK-MB, myoglobin, and troponin offers rapid (15-minute turnaround time) and accurate results and a new option for patient evaluation in the emergency room. Lateral-flow/flow-through rapid assays are now available for troponins, myoglobin, and CK-MB. Only qualitative cTnT assays are available as point-of-care tests, but both quantitative and qualitative point-of-care cTnI assays are currently marketed. Point-of-care tests are also available in combinations of two or three markers, such as cTnI, myoglobin, and CK-MB, on a single card. With such combination cards, in a single skin prick, all of the three important cardiac markers can be tested. The time required to develop the test lines gives some idea of the levels of these markers. Careful attention to the timing of the appearance of a positive bedside test result may give information to the clinician about the levels of these markers. Use of such combination rapid-test devices will improve the efficacy and safety of decision making in patients with chest pain and can significantly reduce the cost for use in intensive care facilities. Point-of-care assays for CK-MB, myoglobin, cTnI, and cTnT are available.
9 High-Sensitivity Troponin Assays
The conventional cTnT assay is not able to detect low levels of cTnT (<0.01 μg/L) and lacks precision until cTnT levels reach 0.035 μg/L. Furthermore, conventional cardiac troponin assays do not detect cardiac troponins in healthy volunteers. Thus it has been difficult to establish the normal ranges of cardiac troponin levels [48]. Recently, improvements in the technology of cardiac troponin assays have allowed manufacturers to provide fully automated assays that fulfill the recommendations set out by the International Federation of Clinical Chemistry and Laboratory Medicine. These assays have a lower limit of detection that is below the 99th percentile in a normal reference population [49]. In a recent study, use of a high-sensitivity cTnT test with a cutoff value of 0.014 μg/L, instead of a conventional cTnT test with a cutoff value of 0.01 μg/L, increased the proportion of patients diagnosed with non–ST-segment elevation MI (NSTEMI) by 33 % [50].
For diagnosis of AMI, cardiac troponin levels (as measured by fully automated standard assays) are superior to all other clinically available biomarkers, including myoglobin, CK-MB, myeloperoxidase, and HFABP. Newer-generation assays are better able to detect cardiac troponin levels at the time of a patient’s presentation. This allows for more rapid diagnosis of AMI, which could otherwise only be confirmed after prolonged monitoring over a period of 6–12 h and serial blood sampling [51]. In a recent study of 939 patients, 205 (21.8 %) had MI. By 2 h after presentation, the high-sensitivity cTnT assay at the cutoff point of the 99th percentile of the general population (14 ng/L) had sensitivity of 92.2 % (95 % confidence interval [CI] 88.1–95.0) and specificity of 79.7 % (95 % CI 78.6–80.5) for the diagnosis of NSTEMI. The sensitivity of the assay at presentation was 100 % among patients who presented 4–6 h after symptom onset. The high-sensitivity cTnT assay was also found to be superior to the conventional assay in predicting death (hazard ratio [HR] 5.4; 95 % CI 2.7–10.7) and heart failure (HR 27.8; 95 % CI 6.6–116.4) within 1 year, whereas the conventional assay was superior in predicting nonfatal MI within 1 year (HR 4.0; 95 % CI 2.4–6.7). The high-sensitivity cTnT assay at the cutoff point of the 99th percentile was highly sensitive for the diagnosis of MI by 2 h after presentation and had prognostic utility beyond that of the conventional assay [52].
The high-sensitivity cTnI assay has improved early diagnosis of AMI and risk stratification, regardless of the time of chest pain onset [53]. Even mild elevation of cardiac troponin levels is common in elderly patients without AMI, and optimal cutoff levels are substantially higher in elderly patients than in younger patients. High-sensitivity cardiac troponin assays have high diagnostic accuracy in the elderly [54]. They also have a high prognostic value and high diagnostic accuracy in coronary artery disease patients [55]. Utilization of high-sensitivity troponin measurements may be useful for applications other than ACS, including risk stratification of patients with renal insufficiency, heart failure, or cardiac amyloid, and screening of elderly patients [56]. However, when making a diagnosis of type 2 MI, the extreme sensitivity of troponins for detecting myocardial injury/infarction makes it imperative to place all results in a clinical perspective. It is well recognized that troponin levels can be abnormal in the absence of symptoms, electrocardiographic changes, or any gross evidence of myocardial dysfunction or subsequent damage [57].
