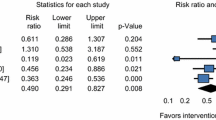
Abstract
Background
Hamstrings injuries are common in sports and the reinjury risk is high. Despite the extensive literature on hamstrings injuries, the effectiveness of the different conservative (i.e., non-surgical) interventions (i.e., modalities and doses) for the rehabilitation of athletes with acute hamstrings injuries is unclear.
Objective
We aimed to compare the effects of different conservative interventions in time to return to sport (TRTS) and/or time to return to full training (TRFT) and reinjury-related outcomes after acute hamstrings injuries in athletes.
Data Sources
We searched CINAHL, Cochrane Library, EMBASE, PubMed, Scopus, SPORTDiscus, and Web of Science databases up to 1 January, 2022, complemented with manual searches, prospective citation tracking, and consultation of external experts.
Eligibility Criteria
The eligibility criteria were multi-arm studies (randomized and non-randomized) that compared conservative treatments of acute hamstrings injuries in athletes.
Data Analysis
We summarized the characteristics of included studies and conservative interventions and analyzed data for main outcomes (TRTS, TRFT, and rate of reinjuries). The risk of bias was judged using the Cochrane tools. Quality and completeness of reporting of therapeutic exercise programs were appraised with the i-CONTENT tool and the certainty of evidence was judged using the GRADE framework. TRTS and TRFT were analyzed using mean differences and the risk of reinjury with relative risks.
Results
Fourteen studies (12 randomized and two non-randomized) comprising 730 athletes (mostly men with ages between 14 and 49 years) from different sports were included. Nine randomized studies were judged at high risk and three at low risk of bias, and the two non-randomized studies were judged at critical risk of bias. Seven randomized studies compared exercise-based interventions (e.g., L-protocol vs C-protocol), one randomized study compared the use of low-level laser therapy, and three randomized and two non-randomized studies compared injections of platelet-rich plasma to placebo or no injection. These low-level laser therapy and platelet-rich plasma studies complemented their interventions with an exercise program. Only three studies were judged at low overall risk of ineffectiveness (i-CONTENT). No single intervention or combination of interventions proved superior in achieving a faster TRTS/TRFT or reducing the risk of reinjury. Only eccentric lengthening exercises showed limited evidence in allowing a shorter TRFT. The platelet-rich plasma treatment did not consistently reduce the TRFT or have any effect on the risk of new hamstrings injuries. The certainty of evidence was very low for all outcomes and comparisons.
Conclusions
Available evidence precludes the prioritization of a particular exercise-based intervention for athletes with acute hamstrings injuries, as different exercise-based interventions showed comparable effects on TRTS/TRFT and the risk of reinjuries. Available evidence also does not support the use of platelet-rich plasma or low-level laser therapy in clinical practice. The currently available literature is limited because of the risk of bias, risk of ineffectiveness of exercise protocols (as assessed with the i-CONTENT), and the lack of comparability across existing studies.
Clinical Trial Registration
PROSPERO CRD42021268499 and OSF (https://osf.io/3k4u2/).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
As exercise-based interventions showed comparable time to return to full training or matches and the risk of reinjuries, no specific strategy needs to be prioritized when rehabilitating athletes with acute hamstrings injuries. Only eccentric lengthening exercises showed limited evidence in allowing a shorter time to return to full training. |
Platelet-rich plasma injections did not consistently reduce the time to return to full training or have any effect on the risk of new hamstrings injuries. Therefore, platelet-rich plasma has no current value in clinical practice. |
The currently available literature is still limited owing to a risk of bias, poor description, the risk of ineffectiveness of exercise protocols, and a lack of comparability across existing studies. Further studies are clearly warranted to allow stronger conclusions. |
1 Introduction
Hamstrings injuries are common across sports involving sprinting or excessive muscle lengthening [1,2,3,4,5,6,7,8,9,10,11], resulting in ~ 17–27 days lost per 1000 h of training and match exposure [1, 5, 10, 12], with players missing up to 80 training sessions and matches per year because of injury [13]. The unavailability of players to compete owing to injury implies a considerable financial burden, for example, a professional soccer player that is absent from competition for 2 weeks because of injury is estimated to cost around €250,000 for clubs participating in the UEFA Champions League [14], while in the Australian Football League the cost of a single hamstrings injury was $A40,021 in 2021 [15]. Reduced player availability may result in a negative impact on team performance [16, 17]. The return to sport still remains a clinical challenge [18, 19], owing to the unacceptably high injury recurrence rate that ranges between 16.0 and 68.0% across different sports [3, 7, 8, 20,21,22], and frequently occurs within the first 2 months after return to play [23]. Hamstrings reinjuries are most common within 1 year of returning to sport (with a higher risk in the first 2 weeks) and tend to be more severe [20, 24, 25]. The high rate of recurrence suggests that athletes may be returning to sport unprepared and prematurely [26]. Even if the rehabilitation strategies are appropriate, perhaps athletes are rushing back to sports without enough time for proper biological healing [26].
Rehabilitation is the usual treatment for hamstrings injuries, with surgical treatment reserved for more complex and severe injuries [27, 28]. It has been suggested that interventions could be structured according to the specific injury site (i.e., semitendinosus vs biceps femoris, intra-tendon or extra-tendon) [29, 30] and may be mediated by inter-individual and intra-individual anatomic and physiologic variations [31], but experimental studies are still needed to sustain these claims. Rehabilitation strategies mostly rely on exercise-based interventions that often comprise multimodal approaches (including movement pattern improvement, progressive strength and sprint training, and strength endurance) [32, 33], but there is no consensus on which exercise modes are more effective. Although hamstrings injuries treatment relies mainly on exercise-based interventions, other therapies such as platelet-rich plasma (PRP) or corticosteroid injections, sacroiliac manipulation and/or non-steroidal anti-inflammatory drugs can be concomitantly used [32, 33]. However, the use of conservative (i.e., non-surgical) non-exercise-related strategies seems poorly substantiated by scientific evidence [32, 33].
There are some systematic reviews addressing hamstrings injuries recovery [33,34,35] and assessing criteria for its rehabilitation progress [18]. Since the last systematic review on the effectiveness of acute hamstrings injuries conservative treatment [33], some new studies have been published [36,37,38,39,40,41], suggesting the need for an update on the topic. Considering that data from systematic reviews may quickly become outdated [42], living reviews provide a regularly updated summary of the most up-to-date evidence [43]. Thus, we performed a living systematic review of conservative rehabilitation strategies after acute hamstrings injuries (excluding complete tears and avulsion injuries) to compare the effects of different interventions in time to return to sport (TRTS) and/or time to return to full training (TRFT) and reinjury-related outcomes.
2 Methods
2.1 Criteria for Administrating and Updating the Review
This living systematic review followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) 2020 [44] and Cochrane guidelines [45], and was performed under the guidance of PERSiST [46]. It will be updated annually on 1 January for a period of 5 years after completion of the initial database searches. These updates will be published in a public OSF project (https://osf.io/3k4u2/) and submitted to publication if new large-scale studies are available and/or new findings significantly change the overall results (e.g., a meta-analysis is possible for existing comparisons or new comparisons are available). Any updates or amendments to the protocol will be fully disclosed.
2.2 Eligibility Criteria
Inclusion and exclusion criteria were set according to the Participants, Intervention, Comparator, Outcome and Study design (PICOS) framework.
2.2.1 Participants
We included athletes of all competitive levels and sports with an acute hamstrings muscle injury, regardless of age, sex, race, or health status. Hamstrings muscle injury had to be diagnosed by physical examination (e.g., palpation, strength tests, range of motion [ROM], among others) and/or confirmed through magnetic resonance imaging (MRI) and/or ultrasound within 10 days of initial injury [18, 33]. Studies that comprised individuals with complete hamstrings muscle ruptures (usually assessed as grade 3, depending on the classification system), avulsion injuries, or hamstrings tendinopathy [18] were excluded. Complete ruptures or avulsion injuries usually undergo surgical procedures, while hamstrings tendinopathy is a chronic injury and therefore the rehabilitation procedures may differ from those applied to acute injuries.
2.2.2 Interventions
We included conservative interventions (i.e., avoiding invasive procedures such as surgery) to treat hamstrings muscle injuries (e.g., exercise training, PRPs).
2.2.3 Comparators
Any other conservative intervention (e.g., different exercise protocols, passive control groups and/or placebo) was accepted as a comparator.
2.2.4 Outcomes
We included studies reporting at least one of the primary outcomes: TRTS, TRFT, or occurrence of hamstrings reinjury or new hamstrings injury. Secondary outcomes were defined in the data items but were not used as eligibility criteria.
2.2.5 Study Design
Only original randomized and non-randomized multi-group study designs, with at least ten participants per group [47, 48], published in any language or date, were accepted.