10 Use of Troponins in Patient Triage
Effective triage of patients presenting to the emergency room with undifferentiated chest pain remains a challenge for the clinician. Prehospital electrocardiographic diagnosis has improved triage and outcomes in patients with acute ST-segment elevation MI (STEMI). However, many patients with unstable angina and NSTEMI present with equivocal electrocardiographic patterns, making prehospital electrocardiographic diagnosis difficult. In addition to being useful for diagnosis, troponins also permit estimation of the prognosis and risk stratification of patients with ACS. A recent study has indicated that prehospital implementation of cTnT quantitative tests with lower detection limits could identify most patients with AMI, irrespective of electrocardiographic changes [58]. Reichlin et al. [59] developed and validated an algorithm to rapidly diagnose or exclude AMI. Use of this simple algorithm, incorporating high-sensitivity cTnT baseline values and absolute changes within the first hour, allowed safe exclusion or accurate diagnosis of AMI within 1 h in 77 % of unselected patients with acute chest pain.
In a study involving triage of patients with acute chest pain lasting for <6 h and a previous history of coronary artery disease, the combination of copeptin and cardiac troponins allowed the diagnosis of AMI to be excluded with a negative predictive value of >95 %. The adjudicated final diagnosis was AMI in 36 patients (8 %), unstable angina in 131 (29 %), and another diagnosis in 284 (63 %) [60].
11 Elevated Cardiac Troponin Levels Unrelated to Acute Coronary Disease
cTnI is a specific marker, which allows diagnosis of even minor myocardial cell injury. However, elevated cardiac troponin levels are also associated with nonischemic cardiac conditions and noncardiovascular conditions, and have been observed in patients with pulmonary embolism. cTnI and cTnT are significantly associated with overall mortality, major clinical events, and recurrence of pulmonary embolism during the hospital stay [61]. In a meta-analysis of 20 studies of acute pulmonary embolism, patients with an elevated cardiac troponin level had more than a fivefold increase in mortality [62]. An important non-ACS diagnosis to consider in a patient presenting with an elevated cardiac troponin level is acute aortic dissection. Elevation of cTnI levels is frequent in patients with type A aortic dissection and might reflect greater hemodynamic stress, but does not necessarily reflect a negative prognosis [63]. In patients with idiopathic acute pericarditis, an increase in cTnI levels is commonly associated with young age, recent infection, male sex, ST-segment elevation, and pericardial effusion at presentation [64, 65].
Besides nonischemic cardiac events, there are many noncardiovascular conditions in which elevated troponin levels can be detected. Increased serum cardiac troponin levels are frequently observed in patients with renal insufficiency, even when the suspicion of active ischemia is relatively low. cTnT levels are elevated in a large number of patients on regular hemodialysis, and this is a significant independent predictor of outcome [66]. Apart from ischemia, septic shock caused by various factors can lead to injury of myocardial cells. Elevated cTnI levels are frequently observed in patients with severe sepsis and septic shock and are associated with left ventricular dysfunction. High serum cTnI levels predict increased severity of sepsis and higher mortality [67]. Elevated troponin levels have also been observed in patients with endocrinologic disorders such as diabetes and hypothyroidism. In a population of patients with type 2 diabetes mellitus [68], elevation of cTnT levels above the 99th percentile measured by a highly sensitive assay were encountered frequently. cTnT levels rise as a result of myocyte necrosis, but do not automatically signify an ACS. Studies have been conducted to determine whether a relationship exists between elevated cardiac troponin levels and acute allograft rejection in patients who have received heart transplants [69]. In a study of 110 consecutive patients who received heart transplants between 1989 and 1997 and survived for at least 1 year after transplantation [69], all recipients had elevated cTnI levels during the first month after transplantation. In addition, cTnI levels in 56 patients (51 %) remained persistently elevated during the first 12 months. These elevated cTnI levels were associated with increased fibrin deposits in the microvasculature and cardiomyocytes (p < 0.001), thus significantly increasing the risk of subsequent development of coronary artery disease and graft failure.
Decompensated heart failure is a common cause of non-ACS-related troponin elevation. In patients without ACS, elevated cTnT levels indicate a high risk of mortality. Clinicians should be cautious about diagnosing ACS in patients with raised cTnT levels [70].
12 Prognostic Potential of Troponins
Unstable angina is a common expression of coronary artery disease [71]. Several studies in patients with unstable angina have shown that the risk of progression to AMI can be up to 20 % during 30-day follow-up [72, 73]. Patients with unstable angina and abnormal cardiac troponin levels have a fivefold higher risk of AMI and cardiac death within 4–6 weeks than patients with normal troponin levels [74]. cTnT is a useful prognostic marker in asymptomatic patients on hemodialysis. Annual measurements of cTnT levels in patients on chronic hemodialysis may be useful both for identifying those at risk of coronary events and as a diagnostic reference level for patients presenting with chest pain and elevated cTnT levels [75]. In a meta-analysis, it was found that increased mortality was significantly associated with elevated troponin levels after percutaneous coronary intervention, and the combined endpoint of mortality and nonfatal MI also occurred more often in patients with postprocedural troponin elevations [76]. In a comparative study of the prognostic values of cTnI and CRP levels in patients with unstable angina, most of whom had proven coronary artery disease, the rate of in-hospital major adverse cardiac events (death, MI, or emergency revascularization) was higher in patients with increased cTnI levels within the first 24 h. Both in unselected patients with unstable angina and in patients with angiographically proven coronary artery disease, the cTnI level within 24 h of admission, but not the CRP level, was an independent predictor of major adverse cardiac events [77]. Elevated cTnI levels (>0.1 μg/mL) during the first 24 h were strongly associated with a higher risk of death or MI at 48 h (3.9 % vs. 0 %; p = 0.01) and at 14 days (13.9 % vs. 2.2 %; p < 0.0001). Elevated cTnI levels also correlated with a higher risk of recurrent ischemia requiring urgent revascularization within 48 h (10.0 % vs. 1.7 %; p = 0.001) and within 14 days (20.6 % vs. 5.6 %; p < 0.0001). Similar results were demonstrated in the FRISC (Fragmin and fast Revascularization during InStability in Coronary artery disease) trial using dalteparin treatment [78]. These results support previous studies demonstrating the prognostic utility of cTnI in patients with non-ST-elevation ACS with no elevation in CK-MB levels.
A recent quantitative troponin elevation study showed that for each approximate tenfold increase in the ratio between peak and baseline troponin levels (i.e. troponin ratios of 0.01 to ≤1, >1 to ≤10, >10 to ≤100, >100 to ≤1000, or >1000), there was an associated increase in the combined incidence of cardiac arrest, sustained ventricular tachycardia, and ventricular fibrillation (1.0 %, 2.4 %, 3.4 %, 5.9 %, and 13.4 %, respectively; p < 0.001 for the linear trend); cardiogenic shock (0.5 %, 1.4 %, 2.0 %, 4.4 %, and 12.7 %, respectively; p < 0.001); new heart failure (2.5 %, 5.1 %, 7.4 %, 11.6 %, and 15.8 %, respectively; p < 0.001); and mortality (0.8 %, 2.2 %, 3.0 %, 5.3 %, and 14.0 %, respectively; p < 0.001). The extent of the of troponin elevation was predictive of early mortality (HR 1.61; 95 % CI 1.44–1.81; p < 0.001 for days 0–14) and longer-term mortality (HR 1.18; 95 % CI 1.07–1.30; p = 0.001 for days 15–180) [79].
The prognosis of suspected unstable angina or NSTEMI patients who are hospitalized for chest pain but do not immediately develop transmural necrosis is signified by serum cTnT levels at hospital admission. cTnT levels ≥0.1 ng/mL signify an almost threefold risk of major events within 3 months after the acute episode [80]. In a statistical model proposed for cTnI as a quantitative predictor of in-hospital mortality, it was suggested that the presence of any detectible cTnI is associated with an increased mortality risk and provide further evidence of the absence of a non-zero risk threshold. The model predicts unadjusted mortality rates of 2.1 % for a cTnI level of 0 μg/L, 5.3 % for a level of 0.08 μg/L (the upper limit of the 99th percentile), and 7.0 % for a level of 0.21 μg/L (the lower limit of positive by the strict 10% coefficient of variation [CV] criterion) [81].