2.3 Information Sources
Initial searches were conducted on 31 August, 2021, and updated on 1 January, 2022, in CINAHL, Cochrane Library, EMBASE, PubMed, Scopus, SPORTDiscus, and Web of Science, without restrictions on language or publication date and no filters applied. Manual searches were conducted by screening the included studies and relevant reviews reference lists. Prospective snowballing citation tracking was performed in Web of Science on 5 October, 2021. Seven external experts (with published research on the topic) were consulted to provide further potentially relevant studies (from which three responded affirmatively as displayed in the Acknowledgements section). The experts accessed our eligibility criteria, but not the search strategy, to avoid biasing their searches. Errata, corrections, corrigenda and/or retractions were sought for the included studies [45] and pre-registered protocols were retrieved when available. If a study had additional and relevant information published in another article, it was used to complement the information.
2.4 Search Strategy and Selection Process
The general search strategy used the following free terms, without filters or limits applied (the full search strategies are displayed in the Electronic Supplementary Material [ESM]): (1) [Ti/Ab] hamstring* OR semitendin* OR semimembran* OR “biceps femoris” OR “femoral biceps” OR “posterior thigh”; AND (2) [Ti/Ab] rehab* OR conserv* OR treat* OR intervention* OR therap* OR manag* OR clinical* OR recover* OR exercis* OR train*; AND (3) [All] injur* OR strain* OR tear* OR ruptur* OR pain OR dysfunction OR trauma; AND (4) [All] athlet* OR sport*. Two authors (JA and SRR) independently screened all database records and performed the manual searches, with disagreements being resolved by a third author (JGC). Automated removal of duplicates was performed using EndNote™ 20.2 for Mac (Clarivate™) and confirmed by manual screening.
2.5 Data Collection
Assessments were planned for the primary outcomes TRTS, TRFT, reinjuries, and new hamstrings injuries. Data on secondary outcomes (pain, strength, strength endurance, power, balance/stability, sprinting, ROM, pre-bilateral and post-bilateral and anteroposterior asymmetries, and adverse effects frequency, type, and severity), study characteristics (e.g., sample size and study design), participant demographics (e.g., age and sex), and sports participation (e.g., sport and competitive level) were also collected. We collected diagnostic characteristics relative to the criteria and methods used as reported by the included studies to determine acute hamstrings injury, imaging techniques applied, number of physicians assessing the images, and specific muscles injured (i.e., semitendinosus, semimembranosus, biceps femoris long head or short head). The programming details of the interventions were defined for exercise-based interventions (e.g., length, weekly frequency, intensity, sets, repetitions, movement types, and muscle actions) and for PRP-based interventions (e.g., number and timing of injections, specific contents, and related information to the PRP-based procedures). The criteria used for progressing in rehabilitation (e.g., time based and/or goal based) and to decide on TRTS/TRFT, co-interventions, funding sources, and competing interests were recorded. Two authors (JOJ and JGC) independently collected data and a third author (FMC) arbitrated in case of disagreements.
2.6 Risk of Bias of Individual Studies
Parallel randomized studies were judged at low risk, some concerns, or high risk of bias in five domains using Cochrane’s Risk of Bias tool, version 2 (RoB 2) [49]: randomization process, deviations from intended interventions (intention-to-treat analysis), missing outcome data, measurement of the outcome, and selection of the reported result. Non-randomized studies were judged at low risk, moderate risk, or critical risk of bias in seven domains using Cochrane’s Risk of Bias In Non-Randomized Studies of Interventions (ROBINS-I) [50]: confounding, selection of the participants, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of the reported result.
The risk of bias was judged at outcome (grouped according to domains, such as reinjuries) and study levels (presenting the worst-case scenario per study). In the absence of a pre-registered protocol, the risk of bias in selection of the reported result was judged, at least, as has having some concerns (RoB 2) or moderate risk (ROBINS-I). Two authors (JA and SRR) independently judged the risk of bias, while a third author (RA) arbitrated when needed. The overall summaries of risk of bias judgments were plotted by the main outcome.
2.7 Data Management
If multiple measurements were available in the included studies, the information provided in the current review refers to the interventions’ endpoint (unless otherwise stated). When data were exclusively provided in figures, two authors (JA and SRR) independently extracted the data using the validated software WebPlotDigitizer version 4.4 [51], and both values are presented in the relevant tables.
2.8 Quality and Completeness of Therapeutic Exercise Program Reporting
This item judged seven domains using the international Consensus on Therapeutic Exercise and Training (i-CONTENT) tool [52]: patient selection, qualified supervisor, type and timing of outcome assessment, dosage parameters (frequency, intensity, time), type of exercise, safety of the exercise program, and adherence to the exercise program. Each domain can be classified as having a low or high risk of ineffectiveness. The specific criteria used to reach the decisions are detailed in the original publication [52]. Two authors (JA and RA) conducted the data collection and a third author (RJF) arbitrated in case of disagreements. If the studies cited other sources to provide relevant information, those publications were viewed.
2.9 Data Synthesis and Analysis
Demographic data were not pooled because of inconsistent and incomplete reporting. Risk-related and continuous variables were treated as risk ratios and mean differences, respectively. Standardized mean differences were planned, but not calculated, as continuous variables used the same units, and we did not pool the data from different studies. Although a pooled quantitative synthesis was not feasible, we computed the between-group mean differences or relative risk for each study within each outcome. Findings were reported narratively because of the very low number of studies per comparison and their clinical heterogeneity precluded us from reliably performing a quantitative synthesis. The planned quantitative analyses can be viewed in the pre-registered protocol (https://osf.io/3k4u2/).
2.10 Certainty of Evidence
Two authors (JA and RA) judged the certainty of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) [53] and disagreements were resolved by consensus. Four of five GRADE dimensions were judged [54, 55]: risk of bias, inconsistency, indirectness, and imprecision. The risk of publication bias was not judged because of an insufficient number of studies per comparison to perform this analysis. Further details on the criteria for judging certainty of evidence can be viewed in the ESM, and the originally planned assessments are available in the study protocol (https://osf.io/3k4u2/).
3 Results
3.1 Study Selection
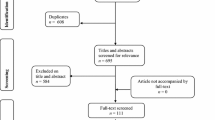
Database searches returned 20,644 records, from which 12,311 were duplicates, and additional searches (included studies’ reference lists, snowballing citation tracking, expert consultations, and updated database searches) did not yield any new studies. Following the titles and abstracts screening, 19 records required a full-text analysis, from which five were excluded because of not fulfilling participants [56, 57] or outcomes [58,59,60] eligibility criteria. Fourteen studies were deemed eligible for inclusion [24, 36,37,38,39,40,41, 57, 61,62,63,64,65,66,67], one study [66] was complemented by previously published information [68], and another [63] had an erratum [69] and a pre-published protocol [70]. Further details on study selection are shown in Fig. 1 and in the ESM.
3.2 Risk of Bias of Individual Studies
Twelve parallel randomized studies were included in the current review [24, 36, 38,39,40, 61,62,63,64,65,66,67], with one study [63] including an erratum [69] and another [66] a letter to the editor [68] that were considered for judging the risk of bias. Nine studies (75.0%) [24, 36, 40, 61,62,63, 65,66,67] and three studies (25.0%) [38, 39, 64] were judged at an overall high and low risk of bias for all primary outcomes, respectively. The study-level assessment (based on the worst-case scenario for each study) and the percentages for each domain are displayed in Fig. 2a and b. Two non-randomized studies [37, 41] were judged at an overall critical risk of bias for all primary outcomes (see Fig. 2c). A more detailed description of am outcome-based and domain-based risk of bias judgment is provided in the ESM.
3.3 Study Characteristics and Results
3.3.1 Publication Details, Funding, and Competing Interests
The studies were published between 2004 and 2020, and five (35.7%) had pre-registered and/or pre-published protocols [38, 39, 63, 64, 66]. Studies were performed mostly in Europe (France, Greece, the Netherlands, Russia, Spain, and Sweden) [36, 40, 41, 61, 62, 65, 66], followed by North America (USA) [24, 37, 67], Asia (Malaysia, Qatar) [63, 64], Oceania (Australia) [38], and South America (Brazil) [39], and no study was performed in Africa. Funding sources were reported in nine studies (64.3%) [24, 37, 38, 61,62,63,64, 66, 67] and unreported in three [36, 41, 65], with two studies reporting no funding [39, 40]. Eight studies (57.1%) displayed no competing interests [38,39,40, 61, 62, 64, 67] or none beyond the public funding [63], while three studies (21.4%) did not address this item [24, 36, 65]. Three studies had potentially relevant competing interests [37, 41, 66] although one of them stated the opposite [66]. More detailed information is provided in the ESM.