In AMI, the extent of myocardial damage is closely linked to the prognosis. Serum levels of cardiac troponins can be used to estimate myocardial infarct size. The ability of cTnT to predict infarct size and left ventricular function in patients with AMI has been studied, and it was found that cTnT levels closely correlated with single-photon emission computed tomography estimates of infarct size and peak serum levels of CK-MB [82]. That study also showed that for estimation of myocardial infarct size, a single measurement of the cTnT level 72 h after the onset of chest pain is superior to measurement of peak CK, CK-MB, or LDH levels, which require serial determinations. The correlation between cardiac troponin levels and infarct size is strong enough to be of clinical utility, and cardiac troponin levels may be employed systematically for crude infarct size estimation in STEMI patients at an early timepoint following the acute event [83]. However, correlations between cardiac troponin levels and infarct size are significantly weaker in NSTEMI patients [84]. The peak cTnT level after primary percutaneous coronary intervention for STEMI offers a good estimation of the infarct size and is a prognostic indicator in patients with their first AMI [85].
To increase the accuracy of MI diagnosis, addition of a combination of biomarkers from numerous pathophysiologic pathways—a multimarker approach—has been studied.
Many studies showed that although multiple biomarkers are associated with a high relative risk of adverse events, even in combination they add only moderately to the prediction of risk in an individual person. The most clinically accurate biomarker for early diagnosis of MI is cTnI alone, rather than a combination of multiple biomarkers, when an analytically robust cardiac troponin assay based on the 99th percentile is used [86–88]. However, in a study of elderly men [89], a multiple biomarker approach with incorporation of cTnI, NT-proBNP, cystatin C, and high-sensitivity CRP into a model with established risk factors improved risk stratification for death from cardiovascular causes. The reason for this discrepancy could be that the relative risk associated with established risk factors (viz. age, systolic blood pressure, use or nonuse of antihypertensive treatment, total cholesterol levels, high-density lipoprotein cholesterol levels, use or nonuse of lipid-lowering treatment, the presence or absence of diabetes, smoking status, and body mass index) has been shown to diminish with advancing age, and the established risk factors would have performed better as prognostic indicators in a sample of younger people who had a lower absolute risk and a lower prevalence of subclinical cardiovascular damage.
In a study of STEMI patients undergoing primary percutaneous coronary intervention, the combination of NT-proBNP levels, glucose levels, CRP levels, the estimated glomerular filtration rate, and cTnT levels identified a high-risk STEMI subgroup with a significantly higher mortality rate than that of an intermediate- or low-risk subgroup (p < 0.001) [90]. In addition to these markers, use of BNP levels adds significant prognostic information to the Killip class and TIMI (Thrombolysis in Myocardial Infarction) risk score in patients with STEMI treated with percutaneous coronary intervention [91].
13 Conclusion
ACS represent a continuum of myocardial ischemia, encompassing angina, reversible tissue injury, unstable angina, myocardial damage, MI, and extensive tissue necrosis. Assessment of coronary artery disease is based on use of World Health Organization diagnostic criteria, electrocardiographic features, and biochemical markers. However, as the diagnostic sensitivity of electrocardiography is as low as 50 %, it is not a perfect instrument for diagnosis. Therefore, cardiac-specific proteins are of paramount importance as markers of myocardial injury associated with cell death, which leads to release of intracellular proteins into the circulation. Sensitive and specific cardiac markers improve AMI diagnosis, therapeutic decision making, and risk stratification. In this regard, CK-MB has been a benchmark for markers, but it is not specific for myocardium. Cardiac-specific isoforms of troponin I and troponin T, together with high-sensitivity assays, have emerged as the most specific and sensitive AMI indicators and, more importantly, as tools for risk stratification of ACS patients. By improving the ability to accurately triage patients presenting with chest pain, use of troponins could reduce mortality rates.
References
World Health Organization. The world health report 2004—changing history [online]. http://www.who.int/whr/2004/en/ (2012). Accessed 8 Nov 2012.
World Health Organization. Cardiovascular diseases (CVDs): fact sheet no. 317 [online]. http://www.who.int/mediacentre/factsheets/fs317/en/index.html (2012). Accessed 8 Nov 2012.
Jabre P, Roger VL, Murad MH, et al. Mortality associated with atrial fibrillation in patients with myocardial infarction: a systematic review and meta-analysis. Circulation. 2011;123:1587–93.
Roger VL, Weston SA, Gerber Y, et al. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation. 2010;121:863–9.
Pegg TJ, Maunsell Z, Karamitsos TD, et al. Utility of cardiac biomarkers for the diagnosis of type V myocardial infarction after coronary artery bypass grafting: insights from serial cardiac MRI. Heart. 2011;97:810–6.
Gibler WB, Young GP, Hedges JR, et al. Acute myocardial infarction in chest pain patients with non-diagnostic ECGs: serial CK-MB sampling in the emergency department. Ann Emerg Med. 1992;21:505–12.
Lott JA, Stang JM. Differential diagnosis of patients with abnormal serum creatine kinase isoenzymes. Clin Lab Med. 1989;9:627–42.
Ramasamy I. Biochemical markers in acute coronary syndrome. Clin Chim Acta. 2011;412:1279–96.
Volz KA, McGillicuddy DC, Horowitz GL, et al. Creatine kinase-MB does not add additional benefit to a negative troponin in the evaluation of chest pain. Am J Emerg Med. 2012;30:188–90.
Licka M, Zimmermann R, Zehelein J, et al. Troponin T concentrations 72 hours after myocardial infarction as a serological estimate of infarct size. Heart. 2002;87:520–4.
Wright RS, Anderson JL, Adams CD, et al. 2011 ACCF/AHA focused update of the guidelines for the management of patients with unstable angina/non–ST-elevation myocardial infarction (updating the 2007 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American College of Emergency Physicians, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;57:1920–59.
Croal BL, Hillis GS, Gibson PH, et al. Relationship between postoperative cardiac troponin I levels and outcome of cardiac surgery. Circulation. 2006;114:1468–75.
Oshima K, Kunimoto F, Takahashi T, et al. Postoperative cardiac troponin I (cTnI) level and its prognostic value for patients undergoing mitral valve surgery. Int Heart J. 2010;51:166–9.
Bonaca M, Scirica B, Sabatine M, et al. Prospective evaluation of the prognostic implications of improved assay performance with a sensitive assay for cardiac troponin I. J Am Coll Cardiol. 2010;55:2118–24.
Debnath M, Prasad GBKS, Bisen PS. Molecular diagnostics: promises and possibilities. 1st ed. Dordrecht: Springer; 2010.
Apple FS, Murakami M, Panteghini M, et al. International survey on the use of cardiac markers. Clin Chem. 2001;47:587–8.
Castaldo AM, Ercolini P, Forino F, et al. Plasma myoglobin in the early diagnosis of acute myocardial infarction. Eur J Clin Chem Clin Biochem. 1994;32:349–53.
Bruins Slot MH, Reitsma JB, Rutten FH, et al. Heart-type fatty acid-binding protein in the early diagnosis of acute myocardial infarction: a systematic review and meta-analysis. Heart. 2010;96:1957–63.
Garcia-Valdecasas S, Ruiz-Alvarez MJ, Garcia De Tena J, et al. Diagnostic and prognostic value of heart-type fatty acid-binding protein in the early hours of acute myocardial infarction. Acta Cardiol. 2011;3:315–21.
Mair J. Glycogen phosphorylase isoenzyme BB to diagnose ischaemic myocardial damage. Clin Chim Acta. 1998;272:79–86.
Després JP. CRP: star trekking the galaxy of risk markers. Lancet. 2011;377:441–2.
Talwalkar SS, Bon-Homme M, Miller JJ, et al. Ischemia modified albumin, a marker of acute ischemic events: a pilot study. Ann Clin Lab Sci. 2008;38:132–7.
Sabatine MS, Morrow DA, de Lemos JA, et al. Acute changes in circulating natriuretic peptide levels in relation to myocardial ischemia. J Am Coll Cardiol. 2004;44:1988–95.
Koglin J, Pehlivanli S, Schwaiblmair M, et al. Role of brain natriuretic peptide in risk stratification of patients with congestive heart failure. J Am Coll Cardiol. 2001;38:1934–41.
de Lemos JA, Morrow DA, Bentley JH, et al. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med. 2001;345:1014–21.
Morrow DA, de Lemos JA, Sabatine MS, et al. Evaluation of B-type natriuretic peptide for risk assessment in unstable angina/non-ST-elevation myocardial infarction: B-type natriuretic peptide and prognosis in TACTICS-TIMI 18. J Am Coll Cardiol. 2003;41:1264–72.
Cardarelli R, Lumicao TG. B-type natriuretic peptide: a review of its diagnostic, prognostic, and therapeutic monitoring value in heart failure for primary care physicians. J Am Board Fam Med. 2003;16:327–33.
Bibbins-Domingo K, Gupta R, Na B, et al. N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. JAMA. 2007;297:169–76.
Pervaiz S, Anderson FP, Lohmann TP, et al. Comparative analysis of cardiac troponin creatine and I kinase-MB as markers of acute myocardial infarction. Clin Cardiol. 1997;20:269–71.