3.3.2 Participant Demographics
We present here a summary of participant characteristics and further information is available in the ESM. Across the 14 studies, 730 participants were included, with sample sizes that ranged from n = 24–90 [39, 64]. Participants’ age ranged from 14 to 49 years [24, 63], with 14–49 years [24] and 22–31 years [36] being the widest and narrowest ranges, respectively. Five studies did not provide the age range [38,39,40, 65, 66] and two studies only provided the average age without a standard deviation [37, 41]. Regarding participants’ sex, 654 were male and 72 were female (89.6% and 9.9%, respectively), with four missing values (0.5%). Studies reported the practiced sport for 726 participants, with soccer as the most represented (n = 346, 47.7%), followed by track and field (n = 166, 22.9%) and American football (n = 74, 10.2%). The competitive level ranged from amateur to professional and was unreported in three studies [24, 38, 65]. Table 1 synthesizes the participants’ characteristics.
3.3.3 Previous Hamstrings or Lower Limb Injuries
Six studies (42.9%) included participants without any previous hamstrings injuries in the same thigh in the previous 6–12 months [40, 41, 61, 62] or ever [24, 65]. Previous ipsilateral or contralateral hamstrings injuries were presented in five studies [24, 38, 63, 64, 66] and unreported in four studies [36, 37, 39, 67]. Previous lower limb injuries (other than hamstrings injuries) were unreported in nine studies [24, 36, 37, 39, 61,62,63,64, 67]. One hundred and thirty-two participants (18.1%) presented previous hamstrings injuries and another seven participants (~ 1%) had previous anterior cruciate ligament reconstruction using hamstrings autografts (further information in the ESM).
3.3.4 Injury Classification and Diagnosis
Studies only included participants with acute hamstrings injuries and adopted a wide range of classification systems. Two studies did not report the type of injury and classification system, but a complete disruption or avulsion were excluded [38, 67]. Participants were diagnosed within 2–10 days post-injury. One study did not report the timeline of diagnosis [37], but it can be assumed that examination took place within 48 h, as the interventions started within 24–48 h after injury. Criteria and methods to determine acute hamstrings injury varied substantially across studies and the criteria were unclear in two studies [36, 64]. When MRI was used to confirm the diagnostic findings [36, 37, 41, 61, 62, 64, 66, 67], it was performed within 2–10 days [36, 67] after the acute injury (information unreported in one study [37]). Two studies used ultrasound in addition to MRI [36, 41], four studies only used ultrasound performed within 2–7 days of injury onset [38, 63, 65], and two studies used no imaging [24, 39]. Detailed accounts are presented in the ESM.
The biceps femoris (even if the injured long or short head was not always reported) represented 61.8–87.5% [39, 41], the semitendinosus 6.9–21.7% [37, 67], and the semimembranosus 7.0–26.5% [41, 62] of all injuries. In the two studies that classified sprinting versus stretching-type injuries and reported the specific muscles injured, the biceps femoris long head and the semitendinosus corresponded to 85.0–94.0% [61, 62] and 76.0–100.0% [61, 62] of sprinting-type injuries, respectively. The specific muscles injured were not reported in five studies [24, 40, 64,65,66].
3.3.5 Interventions, Comparators, and Co-interventions
Seven randomized studies compared different therapeutic exercise-based interventions, particularly the L-protocol (focused on the lengthening phase of the hamstrings actions) versus the C-protocol (focused on the hamstrings actions shortening phase) [61, 62], pain-free versus pain-threshold exercise [38], single versus four stretching daily sessions [65], multimodal individualized exercise versus a general exercise program [40], progressive agility and trunk stabilization (PATS) versus hamstrings stretching and strengthening (STST) [24], and PATS versus progressive running and hamstrings eccentric strengthening (PRES) [67]. A single randomized trial compared low-level (LLLT) to placebo laser therapy [39]. Three randomized studies compared PRP to placebo injections [36, 66] or no injection [63], one trial compared all these three conditions [64], and the two non-randomized trials compared PRP injections to no injection [37, 41]. A complete description is reported in Tables S4 and S5 of the ESM.
The intervention length was not predetermined in any study and rehabilitation was progressive and stepwise depending on goal-based criteria to progress (which could be assessed through TRTS and/or TRFT). Commonly, daily [36] and weekly [24, 61,62,63,64, 67] assessments were performed, or even prior to every rehabilitation session [38, 39]. Follow-up (when existing and described) ranged from 4 to 12 months after return to full sports training [24, 41, 61, 62, 66, 67], but was unclear in two studies [37, 65]. All studies included therapeutic exercise as intervention or co-intervention (the details of each intervention and comparator, including information dosage, is shown in the ESM).
3.3.6 Quality and Completeness of Therapeutic Exercise Program Reporting
The completeness and quality of exercise and physical rehabilitation protocols were appraised for all studies as either interventions or co-interventions (Fig. 3). Overall, only three studies (21.4%) were judged with a low risk of ineffectiveness in all seven domains [38, 39, 64]. The remaining 11 studies (78.6%) had a high risk of ineffectiveness in two [40, 63, 66, 67], three [24, 61, 62], four [65], five [36, 41], or six domains [37]. A detailed analysis is provided in the ESM.
3.3.7 Primary Outcomes
Eleven studies (78.6%) assessed TRFT [24, 36, 38, 40, 41, 61,62,63,64,65,66], with one study assessing both TRTS and TRFT (but the information for the latter was unclear [37]), and two studies focusing only on TRTS [39, 67]. Terminology varied, sometimes even within the same study (e.g., return to play, full return to sports, return to full training, return to full participation in the training process). Eleven studies (78.6%) assessed reinjuries and/or new injuries, from which six studies reported reinjury rates < 5.0% [36, 37, 39, 41, 61, 62]. Details are presented in the ESM.
3.3.8 Secondary Outcomes
Few studies reported pre-intervention to post-intervention changes in strength and ROM [39, 67], bilateral asymmetries [66, 67], and pain [36, 66]. Overall, no between-group differences could be detected for secondary outcomes. Adverse effects (beyond new hamstrings injuries or reinjuries) were unreported in six studies (42.9%) [24, 39, 40, 61, 62, 65]. In the remaining studies, there were either no adverse effects to report or these were mostly minor and/or isolated cases. A detailed account is provided in the ESM.
3.4 Narrative Synthesis
3.4.1 Studies Comparing Therapeutic Exercise-Based Interventions
Therapeutic exercise-based interventions were diverse, with only a few comparisons available for each program. Two studies compared the L-protocols and the C-protocols [61, 62], with one study [62] also including a running and stationary cycling program. The L-protocol compared with the C-protocol showed faster TRFT (28 ± 15 days vs 51 ± 21 days [61]; 49 ± 26 days vs 86 ± 34 days [62]), but data were compromised because of a risk of selection (randomization) and detection bias. All negative-MRI participants were purposely allocated to the L-protocol, with implications for the recovery time: in one study [61], negative-MRI participants had an average TRFT of 6 days, and four of the 11 soccer players recovered within 5 days of injury and did not even perform the L-protocol. In the other study [62], the negative-MRI participants returned after 15 days, compared with 45 days for the other participants. Moreover, the assessors were unblinded to the intervention and the authors explicitly stated that this knowledge could have influenced the Askling H test, which determined discharge to return to sport. Therefore, the evidence from these two studies is associated with important methodological problems that could have influenced the results. One reinjury (0.8%) [61] and two reinjuries (3.6%) were registered [62] in the C-protocol, and none in the L-protocol.
Two studies analyzed the PATS [24, 67], with one comparing PATS to PRES (based on running and eccentric strengthening) [67]. Both intervention and comparator were home based and performed daily (with only one weekly supervised session guaranteed and poor adherence-related measures). No differences were detected in TRTS between PATS and PRES groups, but one versus three reinjuries (6.3 vs 23.1%) were registered in the PATS and PRES groups, respectively. The other study [24] compared PATS to STST (based on hamstrings stretching and concentric, eccentric, and isometric strengthening), again in a mostly home-based setting with very poor control of adherence. The PATS group had a shorter TRFT (22.2 ± 8.3 days versus 37.4 ± 27.6 days), but a closer analysis raised concerns, as 72.0% of participants in the PATS groups had a grade 2 injury versus only 36.0% of participants in the STST group. These baseline differences suggest problems with the randomization process. Furthermore, the authors planned an intention-to-treat analysis, but instead performed a per-protocol analysis, suggestive of bias due to selective reporting. This study also presented an outlier value of reinjuries in the STST group (n = 7, 63.6%) versus a single reinjury (7.7%) in the PATS group.
A single study compared one versus four daily sessions of hamstrings static stretching [65]. The single daily session group took longer to return to training than the other group (15.1 ± 0.8 days vs 13.3 ± 0.7 days) and, although this difference was statistically significant, in absolute values there was only a 2-day difference in recovery time. Reinjuries were not reported, and the study [65] was judged at a high risk of bias in all domains and a high risk of ineffectiveness in several i-CONTENT domains (type of exercise program, qualified supervisor, type and timing of outcome assessment, and adherence to the exercise program).
One study compared an individualized and multifactorial criteria-based algorithm to a general rehabilitation protocol including a running-based program and the L-protocol [40], with no description of the general rehabilitation components or the running program specifications. There were no differences in TRFT, but there were fewer reinjuries in the individualized group versus the general rehabilitation group (n = 1, 4.0% vs n = 6, 25.0%). Finally, one study applied a strength-based exercise program (combined with running) performed at different pain-threshold intensities (0 and ≤ 4 out of a ten-point scale) [38]. No differences were found in TRFT between groups and two reinjuries were registered in each group (9.1% and 9.5% in the pain-free and pain-threshold groups, respectively).
In summary, few studies have assessed each exercise program type (e.g., PATS and L-protocol), relevant heterogeneity was observed regarding their study populations, diagnosis and criteria for progressing in rehabilitation, and methodological problems associated with these studies were detected. Currently, the available evidence does not allow us to confidently assume or suggest a superiority of one exercise program over another in terms of TRTS/TRFT or reinjuries.
3.4.2 Studies Comparing PRP Injections to Placebo or Control
Two randomized studies compared PRP to placebo injections [36, 66], one contrasted PRP with no injection [63], and another compared the three conditions [64]. Because the three studies comparing PRP to placebo injections [36, 64, 66] were very heterogeneous (regarding participants, injury diagnosis, injection contents and dosages, and co-interventions), a quantitative pooled synthesis was not accomplished. Most studies applied a single PRP injection [36, 63, 64] and one study [66] applied two injections 5–7 days apart, with dosages varying from 3 to 8-mL single applications [36, 63] to 1-mL injections in three sites [64, 66]. Injection platelet count varied from unreported [63, 64, 66] to 700,000 per 1 mL [36] and activation agents varied from none [63, 64] to 20 µL per mL of plasma [36] (or were unreported [66]). Placebo injections also varied in content (0.9% NaCl [36], isotonic saline solution [66], platelet-poor plasma [64]), quantity (1–2 [36, 64, 66]), dosage (8 mL or 3 × 1 mL [36, 64, 66]), activating agents (unreported [36, 66] and no agent used [64]), and number of injection sites (single to three locations [36, 64, 66]).
A shorter TRFT was observed in the PRP group compared with the placebo group (11.4 ± 1.2 days vs 21.3 ± 2.7 days) in one study [36] and no reinjuries were registered; information was insufficient to assess baseline differences between groups. Another study showed a faster TRFT in the PRP group than in the platelet-poor plasma group (median 21 vs 27 days and interquartile range 16–33 vs 19–33) [64]. Both groups sustained two reinjuries (6.7% and 8.0%) at the 2-month follow-up, with an additional reinjury in the platelet-poor plasma group at the 6-month follow-up. Last, there were no differences in TRFT between PRP and placebo injections in a third study [66]. In this study, at the 1-year follow-up, 10 and 11 players (27.0% and 30.0%), respectively, in the PRP and placebo groups sustained a reinjury.
One study reported faster TRFT (26.7 ± 7.0 vs 42.5 ± 20.6 days) when comparing PRP to no injection groups [63], but there were important baseline differences between the groups (particularly the fact that 42.9 vs 78.6% of participants had reinjuries and 57.1 vs 78.6% of them had biceps femoris injuries, respectively). A study with three groups (PRP, placebo, no injection) showed a faster TRFT with PRP injection versus placebo (platelet-poor plasma) [64]; however, the same study reported no differences between PRP and the group taking no injections. This study [64] also reported two reinjuries in each group at the 2-month follow-up (with an additional reinjury in the no injection group at the 6-month follow-up).
The two non-randomized cohort studies compared PRP to no injection [37, 41]. One study [37] applied one to three leukocyte-poor PRP injections and both groups engaged in poorly defined physiotherapy and physical therapy protocols. There were no differences in the TRTS and in the number of days off, and each group sustained one reinjury. The authors mentioned lost games, but this may have been affected by match scheduling or coaching decisions. The other non-randomized cohort trial [41] compared a single PRP injection to no injection, with both groups engaging in unclear physiotherapy protocols and exercise programs with undisclosed dosage. There were no differences in TRFT and there were no reinjuries to report. Both non-randomized studies were judged at high risk of ineffectiveness and at critical risk of bias. Overall, the evidence on PRP injections is contentious and they may not result in a faster TRTS/TRFT or reduced reinjury rates than placebo injections or no injection.
3.4.3 Single Study Assessing Low-Level Laser Therapy
One study compared three weekly sessions of LLLT (60 s and 30 J per site, 850-nm wavelength, and continuous frequency) to placebo LLLT (with the device turned off), both supplemented by a rehabilitation exercise program focused on hamstrings strength, trunk stabilization, and agility [39]. There were no differences between the groups in TRTS, TRFT was not assessed, and no reinjuries were reported. Lack of imaging techniques (such as MRI or ultrasound) to confirm diagnosis may have resulted in the inclusion of low-grade or unequal injuries, potentially influencing recovery time. Conversely, the study [39] was judged at low risk of bias in all domains and at low risk of ineffectiveness in all i-CONTENT domains.
3.5 Certainty of Evidence
Three studies reported TRTS [37, 39, 67], 11 studies referred to TRFT [24, 36, 38, 40, 41, 61,62,63,64,65,66], and 12 studies identified reinjuries or new injuries [24, 36,37,38,39,40,41, 61, 62, 64, 66, 67]. The reduced number of studies for each comparison (one to three studies), the high risk and critical risk of bias, the serious inconsistency, and the high imprecision resulted in a very low certainty of evidence for all outcomes and comparisons analyzed. Therefore, no current recommendation can be provided based on existing evidence (Table 2 synthesizes the main findings, including the GRADE judgments).
4 Discussion
The high incidence of hamstrings injuries and their associated financial costs and performance losses [14,15,16,17] require effective rehabilitation protocols to facilitate return to sport and reduce the reinjury risk. We systematically reviewed 14 studies (n = 730) that assessed the impact of different conservative rehabilitation strategies to treat acute hamstrings injuries on the TRTS/TRFT and reinjuries.
4.1 What Does the Current Literature Tell Us?
Based on the existing evidence, it is unclear which conservative approaches are more effective in allowing a faster TRTS/TRFT or reducing the reinjury risk. Our results support the findings of a previous systematic review [33] where the authors did not find any effect of the PRP interventions and reported limited evidence for exercise-based interventions. In our systematic review, we included six new studies [36,37,38,39,40,41] and excluded two studies that did not meet the eligibility criteria for population [56] and outcomes [58]. In contrast to the previous systematic review [33], we considered that the reduced number of studies for each comparison and their clinical heterogeneity advised against performing a meta-analysis; we thus avoided pooling data from different studies and reported the between-group differences for each study and described them in a narrative manner.
When comparing different exercise-based interventions, it is unclear which exercise modalities are more effective (although there is some support for eccentric training) and even less is known concerning dose–response relationships, which aligns with the main findings of the previous review [33]. Also aligned with this review [33], the effectiveness of PRPs and placebo injections remains unclear. Independent of participants’ age, sex, sport background, or injury characteristics (e.g., severity, anatomical location, and etiology), no recommendations on the best rehabilitation strategy can be provided based on current knowledge for TRTS/TRFT and reinjuries.
The literature has devoted considerable attention to the incidence of hamstrings injuries and reinjuries [3, 4, 7, 12] as well as to their financial and performance-related costs [14,15,16,17]. The literature has also extensively focused on the primary prevention of hamstrings injuries [5, 71,72,73,74,75,76], but their number continues to grow [2, 77], and thus we assumed that many studies would be found focusing on rehabilitation strategies. However, only 14 studies fulfilled eligibility criteria (12 randomized and two non-randomized with 730 participants), with a maximum of three studies per comparison. Most randomized studies (75.0%) were judged at high risk of bias, both non-randomized studies (100.0%) were judged at critical risk (i.e., above serious risk), and 78.6% of the exercise programs were judged at high risk of ineffectiveness. These findings corroborate the poor reporting of exercise interventions in the context of hamstrings injury rehabilitation that was recently highlighted by a scoping review [78]. The GRADE judgments denoted very low confidence in the existing published data.
These findings emphasize it is not possible to determine which are the most effective conservative interventions for recovery after acute hamstrings injuries. Studies have focused mostly on exercise-based interventions and on comparing PRP to placebo or no injection (with exercise as a co-intervention). The benefits of adding PRP to exercise interventions remain unclear (conflicting findings) and the most appropriate exercise modalities and dosages are not yet known. Although rehabilitation should be customized to individual needs, it is unknown whether the most appropriate conservative interventions may vary depending on injury mechanism, injury classification and severity, type of sport, competitive level, sex, age, or other individual characteristics. Although it has been hypothesized that the specific injury location, especially if affecting the intramuscular tendon, may change the length of recovery [29, 30, 79], the evidence is still preliminary and it is unclear how conservative interventions could be adapted.