Lee-Lewandrowski E, Januzzi JL, Grisson R, et al. Evaluation of first-draw whole blood, point-of-care cardiac markers in the context of the universal definition of myocardial infarction: a comparison of a multimarker panel to troponin alone and to testing in the central laboratory. Arch Pathol Lab Med. 2011;135:459–63.
Thygesen K, Alpert JS, Jaffe AS, et al. The writing group on behalf of the joint ESC/ACCF/AHA/WHF task force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–35.
Ryan TJ, Anderson JL, Antman EM, et al. ACC/AHA guidelines for the management of patients with acute myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Acute Myocardial Infarction). J Am Coll Cardiol. 1996;28:1328–428.
Wu AHB. Introduction to coronary artery disease (CAD) and biochemical markers. In: Wu AHB, editor. Cardiac markers. Totawa: Humana Press. 1998. p. 3–20.
Jaffe AS, Babuin L, Apple FS. Biomarkers in acute cardiac disease: the present and the future. J Am Coll Cardiol. 2006;48:1–11.
Katus HA, Scheffold T, Remppis A, et al. Proteins of the troponin complex. Lab Med. 1992;23:311–7.
Farah CS, Reinach FC. The troponin complex and regulation of muscle contraction. FASEB J. 1995;9:755–67.
Dawie J, Chawla R, Worku Y, et al. Diagnosis of ischemic heart disease using CK-MB, troponin-I and ischemia modified albumin. Ethiop Med J. 2011;49:25–33.
Desai PH, Kurian D, Thirumavalavan N, et al. A randomized clinical trial investigating the relationship between aprotinin and hypercoagulability in off-pump coronary surgery. Anesth Analg. 2009;109:1387–94.
Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858–67.
Meune C, Drexler B, Haaf P, et al. The GRACE score’s performance in predicting in-hospital and 1-year outcome in the era of high-sensitivity cardiac troponin assays and B-type natriuretic peptide. Heart. 2011;97:1479–83.
Hamm CW, Goldmann BU, Heeschen C, et al. Emergency room triage of patients with acute chest pain by means of rapid testing for cardiac troponin T or troponin I. N Engl J Med. 1997;337:1648–53.
Peetz D, Post F, Schinzel H, et al. Glycogen phosphorylase BB in acute coronary syndromes. Clin Chem Lab Med. 2005;43(12):1351–8.
Ebell MH, Flewelling D, Flynn CA. A systematic review of troponin T and I for diagnosing acute myocardial infarction. J Fam Pract. 2000;49(6):550–6.
Pope JH, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342:1163–70.
Myocardial infarction redefined—a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the Redefinition of Myocardial Infarction. J Am Coll Cardiol. 2000;36:959–69.
Cummins B, Auckland ML, Cummins P. Cardiac-specific troponin-I radioimmunoassay in the diagnosis of acute myocardial infarction. Am Heart J. 1987;113:1333–44.
Bodor GS, Porter S, Landt S, et al. Development of monoclonal antibodies for an assay of cardiac troponin-I and preliminary results in suspected cases of myocardial infarction. Clin Chem. 1992;38:2203–14.
Mueller C, Giannitsis E. Clinical benefits of the cardiac troponin T-high sensitive assay in acute coronary syndrome. Eur Cardiol. 2011;7(1):14–7.
Mingels A, Jacobs L, Michielsen E, et al. Reference population and marathon runner sera assessed by highly sensitive cardiac troponin T and commercial cardiac troponin T and I assays. Clin Chem. 2009;55:101–8.
Ndrepepa G, Braun S, Schulz S, et al. Comparison of prognostic value of high-sensitivity and conventional troponin T in patients with non-ST-segment elevation acute coronary syndromes. Clin Chim Acta. 2011;412:1350–6.
Giannitsis E, Katus HA. Current recommendations for interpretation of the highly sensitive troponin T assay for diagnostic, therapeutic and prognostic purposes in patients with a non-ST-segment-elevation acute coronary syndrome. Eur Cardiol. 2009;5:44–7.
Aldous AJ, Richards M, Cullen L, et al. Diagnostic and prognostic utility of early measurement with high-sensitivity troponin T assay in patients presenting with chest pain. CMAJ. 2012;184:E260–8.
Keller T, Zeller T, Peetz D, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868–77.