Very recent clinical practice guidelines [80] maintained that moderate-level evidence supports faster TRTS/TRFT with interventions focusing on eccentric training, added to stretching, general strengthening, stabilization, and progressive running programs. Likewise, the guidelines [80] reported moderate-level evidence in support of PATS in addition to stretching, general strengthening, and “functional” exercises to reduce reinjury risk after an acute hamstrings injury. The results of our systematic review do not fully support either of these claims and, with the scarce and limited available data, no recommendations can be made on which is the best rehabilitation strategy for acute hamstrings injuries.
Notwithstanding the limitations discussed above and the very low certainty of evidence, some clinical findings may temporarily guide clinical practice: (1) adding eccentric lengthening exercises seems superior to conventional stretching and strengthening exercises for returning sooner to full training [61, 62], but these findings are from a single research group and require confirmation by replication studies; (2) no intervention was superior in reducing the reinjury risk, thus no intervention should be prioritized over any other for purposes of secondary prevention; and (3) PRP did not consistently allow a faster TRFT or reduce the reinjury risk and therefore seems to add no value in accelerating recovery from hamstrings injuries.
4.2 Are We Comparing the Same Things?
Our systematic review highlighted extensive heterogeneity in intervention and comparators (including mode, dosage and supervision of the exercise programs, and content and dosages of PRP and placebo injections) and outcome registration. Even within a single group of the same study, the ranges of TRTS/TRFT exhibited extreme interindividual variation, which may be partially related to interindividual variations in hamstrings anatomy and physiology [31], but also to age. Indeed, the age range within a single study was as wide as 14–49 years [24]. As older players are at a higher risk of injury than their younger counterparts [81, 82], investigating and comparing samples with large age variations may affect the results. Moreover, because women comprise only a minority of the studied samples (~ 10%), it is currently unclear if there are relevant sex-related differences that can affect the recovery process and/or the risk of reinjury.
The competitive level was not reported uniformly across studies, a problem that creates difficulty performing between-study comparisons and to which a recent solution has been proposed [83]. Still, it could be easily identified that the competitive level in the included studies ranged from amateur practitioners to professional athletes. Comparing rehabilitation processes between very distinct competitive levels is tricky, as there are other factors that can come into play, such as higher motivation from professional players or their easier access to high-quality resources and rehabilitation. The pressure on an early return to competition may be superior at the highest levels, considering the performance and financial stakes. Of note, nearly 50% of research on the topic is focused on soccer, followed by track and field (~ 22%) and American football (~ 10%), with only scarce information on return to sports and reinjury risk being available for other sports.
With regard to secondary outcomes, nearly nothing is known about how effective these interventions are for other relevant variables such as strength, ROM, bilateral and anteroposterior asymmetries, balance, power, speed, endurance, and adverse effects. Even exercise-based interventions largely neglected these outcomes or assessed them as solely post-intervention values. Recent clinical practice guidelines further underline the need to evaluate the ability to walk, run, and sprint [80].
The included studies also varied considerably with respect to injury classification systems, diagnostic and inclusion criteria, criteria for returning to sport, and assessments timing, contributing to clinical heterogeneity and making between-study comparisons very difficult, as had been previously pinpointed elsewhere [84]. The sheer diversity of classification systems denotes a lack of agreement and consistency across studies and may contribute to increasing the heterogeneity of findings, which is further exacerbated by mixing grade I and II injuries and MRI-positive with MRI-negative participants. The absence of imaging in some studies, although justified by the authors, cannot completely rule out avulsion or complete rupture, which was an exclusion criterion of our review.
Different sports have specific physical demands and may require different rehabilitation protocols. Perhaps the time has come for a complete and more definitive international consensus on the classification of hamstrings injuries, diagnostic criteria, and criteria for return to full training. Finally, more than half of the studies mixed participants with and without previous hamstring injuries, which represents a relevant confounder for the results of the interventions, as the history of a hamstrings injury greatly increases the risk of having a reinjury [75, 82, 85, 86].
4.3 Room for Improvement: Priorities for Future Research
Although men are up to 60.0% more likely to sustain hamstrings injuries than women [3, 87, 88], they comprise 90% of the research sample. With the rapid increase in female sports participation [89, 90], further research on hamstrings injuries rehabilitation focusing on women is needed. We further identified several features that require more detailed reporting in future studies: (1) explicitly report previous hamstrings injuries or their absence, as well as surgeries involving hamstrings autografts; (2) different hamstrings muscles may be injured differently (mechanisms, consequences, recovery) and proper reporting of which muscles were injured is advised; (3) provide means and standard deviations (or medians and interquartile ranges, when appropriate) for participants’ age and preferably also the range; (4) use the i-CONTENT tool [52] to more completely report on the exercise-based interventions; (5) openly monitor and report adverse effects; and (vi) assess pre-intervention to post-intervention changes in secondary outcomes (such as strength and ROM), providing measures of assessment reliability and reporting the smallest worthwhile changes.
Future research should strive to reduce the risk of bias by following simple procedures: (1) pre-register or pre-publish the research protocols; (2) describe how randomization was achieved, explicitly state whether allocation sequence was concealed and attempt to guarantee balanced baseline values for the most relevant variables (e.g., using minimization techniques when randomizing the participants); (3) attempt to equate the intervention and comparator dosages; (4) ensure proper supervision and monitoring of adherence and/or compliance; in largely home-based interventions, strategies such as regular texting, daily logs, and video calls may help to improve compliance; (5) blind the outcome assessors to eliminate the risk of detection bias; and (6) provide data on the inter-assessor reliability of outcome measurement or, when applicable, measures of error for the used devices (e.g., coefficient of variation, typical error of measures).
Research on conservative intervention to treat acute hamstrings injuries in athletes has been continuing at a slow pace. There is a clear need for more homogeneous studies to allow comparisons and achieve a valid pooled estimate of TRTS/TRFT and reinjuries. To reliably compare the reinjury risk across different conservative interventions, studies with a very large sample size are needed, but these studies are not easy to conduct in real-world club-based sports. We need combined efforts from several clubs implementing the same conservative strategies to increase the comparability and statistical power before clinical practice guidelines can be reliably established. Because we are performing a living systematic review, future updates will reveal changes to the status quo.
4.4 Limitations
The inclusion of non-randomized trials may be interpreted as a weakness of our systematic review, but these trials were analyzed separately from randomized trials and so we provide a more complete picture without mixing the findings from two fundamentally different study designs. Furthermore, randomized trials are not always feasible, especially with high-level athletes. As previously mentioned, some studies lacked imaging to confirm diagnosis, and thus complete rupture or avulsion could not be completely ruled out. Despite planned at the protocol stage, we opted to not perform a meta-analysis or network meta-analysis because of the wide clinical heterogeneity in populations, interventions, comparators, and other methodological features of the studies (including how the outcomes were assessed), which precluded us from confidently pooling the data from different studies and is coherent with Cochrane’s guidelines [45]. Still, to provide useful information, we calculated the between-group mean differences and relative risks for each study within each outcome and provide a narrative summary that is supported with a best evidence synthesis using the GRADE framework.
5 Conclusions
No single intervention or combination of interventions proved superior in achieving a faster return to sports or reducing the reinjury risk. Exercise-based interventions seem comparable and no specific strategy needs to be prioritized when rehabilitating athletes with acute hamstrings injuries. Only eccentric lengthening exercises showed limited evidence (very low certainty) in allowing a shorter TRFT. Platelet-rich plasma did not consistently reduce the TRFT or the reinjury risk and, at the moment, has no value in clinical practice. The use of passive interventions (LLLT) also did not yield any clinical value when added to exercise-based rehabilitation. The currently available literature is limited owing to the risk of bias, the high risk of ineffectiveness of exercise protocols (especially due to poor or uncontrolled adherence, absence of proper supervision, and incomplete information to assess dosage of the prescribed exercise program), and the lack of comparability across existing studies. Future studies should strive to overcome these limitations and provide a pool of evidence that allows meaningful comparisons and stronger clinical directions to be achieved. Our living review will be attentive and update the knowledge synthesis on an annual basis.
Registration and Protocol
The protocol was created (https://osf.io/3k4u2/) and pre-registered (https://osf.io/dxe2t) as an OSF project (public since 30 August, 2021), and also pre-registered in PROSPERO (CRD42021268499, attributed on 22 August, 2021). Changes to the original protocol: (1) an age-related inclusion criterion was removed following a suggestion from one of the external experts before submission of the manuscript. This did not, however, change the studies included in the review, as none of those studies had been excluded based on that criterion; (2) the remaining changes were properly identified in the methods (e.g., absence of quantitative synthesis).
References
Roe M, Murphy JC, Gissane C, Blake C. Hamstring injuries in elite Gaelic football: an 8-year investigation to identify injury rates, time-loss patterns and players at increased risk. Br J Sports Med. 2018;52(15):982–8. https://doi.org/10.1136/bjsports-2016-096401.
Ekstrand J, Waldén M, Hägglund M. Hamstring injuries have increased by 4% annually in men’s professional football, since 2001: a 13-year longitudinal analysis of the UEFA Elite Club injury study. Br J Sports Med. 2016;50(12):731–7. https://doi.org/10.1136/bjsports-2015-095359.
Diemer WM, Winters M, Tol JL, Pas H, Moen MH. Incidence of acute hamstring injuries in soccer: a systematic review of 13 studies involving more than 3800 athletes with 2 million sport exposure hours. J Orthop Sports Phys Ther. 2021;51(1):27–36. https://doi.org/10.2519/jospt.2021.9305.
Elliott MC, Zarins B, Powell JW, Kenyon CD. Hamstring muscle strains in professional football players: a 10-year review. Am J Sports Med. 2011;39(4):843–50. https://doi.org/10.1177/0363546510394647.
Brooks JH, Fuller CW, Kemp SP, Reddin DB. Incidence, risk, and prevention of hamstring muscle injuries in professional rugby union. Am J Sports Med. 2006;34(8):1297–306. https://doi.org/10.1177/0363546505286022.
Ribeiro-Alvares JB, Dornelles MP, Fritsch CG, de Lima ESFX, Medeiros TM, Severo-Silveira L, et al. Prevalence of hamstring strain injury risk factors in professional and under-20 male football (soccer) players. J Sport Rehabil. 2020;29(3):339–45. https://doi.org/10.1123/jsr.2018-0084.
Zachazewski J, Silvers H, Li B, Pohlig R, Ahmad C, Mandelbaum B. Prevalence of hamstring injuries in summer league baseball players. Int J Sports Phys Ther. 2019;14(6):885–97.
Roe M, Murphy JC, Gissane C, Blake C. Time to get our four priorities right: an 8-year prospective investigation of 1326 player-seasons to identify the frequency, nature, and burden of time-loss injuries in elite Gaelic football. PeerJ. 2018;6: e4895. https://doi.org/10.7717/peerj.4895.
Opar DA, Drezner J, Shield A, Williams M, Webner D, Sennett B, et al. Acute hamstring strain injury in track-and-field athletes: a 3-year observational study at the Penn Relay Carnival. Scand J Med Sci Sports. 2014;24(4):e254–9. https://doi.org/10.1111/sms.12159.
Tabben M, Eirale C, Singh G, Al-Kuwari A, Ekstrand J, Chalabi H, et al. Injury and illness epidemiology in professional Asian football: lower general incidence and burden but higher ACL and hamstring injury burden compared with Europe. Br J Sports Med. 2021. https://doi.org/10.1136/bjsports-2020-102945.
Dalton SL, Kerr ZY, Dompier TP. Epidemiology of hamstring strains in 25 NCAA sports in the 2009–2010 to 2013–2014 academic years. Am J Sports Med. 2015;43(11):2671–9. https://doi.org/10.1177/0363546515599631.
Ahmad CS, Dick RW, Snell E, Kenney ND, Curriero FC, Pollack K, et al. Major and Minor League Baseball hamstring injuries: epidemiologic findings from the Major League Baseball Injury Surveillance System. Am J Sports Med. 2014;42(6):1464–70. https://doi.org/10.1177/0363546514529083.
Ekstrand J, Hägglund M, Waldén M. Epidemiology of muscle injuries in professional football (soccer). Am J Sports Med. 2011;39(6):1226–32. https://doi.org/10.1177/0363546510395879.
Ekstrand J. Keeping your top players on the pitch: the key to football medicine at a professional level. Br J Sports Med. 2013;47(12):723. https://doi.org/10.1136/bjsports-2013-092771.
Hickey J, Shield AJ, Williams MD, Opar DA. The financial cost of hamstring strain injuries in the Australian Football League. Br J Sports Med. 2014;48(8):729–30. https://doi.org/10.1136/bjsports-2013-092884.
Hägglund M, Waldén M, Magnusson H, Kristenson K, Bengtsson H, Ekstrand J. Injuries affect team performance negatively in professional football: an 11-year follow-up of the UEFA Champions League injury study. Br J Sports Med. 2013;47(12):738. https://doi.org/10.1136/bjsports-2013-092215.
Eliakim E, Morgulev E, Lidor R, Meckel Y. Estimation of injury costs: financial damage of English Premier League teams’ underachievement due to injuries. BMJ Open Sport Exerc Med. 2020;6(1): e000675. https://doi.org/10.1136/bmjsem-2019-000675.
Hickey J, Timmins RG, Maniar N, Williams MD, Opar DA. Criteria for progressing rehabilitation and determining return-to-play clearance following hamstring strain injury: a systematic review. Sports Med. 2017;47(7):1375–87. https://doi.org/10.1007/s40279-016-0667-x.
Taberner M, Haddad FS, Dunn A, Newall A, Parker L, Betancur E, et al. Managing the return to sport of the elite footballer following semimembranosus reconstruction. BMJ Open Sport Exerc Med. 2020;6(1): e000898. https://doi.org/10.1136/bmjsem-2020-000898.
Orchard J, Seward H. Epidemiology of injuries in the Australian Football League, seasons 1997–2000. Br J Sports Med. 2002;36(1):39. https://doi.org/10.1136/bjsm.36.1.39.
Ekstrand J, Healy JC, Waldén M, Lee JC, English B, Hägglund M. Hamstring muscle injuries in professional football: the correlation of MRI findings with return to play. Br J Sports Med. 2012;46(2):112. https://doi.org/10.1136/bjsports-2011-090155.
Ekstrand J, Krutsch W, Spreco A, van Zoest W, Roberts C, Meyer T, et al. Time before return to play for the most common injuries in professional football: a 16-year follow-up of the UEFA Elite Club Injury Study. Br J Sports Med. 2020;54(7):421. https://doi.org/10.1136/bjsports-2019-100666.
Wangensteen A, Tol JL, Witvrouw E, Van Linschoten R, Almusa E, Hamilton B, et al. Hamstring reinjuries occur at the same location and early after return to sport: a descriptive study of MRI-confirmed reinjuries. Am J Sports Med. 2016;44(8):2112–21. https://doi.org/10.1177/0363546516646086.
Sherry MA, Best TM. A comparison of 2 rehabilitation programs in the treatment of acute hamstring strains. J Orthop Sports Phys Ther. 2004;34(3):116–25. https://doi.org/10.2519/jospt.2004.34.3.116.
Warren P, Gabbe BJ, Schneider-Kolsky M, Bennell KL. Clinical predictors of time to return to competition and of recurrence following hamstring strain in elite Australian footballers. Br J Sports Med. 2010;44(6):415–9. https://doi.org/10.1136/bjsm.2008.048181.
Pieters D, Wezenbeek E, Schuermans J, Witvrouw E. Return to play after a hamstring strain injury: it is time to consider natural healing. Sports Med. 2021;51(10):2067–77. https://doi.org/10.1007/s40279-021-01494-x.
Bodendorfer BM, Curley AJ, Kotler JA, Ryan JM, Jejurikar NS, Kumar A, et al. Outcomes after operative and nonoperative treatment of proximal hamstring avulsions: a systematic review and meta-analysis. Am J Sports Med. 2018;46(11):2798–808. https://doi.org/10.1177/0363546517732526.
Brukner P, Connell D. ‘Serious thigh muscle strains’: beware the intramuscular tendon which plays an important role in difficult hamstring and quadriceps muscle strains. Br J Sports Med. 2016;50(4):205. https://doi.org/10.1136/bjsports-2015-095136.
Macdonald B, McAleer S, Kelly S, Chakraverty R, Johnston M, Pollock N. Hamstring rehabilitation in elite track and field athletes: applying the British Athletics Muscle Injury Classification in clinical practice. Br J Sports Med. 2019;53(23):1464. https://doi.org/10.1136/bjsports-2017-098971.
Pollock N, Kelly S, Lee J, Stone B, Giakoumis M, Polglass G, et al. A 4-year study of hamstring injury outcomes in elite track and field using the British Athletics rehabilitation approach. Br J Sports Med. 2022;56(5):257–63. https://doi.org/10.1136/bjsports-2020-103791.
Afonso J, Rocha-Rodrigues S, Clemente FM, Aquino M, Nikolaidis PT, Sarmento H, et al. The hamstrings: anatomic and physiologic variations and their potential relationships with injury risk. Front Physiol. 2021;12(1049):694604. https://doi.org/10.3389/fphys.2021.694604.
Silvers-Granelli HJ, Cohen M, Espregueira-Mendes J, Mandelbaum B. Hamstring muscle injury in the athlete: state of the art. J ISAKOS. 2021;6(3):170. https://doi.org/10.1136/jisakos-2017-000145.