Reiter M, Twerenbold R, Reichlin T, et al. Early diagnosis of acute myocardial infarction in the elderly using more sensitive cardiac troponin assays. Eur Heart J. 2011;32:1379–89.
Reiter M, Twerenbold R, Reichlin T, et al. Early diagnosis of acute myocardial infarction in patients with pre-existing coronary artery disease using more sensitive cardiac troponin assays. Eur Heart J. 2012;33:988–97.
Christenson RH, Phillips D. Sensitive and high sensitivity next generation cardiac troponin assays: more than just a name. Pathology. 2011;43:213–9.
Pierpont GL, McFalls EO. Interpreting troponin elevations: do we need multiple diagnoses? Eur Heart J. 2009;30:135–8.
Sørensen JT, Terkelsen CJ, Steengaard C, et al. Prehospital troponin T testing in the diagnosis and triage of patients with suspected acute myocardial infarction. Am J Cardiol. 2011;107:1436–40.
Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;13:1–8.
Ray P, Charpentier S, Chenevier-Gobeaux C, et al. Combined copeptin and troponin to rule out myocardial infarction in patients with chest pain and a history of coronary artery disease. Am J Emerg Med. 2012;30:440–8.
Konstantinides S, Geibel A, Olschewski M, et al. Importance of cardiac troponins I and T in risk stratification of patients with acute pulmonary embolism. Circulation. 2002;106:1263–8.
Becattini C, Vedovati MC, Agnelli G. Prognostic value of troponins in acute pulmonary embolism: a meta-analysis. Circulation. 2007;116:427–33.
Bonnefoy E, Godon P, Kirkorian G, et al. Significance of serum troponin I elevation in patients with acute aortic dissection of the ascending aorta. Acta Cardiol. 2005;60:165–70.
Bonnefoy E, Godon P, Kirkorian G, et al. Serum cardiac troponin I and ST-segment elevation in patients with acute pericarditis. Eur Heart J. 2000;21:832–6.
Imazio M, Demichelis B, Cecchi E, et al. Cardiac troponin I in acute pericarditis. J Am Coll Cardiol. 2003;42:2144–8.
Stolear JC, Georges B, Shita A, et al. The predictive value of cardiac troponin T measurements in subjects on regular haemodialysis. Nephrol Dial Transplant. 1999;14(8):1961–7.
ver Elst KM, Spapen HD, Nguyen DN, et al. Cardiac troponins I and T are biological markers of left ventricular dysfunction in septic shock. Clin Chem. 2000;46(5):650–7.
Hallén J, Johansen OE, Birkeland KI, et al. Determinants and prognostic implications of cardiac troponin T measured by a sensitive assay in type 2 diabetes mellitus. Cardiovasc Diabetol. 2010;9:52.
Labarrere CA, Nelson DR, Cox CJ, et al. Cardiac-specific troponin I levels and risk of coronary artery disease and graft failure following heart transplantation. JAMA. 2000;284:457–64.
Wong P, Murray S, Ramsewak A, et al. Raised cardiac troponin T levels in patients without acute coronary syndrome. Postgrad Med J. 2007;83:200–5.
Ding YY, Kader B, Christiansen CL, et al. Patient factors associated with transfusion practices in Veterans Affairs intensive care units: implications for further research. J Crit Care. 2011;26:431.e1–9.
Platelet Receptor Inhibition in Ischemic Syndrome Management (PRISM) Study Investigators. A comparison of aspirin plus tirofiban with aspirin plus heparin for unstable angina. N Engl J Med. 1998;338:1498–505.
The RESTORE Investigators. Effects of platelet glycoprotein IIb/IIIa blockade with tirofiban on adverse cardiac events in patients with unstable angina or acute myocardial infarction undergoing coronary angioplasty. Randomized Efficacy Study of Tirofiban for Outcomes and Restenosis. Circulation. 1997;96:1445–53.
Dorbala S, Giugliano RP, Logsetty G, et al. Prognostic value of SPECT myocardial perfusion imaging in patients with elevated cardiac troponin I levels and atypical clinical presentation. J Nucl Cardiol. 2007;14:53–8.
Conway B, McLaughlin M, Sharpe P, et al. Use of cardiac troponin T in diagnosis and prognosis of cardiac events in patients on chronic haemodialysis. Nephrol Dial Transplant. 2005;20:2759–64.
Nienhuis MB, Ottervanger JP, Bilo HJ, et al. Prognostic value of troponin after elective percutaneous coronary intervention: a meta-analysis. Catheter Cardiovasc Interv. 2008;71:318–24.