Pas HI, Reurink G, Tol JL, Weir A, Winters M, Moen MH. Efficacy of rehabilitation (lengthening) exercises, platelet-rich plasma injections, and other conservative interventions in acute hamstring injuries: an updated systematic review and meta-analysis. Br J Sports Med. 2015;49(18):1197–205. https://doi.org/10.1136/bjsports-2015-094879.
Reurink G, Goudswaard GJ, Tol JL, Verhaar JA, Weir A, Moen MH. Therapeutic interventions for acute hamstring injuries: a systematic review. Br J Sports Med. 2012;46(2):103–9. https://doi.org/10.1136/bjsports-2011-090447.
Mason DL, Dickens V, Vail A. Rehabilitation for hamstring injuries. Cochrane Database Syst Rev. 2012;12:CD004575. https://doi.org/10.1002/14651858.CD004575.pub3.
Bezuglov E, Maffulli N, Tokareva A, Achkasov E. Platelet-rich plasma in hamstring muscle injuries in professional soccer players: a pilot study. Muscles Ligaments Tendons J. 2019;9(1):112–8. https://doi.org/10.32098/mltj.01.2019.20.
Bradley JP, Lawyer TJ, Ruef S, Towers JD, Arner JW. Platelet-rich plasma shortens return to play in National Football League players with acute hamstring injuries. Orthop J Sports Med. 2020;8(4):2325967120911731. https://doi.org/10.1177/2325967120911731.
Hickey J, Timmins R, Maniar N, Rio E, Hickey P, Pitcher C, et al. Pain-free versus pain-threshold rehabilitation following acute hamstring strain injury: a randomized controlled trial. J Orthop Sports Phys Ther. 2020;50(2):91–103. https://doi.org/10.2519/jospt.2020.8895.
Medeiros DM, Aimi M, Vaz MA, Baroni BM. Effects of low-level laser therapy on hamstring strain injury rehabilitation: a randomized controlled trial. Phys Ther Sport. 2020;42:124–30. https://doi.org/10.1016/j.ptsp.2020.01.006.
Mendiguchia J, Martinez-Ruiz E, Edouard P, Morin JB, Martinez-Martinez F, Idoate F, et al. A multifactorial, criteria-based progressive algorithm for hamstring injury treatment. Med Sci Sports Exerc. 2017;49(7):1482–92. https://doi.org/10.1249/mss.0000000000001241.
Guillodo Y, Madouas G, Simon T, Le Dauphin H, Saraux A. Platelet-rich plasma (PRP) treatment of sports-related severe acute hamstring injuries. Muscles Ligaments Tendons J. 2015;5(4):284–8. https://doi.org/10.11138/mltj/2015.5.4.284.
Shojania KG, Sampson M, Ansari MT, Ji J, Doucette S, Moher D. How quickly do systematic reviews go out of date? A survival analysis. Ann Intern Med. 2007;147(4):224–33. https://doi.org/10.7326/0003-4819-147-4-200708210-00179.
Elliott JH, Turner T, Clavisi O, Thomas J, Higgins JPT, Mavergames C, et al. Living systematic reviews: an emerging opportunity to narrow the evidence-practice gap. PLoS Med. 2014;11(2): e1001603. https://doi.org/10.1371/journal.pmed.1001603.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71. https://doi.org/10.1136/bmj.n71.
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. 2nd ed. Chichester: Wiley; 2019.
Ardern CL, Büttner F, Andrade R, Weir A, Ashe MC, Holden S, et al. Implementing the 27 PRISMA 2020 Statement items for systematic reviews in the sport and exercise medicine, musculoskeletal rehabilitation and sports science fields: the PERSiST (implementing Prisma in Exercise, Rehabilitation, Sport medicine and SporTs science) guidance. Br J Sports Med. 2022;56(4):175–95. https://doi.org/10.1136/bjsports-2021-103987.
van der Vlist AC, Winters M, Weir A, Ardern CL, Welton NJ, Caldwell DM, et al. Which treatment is most effective for patients with Achilles tendinopathy? A living systematic review with network meta-analysis of 29 randomised controlled trials. Br J Sports Med. 2021;55(5):249–56. https://doi.org/10.1136/bjsports-2019-101872.
Winters M, Holden S, Lura CB, Welton NJ, Caldwell DM, Vicenzino BT, et al. Comparative effectiveness of treatments for patellofemoral pain: a living systematic review with network meta-analysis. Br J Sports Med. 2020;55(7):369–77. https://doi.org/10.1136/bjsports-2020-102819.
Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898. https://doi.org/10.1136/bmj.l4898.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355: i4919. https://doi.org/10.1136/bmj.i4919.
Rohatgi A. WebPlotDigitizer, version 4.4. Pacifica (CA); 2020.
Hoogeboom TJ, Kousemaker MC, van Meeteren NLU, Howe T, Bo K, Tugwell P, et al. i-CONTENT tool for assessing therapeutic quality of exercise programs employed in randomised clinical trials. Br J Sports Med. 2021;55(20):1153–60. https://doi.org/10.1136/bjsports-2019-101630.
Guyatt GH, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94. https://doi.org/10.1016/j.jclinepi.2010.04.026.
Zhang Y, Alonso-Coello P, Guyatt GH, Yepes-Nuñez JJ, Akl EA, Hazlewood G, et al. GRADE Guidelines: 19. Assessing the certainty of evidence in the importance of outcomes or values and preferences: risk of bias and indirectness. J Clin Epidemiol. 2019;111:94–104. https://doi.org/10.1016/j.jclinepi.2018.01.013.
Zhang Y, Coello PA, Guyatt GH, Yepes-Nuñez JJ, Akl EA, Hazlewood G, et al. GRADE guidelines: 20. Assessing the certainty of evidence in the importance of outcomes or values and preferences: inconsistency, imprecision, and other domains. J Clin Epidemiol. 2019;111:83–93. https://doi.org/10.1016/j.jclinepi.2018.05.011.
Cibulka MT, Rose SJ, Delitto A, Sinacore DR. Hamstring muscle strain treated by mobilizing the sacroiliac joint. Phys Ther. 1986;66(8):1220–3. https://doi.org/10.1093/ptj/66.8.1220.
Rossi LA, Romoli ARM, Altieri BAB, Flor JAB, Scordo WE, Elizondo CM. Does platelet-rich plasma decrease time to return to sports in acute muscle tear? A randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017;25(10):3319–25. https://doi.org/10.1007/s00167-016-4129-7.
Reynolds JF, Noakes TD, Schwellnus MP, Windt A, Bowerbank P. Non-steroidal anti-inflammatory drugs fail to enhance healing of acute hamstring injuries treated with physiotherapy. S Afr Med J. 1995;85(6):517–22.
Sefiddashti L, Ghotbi N, Salavati M, Farhadi A, Mazaheri M. The effects of cryotherapy versus cryostretching on clinical and functional outcomes in athletes with acute hamstring strain. J Bodyw Mov Ther. 2018;22(3):805–9. https://doi.org/10.1016/j.jbmt.2017.08.007.
Kornberg C, Lew P. The effect of stretching neural structures on grade one hamstring injuries. J Orthop Sports Phys Ther. 1989;10(12):481–7. https://doi.org/10.2519/jospt.1989.10.12.481.
Askling CM, Tengvar M, Thorstensson A. Acute hamstring injuries in Swedish elite football: a prospective randomised controlled clinical trial comparing two rehabilitation protocols. Br J Sports Med. 2013;47(15):953–9. https://doi.org/10.1136/bjsports-2013-092165.
Askling CM, Tengvar M, Tarassova O, Thorstensson A. Acute hamstring injuries in Swedish elite sprinters and jumpers: a prospective randomised controlled clinical trial comparing two rehabilitation protocols. Br J Sports Med. 2014;48(7):532–9. https://doi.org/10.1136/bjsports-2013-093214.
Hamid MSA, Mohamed MR, Yusof A, George J, Lee LPC. Platelet-rich plasma injections for the treatment of hamstring injuries: a randomized controlled trial. Am J Sports Med. 2014;42(10):2410–8. https://doi.org/10.1177/0363546514541540.
Hamilton B, Tol JL, Almusa E, Boukarroum S, Eirale C, Farooq A, et al. Platelet-rich plasma does not enhance return to play in hamstring injuries: a randomised controlled trial. Br J Sports Med. 2015;49(14):943–50. https://doi.org/10.1136/bjsports-2015-094603.
Malliaropoulos N, Papalexandris S, Papalada A, Papacostas E. The role of stretching in rehabilitation of hamstring injuries: 80 athletes follow-up. Med Sci Sports Exerc. 2004;36(5):756–9. https://doi.org/10.1249/01.mss.0000126393.20025.5e.
Reurink G, Goudswaard GJ, Moen MH, Weir A, Verhaar JA, Bierma-Zeinstra SM, et al. Rationale, secondary outcome scores and 1-year follow-up of a randomised trial of platelet-rich plasma injections in acute hamstring muscle injury: the Dutch Hamstring Injection Therapy study. Br J Sports Med. 2015;49(18):1206–12. https://doi.org/10.1136/bjsports-2014-094250.
Silder A, Sherry MA, Sanfilippo J, Tuite MJ, Hetzel SJ, Heiderscheit BC. Clinical and morphological changes following 2 rehabilitation programs for acute hamstring strain injuries: a randomized clinical trial. J Orthop Sports Phys Ther. 2013;43(5):284–99. https://doi.org/10.2519/jospt.2013.4452.
Reurink G, Goudswaard GJ, Moen MH, Weir A, Verhaar JAN, Bierma-Zeinstra SMA, et al. Platelet-rich plasma injections in acute muscle injury. N Engl J Med. 2014;370(26):2546–7. https://doi.org/10.1056/NEJMc1402340.
Hamid MSA, Mohamed Ali MR, Yusof A, George J, Lee LP. Erratum: Platelet-rich plasma injections for the treatment of hamstring injuries: a randomized controlled trial (American Journal of Sports Medicine (2014) 42:10 (2410–2418). DOI: https://doi.org/10.1177/0363546514541540). Am J Sports Med. 2015;43(5):NP13. https://doi.org/10.1177/0363546515583261.
Hamid MSA, Mohamed Ali MR, Yusof A, George J. Platelet-rich plasma (PRP): an adjuvant to hasten hamstring muscle recovery: a randomized controlled trial protocol (ISCRTN66528592). BMC Musculoskelet Disord. 2012;13:138. https://doi.org/10.1186/1471-2474-13-138.
Monajati A, Larumbe-Zabala E, Goss-Sampson M, Naclerio F. The effectiveness of injury prevention programs to modify risk factors for non-contact anterior cruciate ligament and hamstring injuries in uninjured team sports athletes: a systematic review. PLoS ONE. 2016;11(5): e0155272. https://doi.org/10.1371/journal.pone.0155272.
Chebbi S, Chamari K, Van Dyk N, Gabbett T, Tabben M. Hamstring injury prevention for elite soccer players: a real-world prevention program showing the effect of players’ compliance on the outcome. J Strength Cond Res. 2022;36(5):1383–8. https://doi.org/10.1519/JSC.0000000000003505.
Rosado-Portillo A, Chamorro-Moriana G, Gonzalez-Medina G, Perez-Cabezas V. Acute hamstring injury prevention programs in eleven-a-side football players based on physical exercises: systematic review. J Clin Med. 2021;10(9):2029. https://doi.org/10.3390/jcm10092029.
Biz C, Nicoletti P, Baldin G, Bragazzi NL, Crimì A, Ruggieri P. Hamstring strain injury (HSI) prevention in professional and semi-professional football teams: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18(16):8272. https://doi.org/10.3390/ijerph18168272.
Chavarro-Nieto C, Beaven M, Gill N, Hébert-Losier K. Hamstrings injury incidence, risk factors, and prevention in Rugby Union players: a systematic review. Phys Sportsmed. 2021;2021:1–19. https://doi.org/10.1080/00913847.2021.1992601.
Raya-González J, Torres Martin L, Beato M, Rodríguez-Fernández A, Sanchez-Sanchez J. The effects of training based on Nordic hamstring and sprint exercises on measures of physical fitness and hamstring injury prevention in U19 male soccer players. Res Sports Med. 2021;2021:1–16. https://doi.org/10.1080/15438627.2021.2010206.
Claudino JG, Cardoso Filho CA, Bittencourt NFN, Gonçalves LG, Couto CR, Quintão RC, et al. Eccentric strength assessment of hamstring muscles with new technologies: a systematic review of current methods and clinical implications. Sports Med Open. 2021;7(1):10. https://doi.org/10.1186/s40798-021-00298-7.
Breed R, Opar D, Timmins R, Maniar N, Banyard H, Hickey J. Poor reporting of exercise interventions for hamstring strain injury rehabilitation: a scoping review of reporting quality and content in contemporary applied research. J Orthop Sports Phys Ther. 2022;52(3):130–41. https://doi.org/10.2519/jospt.2022.10641.
Shamji R, James SLJ, Botchu R, Khurniawan KA, Bhogal G, Rushton A. Association of the British Athletic Muscle Injury Classification and anatomic location with return to full training and reinjury following hamstring injury in elite football. BMJ Open Sport Exerc Med. 2021;7(2): e001010. https://doi.org/10.1136/bmjsem-2020-001010.
Martin RL, Cibulka MT, Bolgla LA, Koc-Jr TA, Loudon JK, Manske RC, et al. Hamstring strain injury in athletes. J Orthop Sports Phys Ther. 2022;52(3):CPG1-44. https://doi.org/10.2519/jospt.2022.0301.
Edouard P, Branco P, Alonso J-M. Muscle injury is the principal injury type and hamstring muscle injury is the first injury diagnosis during top-level international athletics championships between 2007 and 2015. Br J Sports Med. 2016;50(10):619. https://doi.org/10.1136/bjsports-2015-095559.
Green B, Bourne MN, van Dyk N, Pizzari T. Recalibrating the risk of hamstring strain injury (HSI): a 2020 systematic review and meta-analysis of risk factors for index and recurrent hamstring strain injury in sport. Br J Sports Med. 2020;54(18):1081–8. https://doi.org/10.1136/bjsports-2019-100983.
McKay AKA, Stellingwerff T, Smith ES, Martin DT, Mujika I, Goosey-Tolfrey VL, et al. Defining training and performance caliber: a participant classification framework. Int J Sports Physiol Perform. 2022;17(2):317–31. https://doi.org/10.1123/ijspp.2021-0451.
Ernlund L, Vieira LDA. Hamstring injuries: update article. Rev Bras Ortop (Sao Paulo). 2017;52(4):373–82. https://doi.org/10.1016/j.rboe.2017.05.005.
Arnason A, Sigurdsson SB, Gudmundsson A, Holme I, Engebretsen L, Bahr R. Risk factors for injuries in football. Am J Sports Med. 2004;32(1 Suppl.):5s–16s. https://doi.org/10.1177/0363546503258912.
de Visser HM, Reijman M, Heijboer MP, Bos PK. Risk factors of recurrent hamstring injuries: a systematic review. Br J Sports Med. 2012;46(2):124–30. https://doi.org/10.1136/bjsports-2011-090317.
O’Sullivan L, Tanaka MJ. Sex-based differences in hamstring injury risk factors. J Womens Sport Med. 2021;1(1):20–9. https://doi.org/10.53646/jwsm.v1i1.8.
Cross KM, Gurka KK, Saliba S, Conaway M, Hertel J. Comparison of hamstring strain injury rates between male and female intercollegiate soccer athletes. Am J Sports Med. 2013;41(4):742–8. https://doi.org/10.1177/0363546513475342.
Cheslock JJ. Who's playing college sports? Trends in participation. Research Series. Women's Sports Foundation; 2007.
Mujika I, Taipale RS. Sport science on women, women in sport science. Int J Sports Physiol Perform. 2019;14(8):1013–4. https://doi.org/10.1123/ijspp.2019-0514.
Acknowledgements
José Afonso, Jesús Olivares-Jabalera, Ricardo Fernandes, Filipe Manuel Clemente, Sílvia Rocha-Rodrigues, João Gustavo Claudino, Rodrigo Ramirez-Campillo, Cristina Valente, Renato Andrade, and João Espregueira-Mendes thank Professors Gustaaf Reurink, Jack Hickey, and Noel Pollock for their role as external experts: they verified our eligibility criteria and the list of included studies, providing additional suggestions of potentially relevant studies.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
There was no financial or non-financial support for this review. There were no funders or sponsors in the review.
Conflicts of Interest/Competing Interests
José Afonso, Jesús Olivares‑Jabalera, Ricardo Fernandes, Filipe Manuel Clemente, Sílvia Rocha‑Rodrigues, João Gustavo Claudino, Rodrigo Ramirez‑Campillo, Cristina Valente, Renato Andrade and João Espregueira‑Mendes have no competing interests.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
The data used to inform this review are fully disclosed either in the manuscript or in its Electronic Supplementary Material.
Code Availability
Not applicable.
Authors’ Contributions
JA and RA were responsible for the initial drafting of the article, which was reviewed and edited by all authors. All authors were involved in the conception, design, and interpretation of data. All authors read and reviewed the manuscript critically for important intellectual content and approved the final version to be submitted. Specific contributions pertaining data selection, extraction, and analysis are detailed in the methods section. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Afonso, J., Olivares-Jabalera, J., Fernandes, R.J. et al. Effectiveness of Conservative Interventions After Acute Hamstrings Injuries in Athletes: A Living Systematic Review. Sports Med 53, 615–635 (2023). https://doi.org/10.1007/s40279-022-01783-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-022-01783-z