Benamer H, Steg PG, Benessiano J, et al. Comparison of the prognostic value of C-reactive protein and troponin I in patients with unstable angina pectoris. Am J Cardiol. 1998;82:845–50.
Lindahl B, Venge P, Wallentin L. Troponin Tidentifies patients with unstable coronary artery disease who benefit from long-term antithrombotic protection. Fragmin in Unstable Coronary Artery Disease (FRISC) Study Group. J Am Coll Cardiol. 1997;29:43–8.
Jolly SS, Shenkman H, Brieger D, et al. Quantitative troponin and death, cardiogenic shock, cardiac arrest and new heart failure in patients with non-ST-segment elevation acute coronary syndromes (NSTE ACS): insights from the Global Registry of Acute Coronary Events. Heart. 2011;97:197–202.
Torres R, Monge PB, Toral BS, et al. Prognostic value of troponin T in hospitalized patients with angina or non-ST-segment elevation myocardial infarction. Rev Esp Cardiol. 2003;56:35–42.
Waxman DA, Hecht S, Schappert J, et al. A model for troponin I as a quantitative predictor of in-hospital mortality. J Am Coll Cardiol. 2006;48:1755–62.
Panteghini M, Cuccia C, Bonetti G, et al. Single-point cardiac troponin T at coronary care unit discharge after myocardial infarction correlates with infarct size and ejection fraction. Clin Chem. 2002;48:1432–6.
Hallén J. Troponin for the estimation of infarct size: what have we learned? Cardiology. 2012;121:204–12.
Giannitsis E, Steen H, Kurz K, et al. Cardiac magnetic resonance imaging study for quantification of infarct size comparing directly serial versus single time-point measurements of cardiac troponin T. J Am Coll Cardiol. 2008;51:307–14.
Hassan AK, Bergheanu SC, Hasan-Ali H, et al. Usefulness of peak troponin-T to predict infarct size and long-term outcome in patients with first acute myocardial infarction after primary percutaneous coronary intervention. Am J Cardiol. 2009;103:779–84.
Wang TJ, Gona P, Larson MG, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–9.
Blankenberg S, McQueen MJ, Smieja M, et al. Comparative impact of multiple biomarkers and N-terminal pro-brain natriuretic peptide in the context of conventional risk factors for the prediction of recurrent cardiovascular events in the Heart Outcomes Prevention Evaluation (HOPE) study. Circulation. 2006;114:201–8.
Apple FS, Smith SW, Pearce LA, et al. Assessment of the multiple-biomarker approach for diagnosis of myocardial infarction in patients presenting with symptoms suggestive of acute coronary syndrome. Clin Chem. 2009;55:93–100.
Zethelius B, Berglund L, Sundström J, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–16.
Damman P, Beijk MA, Kuijt WJ, et al. Multiple biomarkers at admission significantly improve the prediction of mortality in patients undergoing primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2011;57(1):29–36.
Grabowski M, Filipiak KJ, Malek LA, et al. Admission B-type natriuretic peptide assessment improves early risk stratification by Killip classes and TIMI risk score in patients with acute ST elevation myocardial infarction treated with primary angioplasty. Int J Cardiol. 2007;115(3):386–90.
Acknowledgments
The authors are indebted to Sushil Mehta, Vice President-SEA, RFCL Limited (Avantor Performance Materials), Dr. P. K. Ghosh, former Advisor, Department of Biotechnology, Government of India, and Dr. N. C. Sharma, former Vice President, Cadila Pharmaceuticals Limited, Ahmadabad, for their suggestions and critical counselling. The authors are thankful to the Council of Scientific and Industrial Research (CSIR), New Delhi, for the award of Emeritus Scientist to Professor P. S. Bisen. No sources of funding were used to prepare this article. The authors have no conflicts of interest that are directly relevant to the content of the article.
Author information
Authors and Affiliations
Corresponding author
Additional information
A. Jain and Z. Khan contributed equally to this work.
Rights and permissions
About this article
Cite this article
Tiwari, R.P., Jain, A., Khan, Z. et al. Cardiac Troponins I and T: Molecular Markers for Early Diagnosis, Prognosis, and Accurate Triaging of Patients with Acute Myocardial Infarction. Mol Diagn Ther 16, 371–381 (2012). https://doi.org/10.1007/s40291-012-0011-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-012-0011-6